The Influence of Rheumatoid Arthritis on Higher Reoperation Rates over Time Following Lumbar Spinal Fusion—A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design and Cohort
3. Results
3.1. Characteristics and Comorbidities between the Two Groups
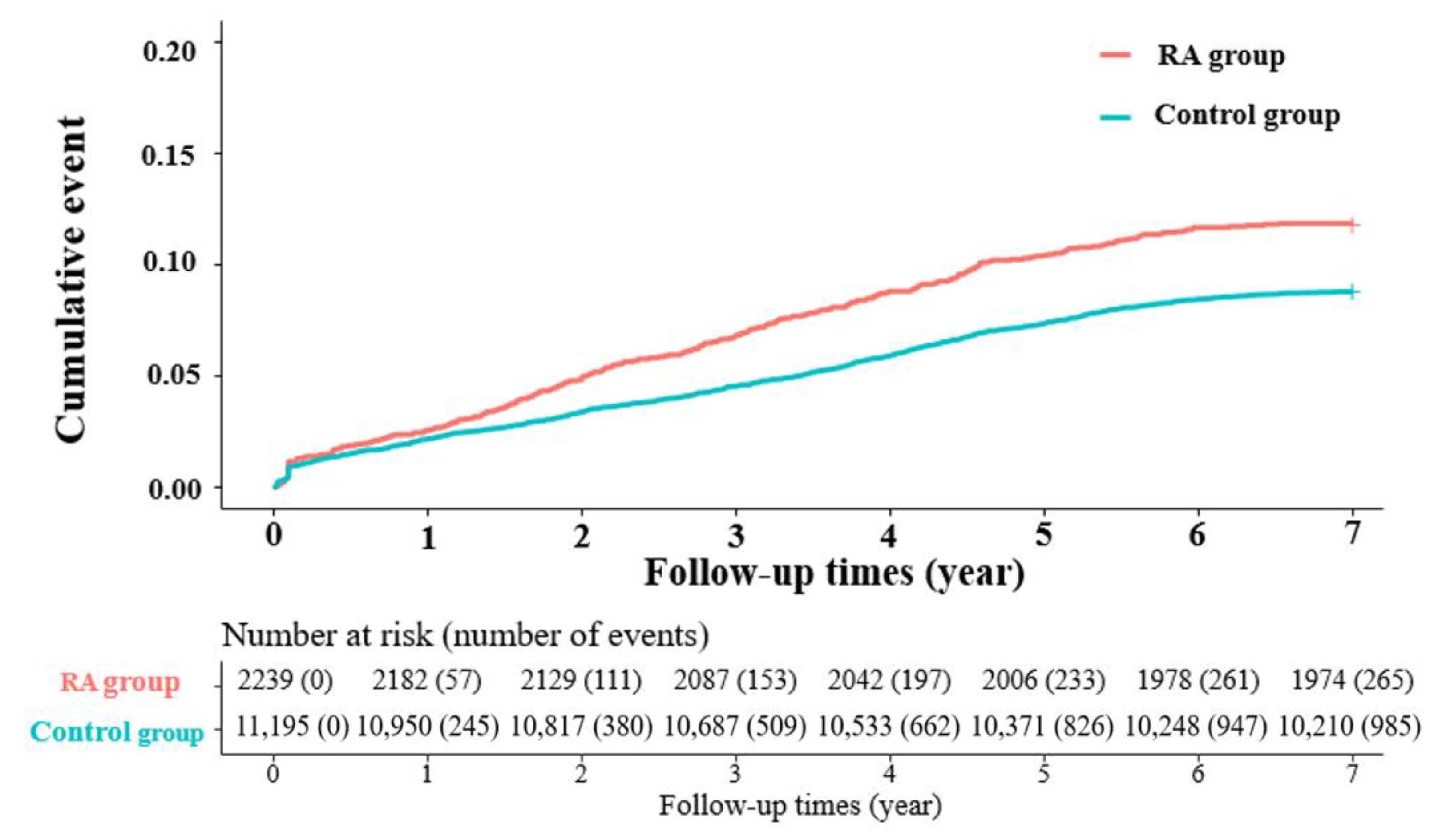
3.2. Risk of Reoperation in RA Patients
3.3. Risk of Reoperation in RA Patients with Progression over Time
3.4. Subgroup Analysis of the Risk of Reoperation in RA Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawaguchi, Y.; Matsuno, H.; Kanamori, M.; Ishihara, H.; Ohmori, K.; Kimura, T. Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. Clin. Spine Surg. 2003, 16, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, M.; Tada, K.; Yonenobu, K. Problems of posterior lumbar interbody fusion (PLIF) for the rheumatoid spondylitis of the lumbar spine. Arch. Orthop. Trauma Surg. 2002, 122, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-N.; Kim, C.; Moon, J. The outcomes of instrumented posterolateral lumbar fusion in patients with rheumatoid arthritis. Bone Jt. J. 2016, 98, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N. The global burden of rheumatoid arthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Hirano, N.; Matsushita, I.; Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Motomura, H.; Suzuki, K.; Yahara, Y.; Watanabe, K. Lumbar spine surgery in patients with rheumatoid arthritis (RA): What affects the outcomes? Spine J. 2018, 18, 99–106. [Google Scholar] [CrossRef]
- Crawford, C.H.; Carreon, L.Y.; Djurasovic, M.; Glassman, S.D. Lumbar fusion outcomes in patients with rheumatoid arthritis. Eur. Spine J. 2008, 17, 822–825. [Google Scholar] [CrossRef]
- Park, J.S.; Jang, H.D.; Hong, J.Y.; Park, Y.S.; Han, K.; Suh, S.W.; Park, S.Y.; Kim, B.T. Impact of ankylosing spondylitis on depression: A nationwide cohort study. Sci. Rep. 2019, 9, 6736. [Google Scholar] [CrossRef]
- Sakai, T.; Sairyo, K.; Hamada, D.; Higashino, K.; Katoh, S.; Takata, Y.; Shinomiya, F.; Yasui, N. Radiological features of lumbar spinal lesions in patients with rheumatoid arthritis with special reference to the changes around intervertebral discs. Spine J. 2008, 8, 605–611. [Google Scholar] [CrossRef]
- Mitsuyama, T.; Kubota, M.; Yuzurihara, M.; Mizuno, M.; Hashimoto, R.; Ando, R.; Okada, Y. The pitfalls in surgical management of lumbar canal stenosis associated with rheumatoid arthritis. Neurol. Med. Chir. 2013, 53, 853–860. [Google Scholar] [CrossRef]
- Koakutsu, T.; Morozumi, N.; Koizumi, Y.; Ishii, Y. Lumbar radiculopathy caused by foraminal stenosis in rheumatoid arthritis. Upsala J. Med. Sci. 2011, 116, 133–137. [Google Scholar] [CrossRef][Green Version]
