Ferric Carboxymaltose Improves the Quality of Life of Patients with Inflammatory Bowel Disease and Iron Deficiency without Anaemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Administration of Intravenous Iron
2.3. Aims of the Study
2.4. HRQoL Assessment
2.5. Efficacy and Safety Analysis
2.6. Statistical Analysis
3. Results
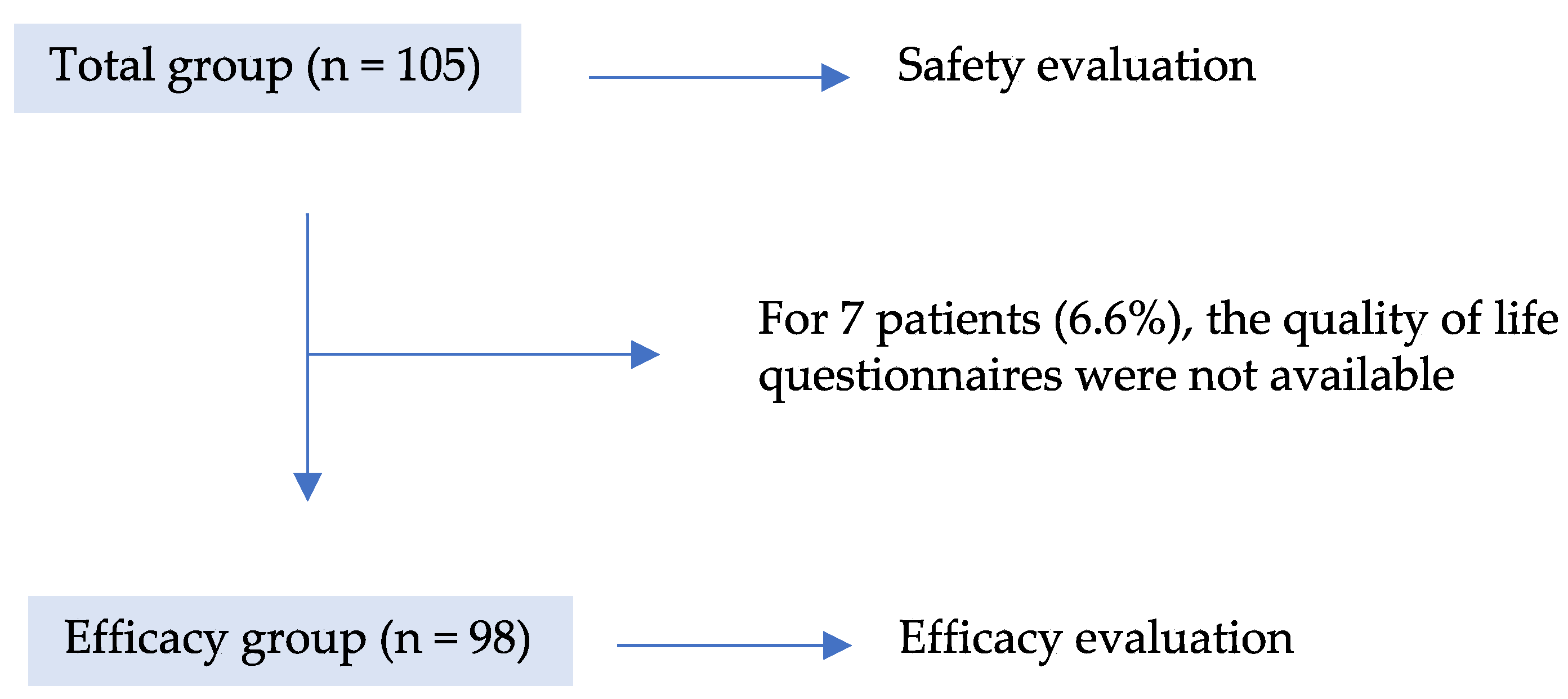
3.1. Patient Population
3.2. Efficacy and Safety of FCM on ID
3.3. Inflammatory Biomarkers and Other Biochemical Parameters
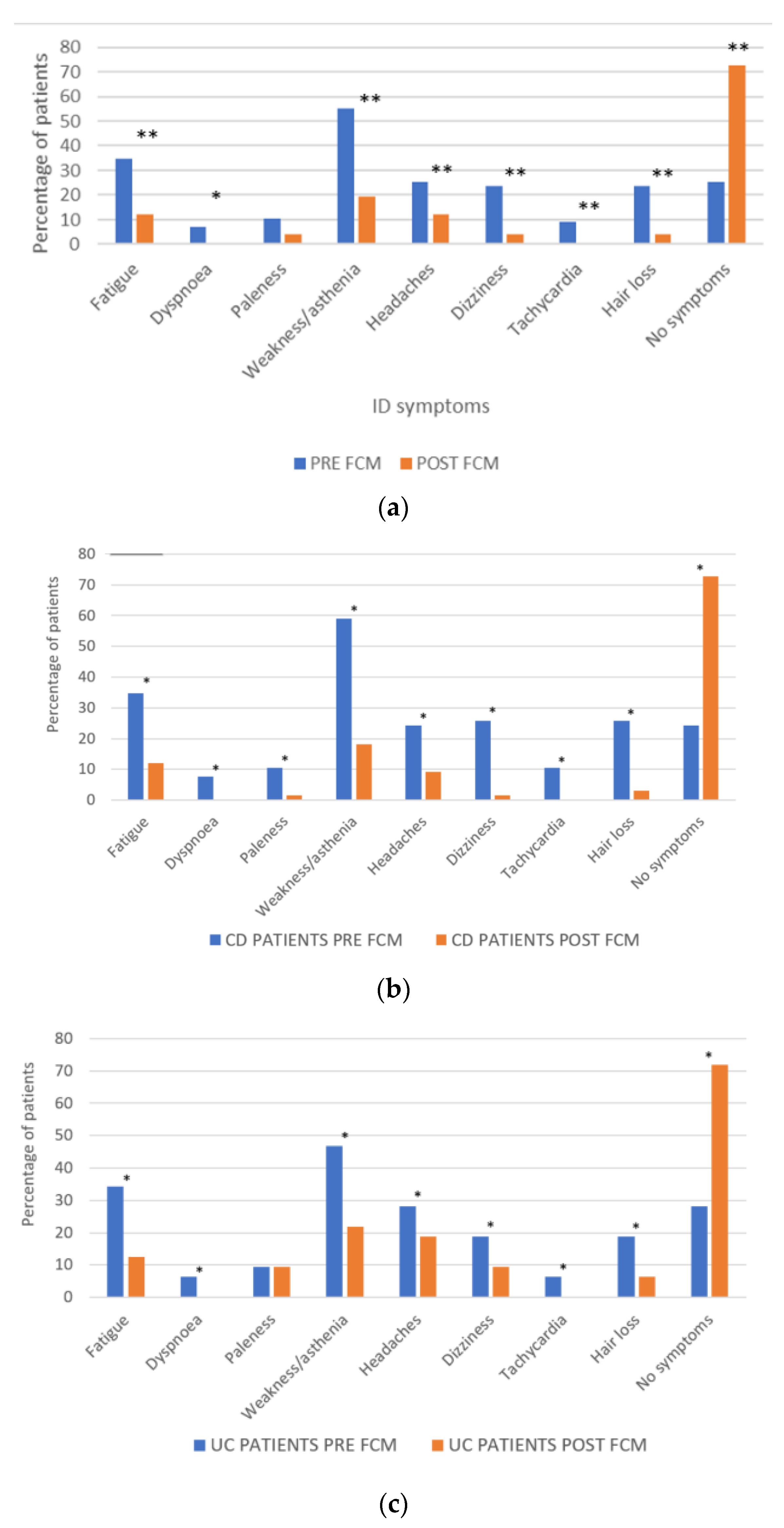
3.4. Impact of FCM on Self-Reported ID Symptoms
3.5. Impact of FCM on Patients’ HRQoL (EQ-5D and SF-12v2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaparro, M.; Barreiro-de Acosta, M.; Benítez, J.M.; Cabriada, J.L.; Casanova, M.J.; Ceballos, D.; Esteve, M.; Fernández, H.; Ginard, D.; Gomollón, F.; et al. Epidemiology, clinical characteristics, evolution and treatments in newly diagnosed inflammatory bowel disease (IBD): Results from the nationwide EpidemIBD study of GETECCU. J. Crohns’s Colitis 2020, 14, S516–S517. [Google Scholar] [CrossRef]
- GBD Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Burisch, J.; Vegh, Z.; Katsanos, K.H.; Christodoulou, D.K.; Lazar, D.; Goldis, A.; O’Morain, C.; Fernandez, A.; Pereira, S.; Myers, S.; et al. Occurrence