Additional Benefit of Intraoperative Electroacupuncture in Improving Tolerance of Deep Brain Stimulation Surgical Procedure in Parkinsonian Patients
Abstract
:1. Introduction
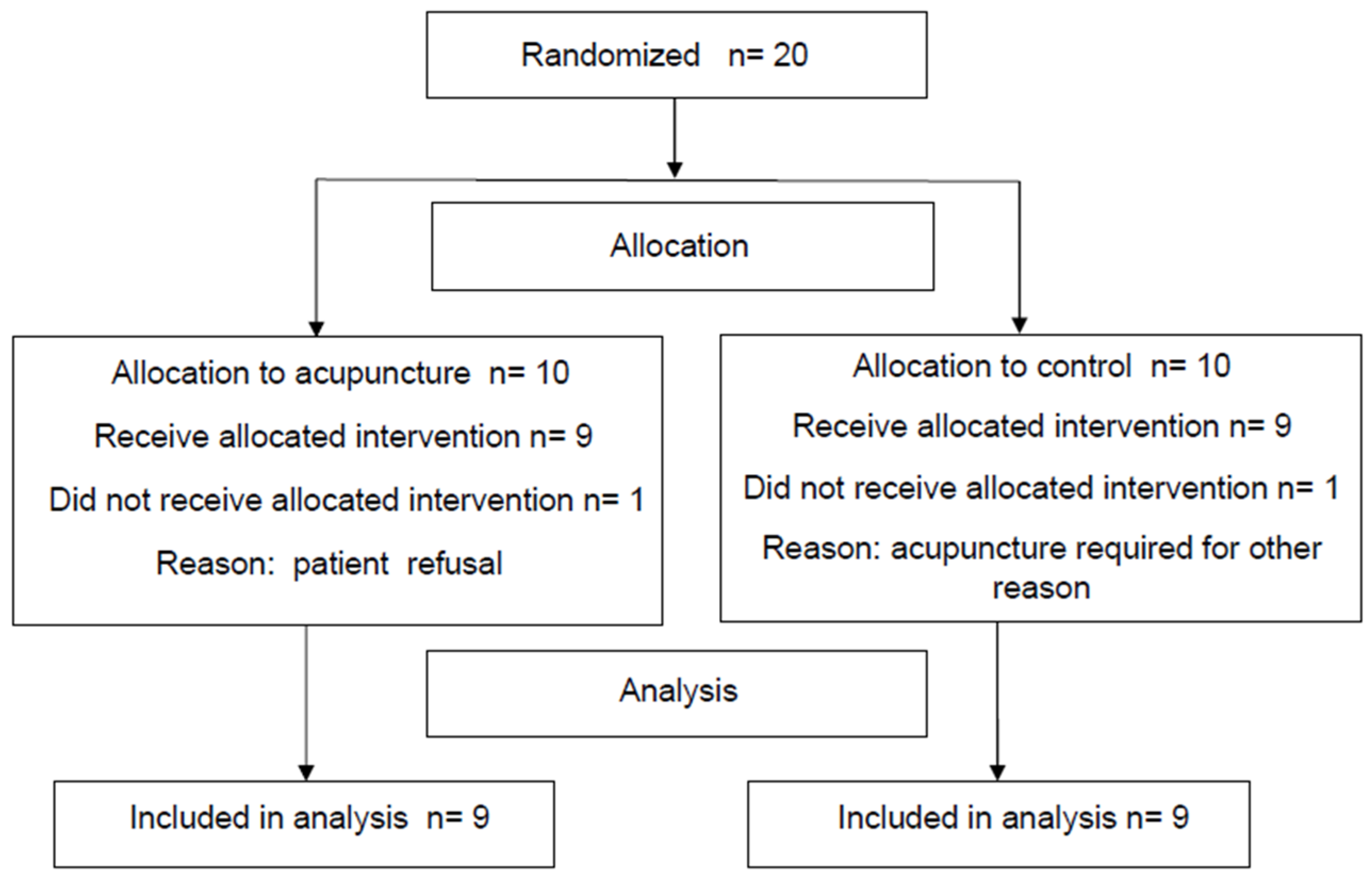
2. Methods
2.1. Study Design
- Parkinsonian patients of both sexes with age ≥18 years.