- Helliwell, P.; Zebouni, L.; Porter, G.; Wright, V. A clinical and radiological study of back pain in rheumatoid arthritis. Rheumatology 1993, 32, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kawaji, H.; Miyamoto, M.; Gembun, Y.; Ito, H. A case report of rapidly progressing cauda equina symptoms due to rheumatoid arthritis. J. Nippon. Med. Sch. 2005, 72, 290–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirohashi, N.; Sakai, T.; Sairyo, K.; Oba, K.; Higashino, K.; Katoh, S.; Yasui, N. Lumbar radiculopathy caused by extradural rheumatoid nodules: Case report. J. Neurosurg. Spine 2007, 7, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Chung, C.K.; Park, C.S.; Choi, B.; Kim, M.J.; Park, B.J. Reoperation rate after surgery for lumbar herniated intervertebral disc disease: Nationwide cohort study. Spine 2013, 38, 581–590. [Google Scholar] [CrossRef]
- Martin, B.I.; Mirza, S.K.; Comstock, B.A.; Gray, D.T.; Kreuter, W.; Deyo, R.A. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine 2007, 32, 382–387. [Google Scholar] [CrossRef]
- Park, J.-S.; Shim, K.-D.; Song, Y.-S.; Park, Y.-S. Risk factor analysis of adjacent segment disease requiring surgery after short lumbar fusion: The influence of rheumatoid arthritis. Spine J. 2018, 18, 1578–1583. [Google Scholar] [CrossRef]
- Olsen, N.J.; Stein, C.M. New drugs for rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2167–2179. [Google Scholar] [CrossRef]
- Saleh, K.J.; Kurdi, A.J.; El-Othmani, M.M.; Voss, B.A.; Tzeng, T.H.; Saleh, J.; Lane, J.M.; Mihalko, W.M. Perioperative treatment of patients with rheumatoid arthritis. JAAOS-J. Am. Acad. Orthop. Surg. 2015, 23, e38–e48. [Google Scholar] [CrossRef]
- Seki, E.; Matsushita, I.; Sugiyama, E.; Taki, H.; Shinoda, K.; Hounoki, H.; Motomura, H.; Kimura, T. Radiographic progression in weight-bearing joints of patients with rheumatoid arthritis after TNF-blocking therapies. Clin. Rheumatol. 2009, 28, 453–460. [Google Scholar] [CrossRef]
- Meng, J.; Li, Y.; Yuan, X.; Lu, Y. Evaluating osteoporotic fracture risk with the Fracture Risk Assessment Tool in Chinese patients with rheumatoid arthritis. Medicine 2017, 96, e6677. [Google Scholar] [CrossRef]
- Ursum, J.; Britsemmer, K.; van Schaardenburg, D.; Lips, P.T.; Dijkmans, B.A.; Lems, W. High prevalence of vertebral deformities in elderly patients with early rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 1512–1513. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Hanyu, T.; Sugitani, H.; Murai, T.; Fujisawa, J.; Nakazono, K.; Kondo, N.; Endo, N. Risk factors for vertebral fracture in menopausal or postmenopausal Japanese women with rheumatoid arthritis: A cross-sectional and longitudinal study. J. Bone Miner. Metab. 