of Anaemia in the First Year of Inflammatory Bowel Disease in a European Population-based Inception Cohort-An ECCO-EpiCom Study. J. Crohns’s Colitis 2017, 11, 1213–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; Morena, F.D.L.; Moum, B.; et al. Prevalence of anemia in inflammatory bowel diseases in european countries: A systematic review and individual patient data meta-analysis. Inflamm. Bowel Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef]
- Gomollon, F.; Gisbert, J.P. Anemia and inflammatory bowel diseases. World J. Gastroenterol. 2009, 15, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Hartmann, F.; Dignass, A.U. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C.; et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, 846–853.e2. [Google Scholar] [CrossRef]
- Garcia-Lopez, S.; Bocos, J.M.; Gisbert, J.P.; Bajador, E.; Chaparro, M.; Castaño, C.; García-Erce, J.A.; Gomollón, F. High-dose intravenous treatment in iron deficiency anaemia in inflammatory bowel disease: Early efficacy and impact on quality of life. Blood Transfus. 2016, 14, 199–205. [Google Scholar]
- Shah, Y.; Patel, D.; Khan, N. Iron deficiency anemia in IBD: An overlooked comorbidity. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 771–781. [Google Scholar] [CrossRef]
- Gonzalez Alayon, C.; Pedrajas Crespo, C.; Marin Pedrosa, S.; Benítez, J.M.; Iglesias Flores, E.; Salgueiro Rodríguez, I.; Medina Medina, R.; García-Sánchez, V. Prevalence of iron deficiency without anaemia in inflammatory bowel disease and impact on health-related quality of life. Gastroenterol. Hepatol. 2018, 41, 22–29. [Google Scholar] [CrossRef]
- Herrera-deGuise, C.; Casellas, F.; Robles, V.; Navarro, E.; Borruel, N. Iron deficiency in the absence of anemia impairs the perception of health-related quality of life of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 1450–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuluvath, A.; Rayapati, D.; Limketkai, B.; Parian, A. Half of IBD patients without anemia have iron deficiency leading to similar rates of fatigue as iron deficiency anemia. Off. J. Am. Coll. Gastroenterol. 2018, 113, S343. [Google Scholar] [CrossRef]
- Gasche, C.; Berstad, A.; Befrits, R.; Beglinger, C.; Dignass, A.; Erichsen, K.; Gomollon, F.; Hjortswang, H.; Koutroubakis, I.; Kulnigg, S.; et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm. Bowel Dis. 2007, 13, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohns’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.who.int/es/publications/i/item/WHO-NMH-NHD-MNM-11.1 (accessed on 10 February 2022).
