Perinatal and Neonatal Outcomes in Fetal Growth Restriction and Small for Gestational Age
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Maternal Data
3.2. Perinatal Data
4. Discussion
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cetin, I.; Sparks, J.W. Determinants of intrauterine growth. In Neonatal Nutrition and Metabolism; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Baschat, A.A. Fetal responses to placental insufficiency: An update. BJOG 2004, 111, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Mandò, C.; Calabrese, S. Maternal predictors of intrauterine growth restriction. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Pels, A.; Beune, I.M.; van Wassenaer-Leemhuis, A.G.; Limpens, J.; Ganzevoort, W. Early-onset fetal growth restriction: A systematic review on mortality and morbidity. Acta Obstet. Gynecol. Scand. 2020, 99, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibley, C.P.; Turner, M.A.; Cetin, I.; Ayuk, P.; Boyd, C.A.; D’Souza, S.W.; Glazier, J.D.; Greenwood, S.L.; Jansson, T.; Powell, T. Placental phenotypes of intrauterine growth. Pediatr. Res. 2005, 58, 827–832. [Google Scholar] [CrossRef]
- Pardi, G.; Cetin, I.; Marconi, A.M.; Lanfranchi, A.; Bozzetti, P.; Farrazzi, E.; Buscaglia, M.; Battaglia, F.C. Diagnostic value of blood sampling in fetuses with growth retardation. N. Engl. J. Med. 1993, 328, 692–696. [Google Scholar] [CrossRef]
- Cetin, I.; Taricco, E.; Mandò, C.; Radaelli, T.; Boito, S.; Nuzzo, A.M.; Giussani, D.A. Fetal Oxygen and Glucose Consumption in Human Pregnancy Complicated by Fetal Growth Restriction. Hypertension 2020, 75, 748–754. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Meschia, G. Fetal Nutrition. Annu. Rev. Nutr. 2003, 8, 43–61. [Google Scholar] [CrossRef]
- Morriss, F.H.; Makowski, E.L.; Meschia, G.; Battaglia, F.C. The glucose/oxygen quotient of the term human fetus. Biol. Neonate 1974, 25, 44–52. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obs. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacós, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef]