- Scheduled DBS intervention targeting the STN.
- Signed informed consent.
- Patients with age ≥ 70 years.
- History of intolerance to acupuncture.
- Unstable psychiatric disorder.
- Legal protection regimen.
2.2. Assessment
- Intraoperative anesthesia monitoring parameters: heart rate, oxygen saturation, and systolic and diastolic blood pressure.
- Total time duration of the surgery.
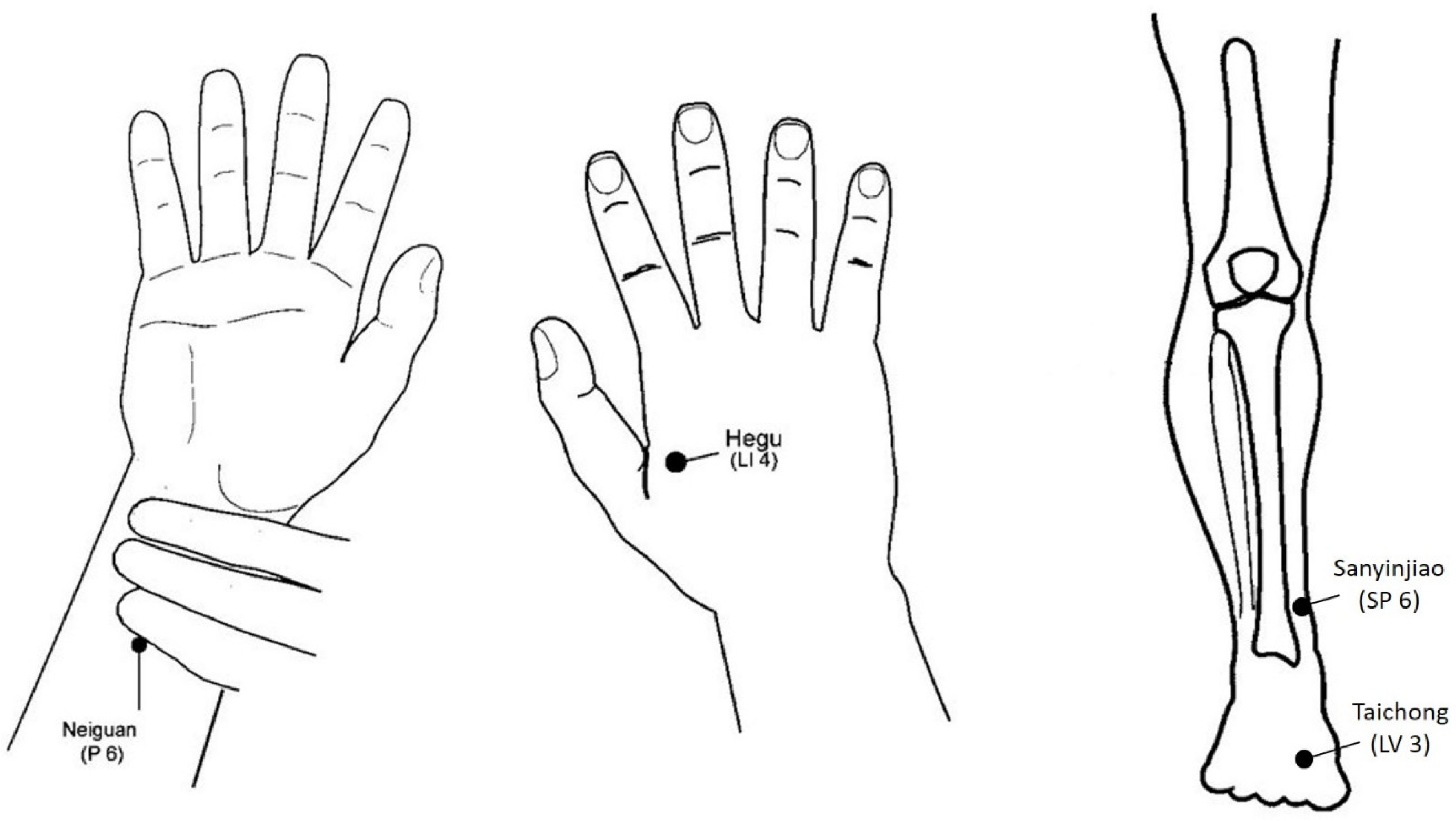
2.3. Acupuncture Procedure
2.4. Statistical Methods
- Baseline/inclusion (incl): the day before the surgery.