- Soppi, E. Iron deficiency without anemia—Common, important, neglected. Clin. Case. Rep. Rev. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Comin-Colet, J.; Enjuanes, C.; Gonzalez, G.; Torrens, A.; Cladellas, M.; Meroño, O.; Ribas, N.; Ruiz, S.; Gómez, M.; Verdú, J.M.; et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur. J. Heart Fail. 2013, 15, 1164–1172. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Rozen-Zvi, B.; Avni, T.; Gafter, U.; Gafter-Gvili, A. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: An updated systematic review and meta-analysis. Am. J. Kidney Dis. 2016, 68, 677–690. [Google Scholar] [CrossRef]
- Çekiç, C.; Ipek, S.; Aslan, F.; Akpınar, Z.; Arabul, M.; Topal, F. The effect of intravenous iron treatment on quality of life in inflammatory bowel disease patients with nonanemic iron deficiency. Gastroenterol. Res. Pract. 2015, 2015, 582163. [Google Scholar] [CrossRef]
- Eliadou, E.; Kini, G.; Huang, J.; Champion, A.; Inns, S.J. Intravenous iron replacement improves quality of life in hypoferritinemic inflammatory bowel disease patients with and without anemia. Dig. Dis. 2017, 35, 444–448. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. 1989, 24 (Suppl. 170), 2–6. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl A), 5A–36A. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Panes, J.; Sandborn, W.J.; Vermeire, S.; Danese, S.; Feagan, B.G.; Colombel, J.F.; Hanauer, S.B.; Rycroft, B. Defining disease severity in inflammatory bowel diseases: Current and future directions. Clin. Gastroenterol. Hepatol. 2016, 14, 348–354.e17. [Google Scholar] [CrossRef] [Green Version]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- European Medicines Agency. Ferinject (Ferric Carboxymaltose) Summary of Product Characteristics; European Medicines Agency: London, UK, 2015; Available online: https://cima.aemps.es/cima/dochtml/ft/69771/FT_69771.html. (accessed on 4 December 2021).
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: www.R-project.org (accessed on 2 December 2020).
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [Green Version]
- Bailie, G.R.; Mason, N.A.; Valaoras, T.G. Safety and tolerability of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Hemodial. Int. 2010, 14, 47–54. [Google Scholar] [CrossRef]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Gafter-Gvili, A.; Paul, M.; Leibovici, L.; Shpilberg, O.; Gafter, U. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: Systematic review and meta-analysis. Am. J. Kidney Dis. 2008, 52, 897–906. [Google Scholar] [CrossRef]
- van Veldhuisen, D.J.; Ponikowski, P.; van der Meer, P.; Metra, M.; Böhm, M.; Doletsky, A.; Voors, A.A.; Macdougall, I.C.; Anker, S.D.; Roubert, B.; et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017, 136, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Alexeeva, O.; Bokemeyer, B.; Chopey, I.; Felder, M.; Gudehus, M.; Iqbal, T.; Khalif, I.; Marteau, P.; Stein, J.; et al. FERGI Study Group. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2013, 11, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Befrits, R.; Wikman, O.; Blomquist, L.; Hjortswang, H.; Hammarlund, P.; Bajor, A.; Klintman, D.; Blom, H. Anemia and iron deficiency in inflammatory bowel disease: An open, prospective, observational study on diagnosis, treatment with ferric carboxymaltose and quality of life. Scand. J. Gastroenterol. 2013, 48, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Lopez, A.; Cummings, J.R.F.; Dignass, A.; Detlie, T.E.; Danese, S. Review article: Treating-to-target for inflammatory bowel disease-associated anaemia. Aliment. Pharmacol. Ther. 2018, 48, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Deloughery, T. Single-dose intravenous iron for iron deficiency: A new paradigm. Hematol. Am Soc Hematol Educ Program 2016, 2016, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.A.; Gaskell, H.; Rose, P.; Allan, J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011, 11, 4. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | n = 98 |
|---|---|
| Mean age (SD), years | 43 (12.41) |
| Gender Female, n (%) | 70 (71.43) |
| Type of disease, n (%) | |
| Crohn’s disease | 66 (67.35) |
| Ulcerative colitis | 32 (32.65) |
| Harvey–Bradshaw index, n (%) | |
| Remission (score 0–4) | 53 (54.07) |
| Mild (score 5–7) | 13 (13.26) |
| Moderate (score 8–16) | 0 |
| Severe (score > 16) | 0 |
| Partial Mayo Scoring Index; n (%) | |
| Remission (score 0–1) | 18 (18.36) |
| Mild (score 2–4) | 14 (14.32) |
| Moderate (score 5–6) | 0 |
| Severe (score 7–9) | 0 |
| Previous surgery, n (%) | |
| Yes | 26 (26.53) |
| No | 72 (73.47) |
| Mean CRP (SD), mg/L a | 4.12 (6.16) |
| Mean faecal calprotectin (SD), µg/g b | 333.21 (647.64) |
| Mean Hb (SD), g/dL | 13.44 (1.02) |
| Mean s-ferritin (SD), μg/L | 48.38 (64.60) |
| Mean s-iron (SD), μg/dL | 51.92 (41.03) |
| Mean TSAT (SD), % | 12.78 (4.19) |
| Mean vitamin B12 (SD), pg/mL c | 346.91 (270.79) |
| Mean folic acid (SD), ng/mL d | 7.01 (3.34) |
| Treatment, n (%) | |
| Mesalazine | 31 (31.63) |
| Steroids | 6 (6.12) |
| Thiopurines | 24 (24.49) |
| Methotrexate | 4 (4.08) |
| Anti-TNF | 38 (38.78) |
| Vedolizumab | 6 (6.12) |
| Ustekinumab | 11 (11.22) |
| Apheresis | 2 (2.04) |
| All Patients (n = 98) | Pre-FCM | Post-FCM | 95% CI | p-Value * |
| Mean s-ferritin, μg/L n (pre/post) 1 = 87 | 48.4 | 175.0 | 126.6 (97.5, 135) | <0.001 |
| Mean s-iron, μg/L n (pre/post) 1 = 84 | 51.9 | 84.4 | 32.5 (28.5, 44) | <0.001 |
| Mean TSAT, % n (pre/post) 1 = 75 | 12.8 | 27.2 | 14.4 (11.6, 17) | <0.001 |
| Patients with CD (n = 66) | Pre-FCM | Post-FCM | 95% CI | p-Value * |
| Mean s-ferritin, μg/L n (pre/post) 1 = 56 | 44.7 | 175.0 | 130.3 (94.5, 142.5) | <0.01 |
| Mean s-iron, μg/L n (pre/post) 1 = 51 | 47.6 | 76.3 | 28.7 (22.0, 38.5) | <0.01 |
| Mean TSAT, % n (pre/post) 1 = 44 | 13.1 | 24.9 | 11.8 (11.6, 17.0) | <0.01 |
| Patients with UC (n = 32) | Pre-FCM | Post-FCM | 95% CI | p-Value * |
| Mean s-ferritin, μg/L n (pre/post) 1 = 28 | 56.4 | 174.8 | 118.4 (78.5, 154.0) | <0.01 |
| Mean s-iron, μg/L n (pre/post) 1 = 28 | 60.6 | 99.9 | 39.3 (33.5, 65.5) | <0.01 |