- Bertino, E.; Spada, E.; Occhi, L.; Coscia, A.; Giuliani, F.; Gagliardi, L.; Gilli, G.; Bona, G.; Fabris, C.; De Curtis, M.; et al. Neonatal Anthropometric Charts: The Italian Neonatal Study Compared With Other European Studies. J. Pediatric Gastroenterol. Nutr. 2010, 51, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damhuis, S.E.; Ganzevoort, W.; Gordijn, S.J. Abnormal Fetal Growth Small for Gestational Age, Fetal Growth Restriction, Large for Gestational Age: Definitions and Epidemiology. Obstet. Gynecol. Clin. N. Am. 2021, 48, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Pallotto, E.K.; Kilbride, H.W. Perinatal Outcome and Later Implications of Intrauterine Growth Restriction. Clin. Obstet. Gynecol. 2006, 49, 257–269. [Google Scholar] [CrossRef]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status With Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatrics 2020, 174, 1. [Google Scholar] [CrossRef] [PubMed]
- Henrichs, J.; de Jonge, A.; Westerneng, M.; Verfaille, V.; Franx, A.; van der Horst, H.E.; Bosmans, J.E.; IRIS Study Group. Cost-Effectiveness of Routine Third Trimester Ultrasound Screening for Fetal Growth Restriction Compared to Care as Usual in Low-Risk Pregnancies: A Pragmatic Nationwide Stepped-Wedge Cluster-Randomized Trial in The Netherlands (the IRIS Study). Int. J. Environ. Res. Public Health 2022, 19, 3312. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, U.; Todros, T.; Ferrazzi, E.; Zorzoli, A.; Groli, C.; Zucca, S.; Tinti, A.; Dodero, D.; Destro, F.; Ceccarello, P. Transverse fetal growth curves. A multicenter study. Minerva Ginecol. 1986, 38, 873–887. [Google Scholar]
- Maršál, K.; Persson, P.H.; Larsen, T.; Lilja, H.; Selbing, A.; Sultan, B.L. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996, 85, 843–848. [Google Scholar] [CrossRef]
- Farsetti, D.; Pometti, F.; Tiralongo, G.M.; Lo Presti, D.; Pisani, I.; Gagliardi, G.; Vasapollo, B.; Novelli, G.P.; Valensise, H. Fetal Umbilical Vein Flow in the Classification of Fetuses with Growth Restriction. Reprod. Med. 2021, 2, 50–56. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacos, E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 48–58. [Google Scholar] [CrossRef]
- RCOG. The Investigation and Management of the Small–for–Gestational–Age Fetus. Green-Top Guideline, 2nd ed.; Royal College of Obstetricians and Gynaecologists: London, UK, 2014. [Google Scholar]
- Doctor, B.A.; O’Riordan, M.A.; Kirchner, H.L.; Shah, D.; Hack, M. Perinatal correlates and neonatal outcomes of small for gestational age infants born at term gestation. Am. J. Obs. Gynecol. 2001, 185, 652–659. [Google Scholar] [CrossRef]