- Positioning/installation (inst): a few minutes after the patient rested quietly on the operating table.
- After placement of the stereotactic frame (post SF).
- Immediately after completion of the first burr hole (post BH1).
- Immediately after completion of the second burr hole (post BH2).
- One hour after completion of the second burr hole (1H post BH2).
- After ablation of the stereotactic frame at the end of the procedure (End).
- 2 days after surgery (D2).
3. Results
- -
- “pain”, from post SF to end in both groups (mean (95% confidence interval) difference = 1.24 (−0.80, 3.29) to 4.43 (2.39, 6.48));
- -
- “tiredness”, from post BH2 or 1H post BH2 to end in both groups (2.93 (−0.43, 5.43) to 5.69 (3.19, 8.19));
- -
- “lack of appetite”, from post SF to end in the control group only (4.19 (1.47, 6.91) to 5.04 (2.32, 7.77));
- -
- ESAS total score, from post BH1 to end in the control group only (10.79 (1.29, 20.29) to 18.07 (8.57, 27.57));
- -
- ESAS physical subscore, from post BH1 to end in the control group only (9.51 (2.59, 16.43) to 17.40 (10.48, 24.32)) (Figure 3).
- -
- “anxiety” at D2 in both groups (−2.51 (−0.29, −4.73) to −2.70 (−0.48, −4.92));
- -
- ESAS emotional subscore at D2 in both groups (−2.66 (−0.06, −5.25) to −2.70 (−0.11, −5.29));
- -
- “wellbeing” at D2 in the EA group only (−2.43 (−0.27, −4.60)) (Figure 3).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benabid, A.; Pollak, P.; Louveau, A.; Henry, S.; De Rougemont, J. Combined (Thalamotomy and Stimulation) Stereotactic Surgery of the VIM Thalamic Nucleus for Bilateral Parkinson Disease. Ster. Funct. Neurosurg. 1987, 50, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Benabid, A.L.; Krack, P.P.; Benazzouz, A.; Limousin, P.; Koudsie, A.; Pollak, P. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: Methodologic aspects and clinical criteria. Neurology 2000, 55, S40–S44. [Google Scholar] [PubMed]
- Goodman, R.R.; Kim, B.; McClelland, S.; Senatus, P.B.; Winfield, L.M.; Pullman, S.L.; Yu, Q.; Ford, B.; McKhann, G.M. Operative techniques and morbidity with subthalamic nucleus deep brain stimulation in 100 consecutive patients with advanced Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuepbach, W.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Hälbig, T.; Hesekamp, H.; Navarro, S.; Meier, N.; et al. Neurostimulation for Parkinson’s Disease with Early Motor Complications. N. Engl. J. Med. 2013, 368, 610–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, B.L.; Rn, S.R.J.; Rn, A.C.; Karlsborg, M.; Jespersen, B.; Løkkegaard, A. Deep Brain Stimulation in Parkinson’s Disease: Still Effective after More Than 8 Years. Mov. Disord. Clin. Pract. 2020, 7, 788–796. [Google Scholar] [CrossRef]
- Maarouf, M.; Wojtecki, L.; Groiss, S.J.; Florin, E.; Sturm, V.; Fink, G.R.; Schnitzler, A.; Timmermann, L.; Reck, C. Clinical Outcome of Subthalamic Stimulation in Parkinson’s Disease is Improved by Intraoperative Multiple Trajectories Microelectrode Recording. J. Neurol. Surg. Part A Central Eur. Neurosurg. 2012, 73, 377–386. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Gurruchaga, J.-M.; Pollin, B.; Von Raison, F.; Mohsen, N.; Shin, M.; Ménard-Lefaucheur, I.; Oshino, S.; Kishima, H.; Fénelon, G.; et al. Outcome of Bilateral Subthalamic Nucleus Stimulation in the Treatment of Parkinson’s Disease: Correlation with Intra-Operative Multi-Unit Recordings but Not with the Type of Anaesthesia. Eur. Neurol. 2008, 60, 186–199. [Google Scholar] [CrossRef]