2006, 24, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, M.; Kolta, S.; Briot, K.; Fechtenbaum, J.; Paternotte, S.; Roux, C. Prevalence of vertebral fractures in patients with rheumatoid arthritis: Revisiting the role of glucocorticoids. Osteoporos. Int. 2012, 23, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, R.E.; Haugeberg, G.; Uhlig, T.; Mowinckel, P.; Falch, J.A.; Halse, J.I.; Kvien, T.K. Incidence of vertebral deformities in 255 female rheumatoid arthritis patients measured by morphometric X-ray absorptiometry. Osteoporos. Int. 2005, 16, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.N.; Kurucan, E.; Menga, E.N.; Molinari, R.W.; Rubery, P.T.; Mesfin, A. Comparison of adult spinal deformity patients with and without rheumatoid arthritis undergoing primary non-cervical spinal fusion surgery: A nationwide analysis of 52,818 patients. Spine J. 2018, 18, 1861–1866. [Google Scholar] [CrossRef] [PubMed]

| RA Group (n = 2239) | Control Group (n = 11,195) | Total (n = 13,434) | p-Value | |
|---|---|---|---|---|
| Age | 64.5 ± 8.8 | 64.5 ± 8.8 | 64.5 ± 8.8 | 0.96 |
| Sex (male) | 603 (26.9%) | 3033 (27.1%) | 3636 (27.1%) | 0.876 |
| Age group | ||||
| 40–49 | 112 (5.0%) | 561 (5.0%) | 673 (5.0%) | 0.997 |
| 50–59 | 562 (25.1%) | 2785 (24.9%) | 3347 (24.9%) | |
| 60–69 | 839 (37.5%) | 4207 (37.6%) | 5046 (37.6%) | |
| ≥70 | 726 (32.4%) | 3642 (32.5%) | 4368 (32.5%) | |
| Comorbidity | ||||
| DM | 594 (26.5%) | 2710 (24.2%) | 3304 (24.6%) | 0.02 |
| Depression | 308 (13.8%) | 951 (8.5%) | 1259 (9.4%) | <0.001 |
| Osteoporosis | 662 (29.6%) | 2432 (21.7%) | 3094 (23.0%) | <0.001 |
| Parkinson | 25 (1.1%) | 120 (1.1%) | 145 (1.1%) | 0.852 |
| Peripheral vascular | 371 (16.6%) | 1580 (14.1%) | 1951 (14.5%) | 0.003 |
| ESRD | 20 (0.9%) | 99 (0.9%) | 119 (0.9%) | 0.967 |
| Liver disease | 279 (12.5%) | 929 (8.3%) | 1208 (9.0%) | <0.001 |
| HTN | 1305 (58.3%) | 5986 (53.5%) | 7291 (54.3%) | <0.001 |
| Cerebrovascular | 224 (10.0%) | 1029 (9.2%) | 1253 (9.3%) | 0.227 |
| Chronic pulmonary | 560 (25.0%) | 2110 (18.8%) | 2670 (19.9%) | <0.001 |
| Comorbidity number | ||||
| 0 | 596 (26.6%) | 3781 (33.8%) | 4377 (32.6%) | <0.001 |
| 1–2 | 1393 (62.2%) | 6535 (58.4%) | 7928 (59.0%) | |
| ≥3 | 250 (11.2%) | 879 (7.9%) | 1129 (8.4%) | |
| Patient Group | n | Reoperation | Duration | Rate | HR (95% CI) | |
|---|---|---|---|---|---|---|
| Crude HR | Adjusted HR | |||||
| Controls | 11,195 | 958 | 1032.2 ± 700.9 | 8.8 | 1 (ref.) | 1 (ref.) |
| RA patients | 2239 | 265 | 964.8 ± 647.2 | 11.8 | 1.44 (1.24–1.66) | 1.31 (1.10–1.60) |
| <3 Months | 3 Months–1 Year | >1 Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reoperation | Rate | Adjusted HR (95% CI) | p-Value | Reoperation | Rate | Adjusted HR (95% CI) | p-Value | Reoperation | Rate | Adjusted HR (95% CI) | p-Value |
| 77 | 0.69 | 1 (ref.) | 0.699 | 168 | 1.50 | 1 (ref.) | 0.44 | 740 | 6.61 | 1 (ref.) | 0.002 |