- Larkin, J.C.; Chauhan, S.P.; Simhan, H.N. Small for Gestational Age: The Differential Mortality When Detected versus Undetected Antenatally. Am. J. Perinatol. 2017, 34, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Romero, R.; Stampalija, T.; Dall’Asta, A.; DeVore, G.; Prefumo, F.; Frusca, T.; Visser, G.H.; Hobbins, J.; Baschat, A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obs. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Martín-Palumbo, G.; Atanasova, V.B.; Rego Tejeda, M.T.; Antolín Alvarado, E.; Bartha, J.L. Third trimester ultrasound estimated fetal weight for increasing prenatal prediction of small-for-gestational age newborns in low-risk pregnant women. J. Matern. Fetal Neonatal. Med. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Robert Peter, J.; Ho, J.J.; Valliapan, J.; Sivasangari, S. Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database Syst. Rev. 2015, 2015, CD008136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papageorghiou, A.T.; Ohuma, E.O.; Gravett, M.G.; Hirst, J.; Da Silveira, M.F.; Lambert, A.; Carvalho, M.; Jaffer, Y.A.; Altman, D.G.; Noble, J.A.; et al. International standards for symphysis-fundal height based on serial measurements from the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: Prospective cohort study in eight countries On behalf of the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). Obstet. Gynecol. Surv. 2017, 72, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Odibo, A.O.; Nwabuobi, C.; Odibo, L.; Leavitt, K.; Obican, S.; Tuuli, M.G. Customized fetal growth standard compared with the INTERGROWTH-21st century standard at predicting small-for-gestational-age neonates. Acta Obs. Gynecol. Scand. 2018, 97, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Mantel, Ä.; Hirschberg, A.L.; Stephansson, O. Association of Maternal Eating Disorders With Pregnancy and Neonatal Outcomes. JAMA Psychiatry 2020, 77, 285–293. [Google Scholar] [CrossRef]
- Koubaa, S.; Hällström, T.; Brismar, K.; Hellström, P.M.; Hirschberg, A.L. Biomarkers of nutrition and stress in pregnant women with a history of eating disorders in relation to head circumference and neurocognitive function of the offspring. BMC Pregnancy Childbirth 2015, 15, 318. [Google Scholar] [CrossRef] [Green Version]
| Features | AGA (N = 655) | SGA (N = 62) | Early-FGR (N = 132) | Late-FGR (N = 57) |
|---|---|---|---|---|
| Age (yrs) | 33.0 (30–36) | 33.0 (30–37) | 35.0 (30–38) | 33.0 (30–36) |
| Pregestational weight (kg) | 67.0 (60–74) | 56.0 (50–60) | 60.0 (52–70) | 54.0 (41–67) |
| ***, ++ | *** | ***, + | ||