- Bos, M.J.; Sanchez, A.M.A.; Bancone, R.; Temel, Y.; De Greef, B.T.; Absalom, A.R.; Gommer, E.D.; Van Kranen-Mastenbroek, V.H.; Buhre, W.F.; Roberts, M.J.; et al. Influence of Anesthesia and Clinical Variables on the Firing Rate, Coefficient of Variation and Multi-Unit Activity of the Subthalamic Nucleus in Patients with Parkinson’s Disease. J. Clin. Med. 2020, 9, 1229. [Google Scholar] [CrossRef]
- Kalenka, A.; Schwarz, A. Anaesthesia and Parkinson’s disease: How to manage with new therapies? Curr. Opin. Anaesthesiol. 2009, 22, 419–424. [Google Scholar] [CrossRef]
- Mulroy, E.; Robertson, N.; Macdonald, L.; Bok, A.; Simpson, M. Patients’ Perioperative Experience of Awake Deep-Brain Stimulation for Parkinson Disease. World Neurosurg. 2017, 105, 526–528. [Google Scholar] [CrossRef]
- Khatib, R.; Ebrahim, Z.; Rezai, A.; Cata, J.P.; Boulis, N.M.; Doyle, D.J.; Schurigyn, T.; Farag, E. Perioperative Events During Deep Brain Stimulation: The Experience at Cleveland Clinic. J. Neurosurg. Anesthesiol. 2008, 20, 36–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, J.; Volkmann, J.; Krack, P.; Kopper, F.; Pötter, M.; Lorenz, D.; Steinbach, M.; Klebe, S.; Hamel, W.; Schrader, B.; et al. Two-year follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Mov. Disord. 2003, 18, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Vergani, F.; Landi, A.; Pirillo, D.; Cilia, R.; Antonini, A.; Sganzerla, E.P. Surgical, Medical, and Hardware Adverse Events in a Series of 141 Patients Undergoing Subthalamic Deep Brain Stimulation for Parkinson Disease. World Neurosurg. 2010, 73, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Boviatsis, E.J.; Stavrinou, L.C.; Themistocleous, M.; Kouyialis, A.T.; Sakas, D.E. Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir. 2010, 152, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.M.; Coste, J.; Derost, P.; Ulla, M.; Gabrillargues, J.; Durif, F.; Chazal, J.; Lemaire, J.-J. Complications chirurgicales de la stimulation cérébrale profonde: Expérience clinique à propos de 184 cas. Neurochirurgie 2012, 58, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Seijo, F.; Beramendi, S.A.D.E.; Liebana, E.S.; Aragoneses, B.L.; Ayala, A.S.; De Leon, R.F.; Vega, M.A.A. Surgical adverse events of deep brain stimulation in the subthalamic nucleus of patients with Parkinson’s disease. The learning curve and the pitfalls. Acta Neurochir. 2014, 156, 1505–1512. [Google Scholar] [CrossRef]
- Lange, M.; Zech, N.; Seemann, M.; Janzen, A.; Halbing, D.; Zeman, F.; Doenitz, C.; Rothenfusser, E.; Hansen, E.; Brawanski, A.; et al. Anesthesiologic regimen and intraoperative delirium in deep brain stimulation surgery for Parkinson’s disease. J. Neurol. Sci. 2015, 355, 168–173. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Harrington, M.B.; Lucatorto, M.A.; Chester, J.G.; Francis, J.; Shay, K.J. Veterans Affairs and Delirium Working Group Validation of a Medical Record-Based Delirium Risk Assessment. J. Am. Geriatr. Soc. 2011, 59, S289–S294. [Google Scholar] [CrossRef] [Green Version]
- Limousin, P.; Krack, P.; Pollak, P.; Benazzouz, A.; Ardouin, C.; Hoffmann, D.; Benabid, A.-L. Electrical Stimulation of the Subthalamic Nucleus in Advanced Parkinson’s Disease. N. Engl. J. Med. 1998, 339, 1105–1111. [Google Scholar] [CrossRef]
- Krack, P.; Batir, A.; Van Blercom, N.; Chabardes, S.; Fraix, V.; Ardouin, C.; Koudsie, A.; Limousin, P.D.; Benazzouz, A.; LeBas, J.F.; et al. Five-Year Follow-up of Bilateral Stimulation of the Subthalamic Nucleus in Advanced Parkinson’s Disease. N. Engl. J. Med. 2003, 349, 1925–1934. [Google Scholar] [CrossRef] [Green Version]