| Mean TSAT, % n (pre/post) 1 = 26 | 12.1 | 31.2 | 19.1 (11.6, 17.0) | <0.01 |
| Problem (% of Patients) | Pre-FCM | Post-FCM | Difference | p-Value * |
|---|---|---|---|---|
| Mobility Self-care | 23.40 | 16.67 | –6.74 | 0.194 |
| 9.57 | 3.57 | –6.00 | 0.136 | |
| Usual activities | 55.32 | 35.71 | –19.60 | 0.002 |
| Pain/discomfort | 70.21 | 53.57 | –16.64 | 0.003 |
| Anxiety/depression | 55.32 | 28.57 | –26.75 | <0.001 |
| With no problems | 17.02 | 39.29 | +22.26 | 0.008 |
| Pre-FCM | Post-FCM | p-Value * | |
|---|---|---|---|
| All | 0.721 (0.283) | 0.823 (0.236) | <0.001 |
| Women | 0.692 (0.294) | 0.807 (0.240) | <0.001 |
| Men | 0.792 (0.243) | 0.865 (0.225) | na |
| CD | 0.694 (0.320) | 0.816 (0.249) | <0.01 |
| UC | 0.773 (0.187) | 0.835 (0.216) | 0.02 |
| Pre-FCM | Post-FCM | p-Value * | |
|---|---|---|---|
| All | 62.6 (18.8) | 70.1 (18.8) | <0.01 |
| Women | 61.6 (18.9) | 69.0 (19.3) | <0.01 |
| Men | 64.8 (18.7) | 73.0 (17.6) | na |
| CD | 62.1 (19.6) | 69.4 (20.2) | 0.057 |
| UC | 63.4 (17.3) | 71.4 (16.9) | <0.01 |
| Mean SF-12v2 Scores (SD) | |||
|---|---|---|---|
| All Patients | Pre-FCM | Post-FCM | p-Value * |
| Physical score | 42.2 (10.66) | 45.1 (10.68) | 0.002 |
| Mental score | 42.2 (13.08) | 49.5 (12.23) | <0.001 |
| CD | |||
| Physical score | 41.7 (11.0) | 44.3 (10.5) | 0.01 |
| Mental score | 42.7 (12.2) | 48.4 (13.1) | <0.001 |
| UC | |||
| Physical score | 43.2 (10.1) | 46.6 (11.1) | 0.06 |
| Mental score | 41.1 (14.9) | 51.5 (10.5) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huguet, J.M.; Cortés, X.; Boscá-Watts, M.M.; Muñoz, M.; Maroto, N.; Iborra, M.; Hinojosa, E.; Capilla, M.; Asencio, C.; Amoros, C.; et al. Ferric Carboxymaltose Improves the Quality of Life of Patients with Inflammatory Bowel Disease and Iron Deficiency without Anaemia. J. Clin. Med. 2022, 11, 2786. https://doi.org/10.3390/jcm11102786
Huguet JM, Cortés X, Boscá-Watts MM, Muñoz M, Maroto N, Iborra M, Hinojosa E, Capilla M, Asencio C, Amoros C, et al. Ferric Carboxymaltose Improves the Quality of Life of Patients with Inflammatory Bowel Disease and Iron Deficiency without Anaemia. Journal of Clinical Medicine. 2022; 11(10):2786. https://doi.org/10.3390/jcm11102786
Chicago/Turabian StyleHuguet, Jose María, Xavier Cortés, Marta Maia Boscá-Watts, Margarita Muñoz, Nuria Maroto, Marisa Iborra, Esther Hinojosa, María Capilla, Carmina Asencio, Cirilo Amoros, and et al. 2022. "Ferric Carboxymaltose Improves the Quality of Life of Patients with Inflammatory Bowel Disease and Iron Deficiency without Anaemia" Journal of Clinical Medicine 11, no. 10: 2786. https://doi.org/10.3390/jcm11102786
APA StyleHuguet, J. M., Cortés, X., Boscá-Watts, M. M., Muñoz, M., Maroto, N., Iborra, M., Hinojosa, E., Capilla, M., Asencio, C., Amoros, C., & Paredes, J. M. (2022). Ferric Carboxymaltose Improves the Quality of Life of Patients with Inflammatory Bowel Disease and Iron Deficiency without Anaemia. Journal of Clinical Medicine, 11(10), 2786. https://doi.org/10.3390/jcm11102786