| BMI (kg/m²) | 24.7 (22.5–27.3) | 20.7 (19.1–23.3) | 22.3 (19.1–25.4) | 19.8 (18.5–22.8) |
| ***, + | *** | ***, ++ | ||
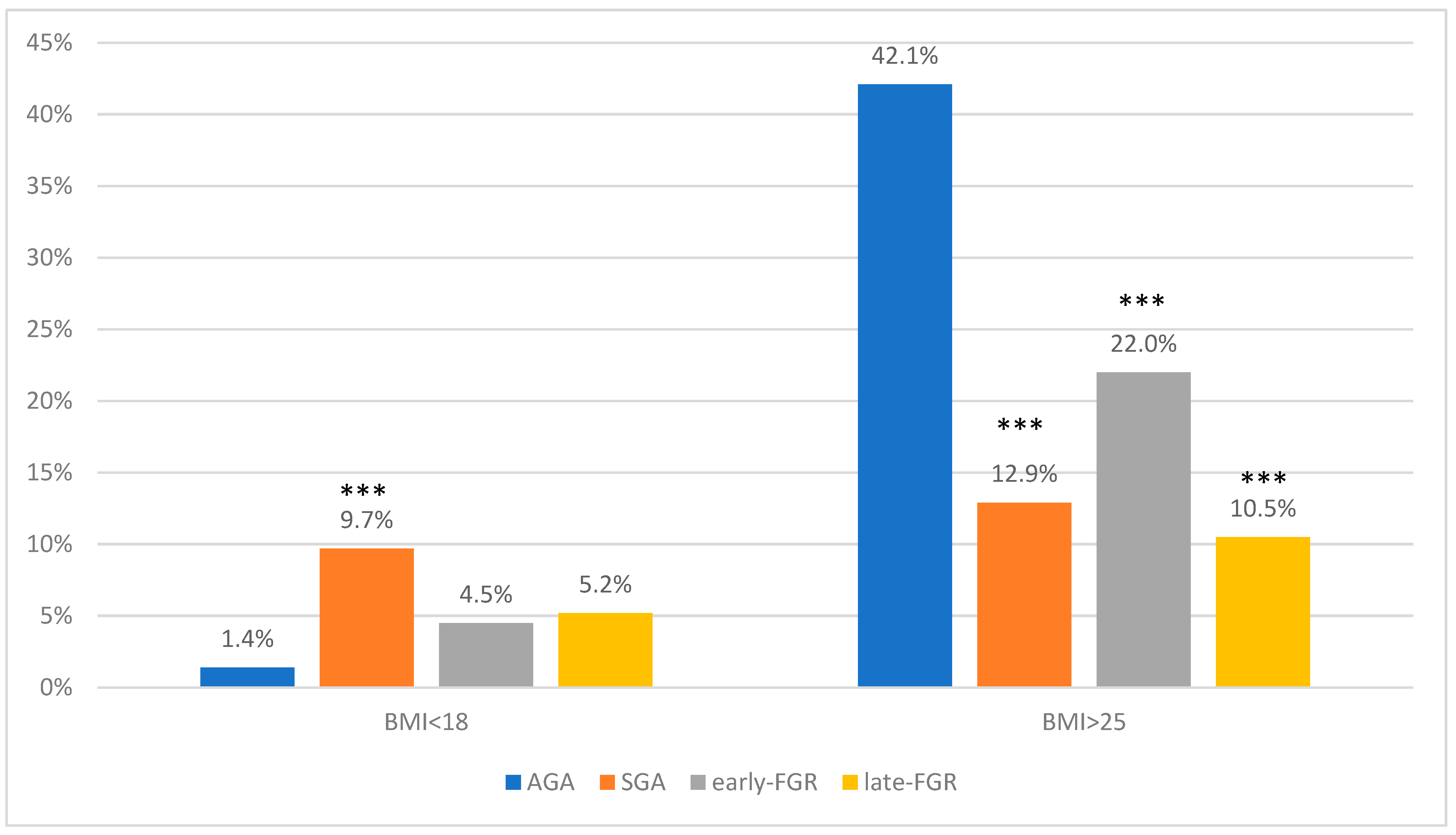
| <18 kg/m² | 9 (1.4%) | 6 (9.7%) | 6 (4.5%) | 3 (5.2%) |
| *** | ||||
| >25 kg/m² | 267 (42%) | 8 (12.9%) | 29 (22%) | 6 (10.5%) |
| *** | *** | *** | ||
| Weight gain (kg) | 11.0 (6–16) | 10.0 (8–13) | 8.5 (7–11.5) | 10.0 (8–13) |
| ***, ^ | * | |||
| Weight gain/gestational age at delivery (g/week) | 282.1 (236.8–368.4) | 259.8 (205.1–325) * | 258.3 (95.3–308.4) *** | 263.2(194.4–333.3) |
| Pregnancy | ||||
| Primiparous | 306 (47%) | 39 (63%) | 98 (78%) | 37 (67%) |
| * | ***, ^ | * | ||
| Multiparous | 349 (53%) | 23 (37%) | 28 (22%) | 18 (33%) |
| Conception | ||||
| ART | 0 | 1 (2%) | 11 (9%) | 4 (8%) |
| Spontaneous | 655 (100%) | 56 (98%) | 119 (91%) | 52 (92%) |
| Ethnicity | ||||
| Caucasian | 500 (77%) | 102 (79%) | 102 (80%) | 49 (87%) |
| African | 11 (2%) | 0 | 6 (5%) | - |
| Asian | 65 (10%) | 4 (6.5%) | 10 (8%) | 1 (2%) |
| Middle Eastern | 30 (5%) | 4 (6.5%) | 4 (3%) | 4 (7%) |
| South American | 38 (6%) | 5 (8%) | 5 (4%) | 2 (4%) |
| Groups | BMI < 18 | BMI > 25 |
|---|---|---|
| OR (95% CI:); p * | OR (95% CI:); p * | |
| SGA | OR 3.5 (1.57–7.77); 0.002 | OR 0.14 (0.69–0.28); 0.0001 |
| early-FGR | OR 3.48 (1.7–7.04); 0.0005 | OR 0.38 (0.25–0.59); 0.0001 |
| late-FGR | OR 4.88 (2.25–10.6); 0.0001 | OR 0.11 (0.05–0.26); 0.001 |
| Features | AGA (N = 655) | SGA (N = 62) | Early-FGR (N = 132) | Late-FGR (N = 57) |
|---|---|---|---|---|
| Gestational age (weeks) | 39 (39–40) | 39 (38–40) | 32 (29–38) | 38 (36–39) |
| ***, ###, ^^^ | ***, ^^^ | |||
| Time from diagnosis to delivery (weeks) | 0 | 0 | 9 (3–15) ### | 3.5 (2–5) |
| Sex | ||||
| Male | 318 (52%) | 38 (61%) | 46 (35%) | 25 (44%) |
| ***, ^^^ | ||||
| Female | 292 (48%) | 24 (39%) | 86 (65%) ***, ^^^ | 32 (56%) |
| Delivery mode | ||||
| Cesarean section | 117 (18%) | 11 (18%) | 95 (72%) | 17 (30%) |
| ***, ###, ^^^ | * | |||
| Vaginal delivery | 538 (82%) | 51 (82%) | 37 (28%) | 40 (70%) |
| Birthweight (gr) | 3330 (3065–3560) | 2690 (2557–2806) | 1252 (930–2260) | 2420 (2052–2620) |
| *** | ***, ^^^ | *** | ||
| Birthweight (percentile) | 53 (52–54) | 5 (3–7.2) | 5 (3–10) | 5 (3–10) |
| *** | *** | *** | ||
| Placental weight (gr) | 560 (500–630) | 440 (405–500) | 280 (200–401.2) | 400 (330–440) |
| *** | ***, ^^^ | *** | ||