| 15 | 0.67 | 1.13 (0.61–2.11) | 42 | 1.88 | 0.87 (0.61–1.24) | 208 | 9.29 | 1.31 (1.11–1.57) | |||
| Variables | <3 Months | 3 Months–1 Year | <1 Year | ||||
|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | ||
| Sex | Female | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Male | 1.04 (0.67–1.66) | 0.860 | 0.70 (0.50–0.97) | 0.031 | 1.12 (0.96–1.32) | 0.153 | |
| Age group | 40–49 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| 50–59 | 1.18 (0.42–3.35) | 0.750 | 0.52 (0.24–1.17) | 0.114 | 0.69 (0.47–1.03) | 0.072 | |
| 60–69 | 1.76 (0.65–4.76) | 0.266 | 0.64 (0.30–1.35) | 0.239 | 0.74 (0.50–1.09) | 0.123 | |
| ≥70 | 1.00 (0.35–2.83) | 0.997 | 0.85 (0.40–1.81) | 0.677 | 0.86 (0.58–1.29) | 0.470 | |
| Osteoporosis | No | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Yes | 2.14 (1.15–4.02) | 0.017 | 1.02 (0.70–1.49) | 0.914 | 1.04 (0.87–1.23) | 0.698 | |
| Parkinson’s | No | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Yes | 1.64 (0.20–13.85) | 0.647 | 1.54 (0.46–5.21) | 0.486 | 1.15 (0.66–2.00) | 0.621 | |
| DM | No | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Yes | 1.42 (0.82–2.43) | 0.209 | 0.82 (0.59–1.13) | 0.225 | 1.05 (0.89–1.24) | 0.571 | |
| Depression | No | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Yes | 2.38 (1.09–5.19) | 0.030 | 1.37 (0.92–2.05) | 0.125 | 0.94 (0.74–1.19) | 0.595 | |
| Comorbidity | 0 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| 1–2 | 0.67 (0.37–1.21) | 0.184 | 0.97 (0.68–1.39) | 0.881 | 0.93 (0.78–1.10) | 0.394 | |
| ≥3 | 0.48 (0.19–1.21) | 0.118 | 0.98 (0.59–1.64) | 0.949 | 0.90 (0.68–1.20) | 0.475 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-S.; Park, S.-J.; Park, J.; Shin, G.; Hong, J.-Y. The Influence of Rheumatoid Arthritis on Higher Reoperation Rates over Time Following Lumbar Spinal Fusion—A Nationwide Cohort Study. J. Clin. Med. 2022, 11, 2788. https://doi.org/10.3390/jcm11102788
Park J-S, Park S-J, Park J, Shin G, Hong J-Y. The Influence of Rheumatoid Arthritis on Higher Reoperation Rates over Time Following Lumbar Spinal Fusion—A Nationwide Cohort Study. Journal of Clinical Medicine. 2022; 11(10):2788. https://doi.org/10.3390/jcm11102788
Chicago/Turabian StylePark, Jin-Sung, Se-Jun Park, Jiwon Park, Gijun Shin, and Jae-Young Hong. 2022. "The Influence of Rheumatoid Arthritis on Higher Reoperation Rates over Time Following Lumbar Spinal Fusion—A Nationwide Cohort Study" Journal of Clinical Medicine 11, no. 10: 2788. https://doi.org/10.3390/jcm11102788
APA StylePark, J.-S., Park, S.-J., Park, J., Shin, G., & Hong, J.-Y. (2022). The Influence of Rheumatoid Arthritis on Higher Reoperation Rates over Time Following Lumbar Spinal Fusion—A Nationwide Cohort Study. Journal of Clinical Medicine, 11(10), 2788. https://doi.org/10.3390/jcm11102788