| Fetal/Placental weight | 5.9 (5.4–6.4) | 5.9 (5.2–6.6) | 5.2 (3.9–6.0) ***, ###, ^^^ | 5.9 (5.5–6.4) |
| Features | AGA (N = 655) | SGA (N = 62) | Early-FGR (N = 132) | Late-FGR (N = 57) |
|---|---|---|---|---|
| Apgar < 7 1′ | 5 (0.8%) | 2 (3.2%) | 44 (33%) | 3 (5.3%) |
| ***, ###, ^^^ | *** | |||
| Apgar < 7 5′ | 1 (0.2%) | 0 | 4 (3%) | 0 |
| *** | ||||
| pH < 7.10 | 18 (2.7%) | 3 (4.8%) | 1 (0.8%) | 2 (3.5%) |
| BE | −3.3 (−5.4–1.1) | −5.0 (−7.1–−2.8) | −2.8 (−5.6–1.0) | −3.4 (−5–−2.51) |
| ***, +++, # | ||||
| Lactate | 3.7 (2.4–5.3) | 4.4 (2.7–5.5) | 3.0 (2.1–4.6) | 3.5 (2.6–4.7) |
| *, ++ |
| Features | SGA (N = 62) | Early-FGR (N = 132) | Late-FGR (N = 57) |
|---|---|---|---|
| Access in NICU | 7 (11%) | 84 (64%) | 25 (44%) |
| ##, ^^^ | ^^^ | ||
| Days in NICU (days) | 3 (2–4) | 28 (4–52) | 4 (3–5) |
| ##, ^^ | ^^ | ||
| Jaundice | 4 (6.4%) | 51 (38.6%) | 4 (7%) |
| ###, ^^^ | |||
| Intraventricular Hemorrhage | 0 | 1 (0.8%) | 1 (1.7%) |
| Anemia | 0 | 37 (28%) | 1 (1.7%) |
| ### | |||
| Hypoglycemia | 6 (9.7%) | 17 (13%) | 9 (16%) |
| Full enteral feeding (days) | 0 | 10 (1–19) ### | 1 (0.25–1.75) |
| RDS | 1 (1.6%) | 56 (42%) | 4 (7%) |
| ###, ^^^ | |||
| Ventilatory-assistance | 2 (3.2%) | 61 (46%) ###, ^^^ | 7 (12%) |
| Apneas | 1 (1.6%) | 22 (17%) #, ^^^ | 2 (3.5%) |
| NEC | 0 | 6 (4.5%) | 0 |
| Sepsis | 0 | 11 (8.3%) | 1 (1.7%) |
| Fetal death | 0 | 1 (0.8%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubrano, C.; Taricco, E.; Coco, C.; Di Domenico, F.; Mandò, C.; Cetin, I. Perinatal and Neonatal Outcomes in Fetal Growth Restriction and Small for Gestational Age. J. Clin. Med. 2022, 11, 2729. https://doi.org/10.3390/jcm11102729
Lubrano C, Taricco E, Coco C, Di Domenico F, Mandò C, Cetin I. Perinatal and Neonatal Outcomes in Fetal Growth Restriction and Small for Gestational Age. Journal of Clinical Medicine. 2022; 11(10):2729. https://doi.org/10.3390/jcm11102729
Chicago/Turabian StyleLubrano, Chiara, Emanuela Taricco, Chiara Coco, Fiorenza Di Domenico, Chiara Mandò, and Irene Cetin. 2022. "Perinatal and Neonatal Outcomes in Fetal Growth Restriction and Small for Gestational Age" Journal of Clinical Medicine 11, no. 10: 2729. https://doi.org/10.3390/jcm11102729
APA StyleLubrano, C., Taricco, E., Coco, C., Di Domenico, F., Mandò, C., & Cetin, I. (2022). Perinatal and Neonatal Outcomes in Fetal Growth Restriction and Small for Gestational Age. Journal of Clinical Medicine, 11(10), 2729. https://doi.org/10.3390/jcm11102729