- Pilitsis, J.G.; Rezai, A.R.; Boulis, N.M.; Henderson, J.M.; Busch, R.M.; Kubu, C.S. A Preliminary Study of Transient Confusional States following Bilateral Subthalamic Stimulation for Parkinson’s Disease. Ster. Funct. Neurosurg. 2005, 83, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Gologorsky, Y.; Ben-Haim, S.; Moshier, E.L.; Godbold, J.; Tagliati, M.; Weisz, D.; Alterman, R.L. Transgressing the Ventricular Wall During Subthalamic Deep Brain Stimulation Surgery for Parkinson Disease Increases the Risk of Adverse Neurological Sequelae. Neurosurgery 2011, 69, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.D.; Neumiller, J.J.; Swain, L.D.; Mark, J.; McLeod, P.; Hirschauer, J. Postoperative delirium in Parkinson’s disease patients following deep brain stimulation surgery. J. Clin. Neurosci. 2014, 21, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, B.; Marcantonio, E.R.; Pascual-Leone, A.; Shaefi, S.; Mueller, A.; Banner-Goodspeed, V.; Talmor, D.; Subramaniam, B. Prevention of Early Postoperative Decline (PEaPoD): Protocol for a randomized, controlled feasibility trial. Trials 2018, 19, 676. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, M.G.V.; Sessler, M.D.I.; Warltier, M.D.C. Perioperative Acupuncture and Related Techniques. Anesthesiology 2005, 102, 1031–1049. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Gan, T.J.; Dubose, J.W.; Habib, A.S. Acupuncture and related techniques for postoperative pain: A systematic review of randomized controlled trials. Br. J. Anaesth. 2008, 101, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.K.; Habib, A.S. P6 Stimulation for the Prevention of Nausea and Vomiting Associated with Cesarean Delivery Under Neuraxial Anesthesia: A Systematic Review of Randomized Controlled Trials. Anesthesia Analg. 2008, 107, 1308–1312. [Google Scholar] [CrossRef]
- Coura, L.E.F.; Manoel, C.H.U.; Poffo, R.; Bedin, A.; Westphal, G.A. Randomised, Controlled Study of Preoperative Eletroacupuncture for Postoperative Pain Control after Cardiac Surgery. Acupunct. Med. 2011, 29, 16–20. [Google Scholar] [CrossRef]
- Wang, J.L.; Xie, W.X.; Zhang, Q. Effects of combined transdermal acupoint electric stimulation and isoflurane anesthesia on isoflurane-induced hypotension and tissue oxygen metabolism in patients undergoing craniotomy. Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2001, 21, 180–182. [Google Scholar]
- Zhang, J.-M.; Zhang, H.; Zhu, X.-D.; Lü, S.; Liu, Z.; Chen, J.; Peng, S. Clinical application of acupunctural anesthesia with new combination principle of acupoints in supratentorial craniocerebral operation of tumor in vital functional area or deep site of brain. Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2004, 24, 969–972. [Google Scholar]
- Wu, Q.; Mo, Y.-C.; Huang, L.-P.; Luo, L.; Wang, J.-L. Effect of transcutaneous acupoint electrical stimulation on stress in brain surgery with propofol target controlled infusion general anesthesia. Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2013, 33, 6121–6125. [Google Scholar]
- Wu, M.-S.; Chen, K.-H.; Chen, I.-F.; Huang, S.K.; Tzeng, P.-C.; Yeh, M.-L.; Lee, F.-P.; Lin, J.-G.; Chen, C. The Efficacy of Acupuncture in Post-Operative Pain Management: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.G.; Wang, E.Z.; Chen, X.Z. A study on combined acupuncture and enflurane anesthesia for craniotomy. Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 1994, 14, 10–13. [Google Scholar]
- Jiang, C. Normalization of acupuncture anesthesia used in operation of neoplasm in functional area or deep site of brain. Zhen Ci Yan Jiu = Acupunct. Res. 1996, 21, 4–7. [Google Scholar]
- Yan, H.; Jiang, C. Application of acupuncture anesthesia during craniocerebral operation in temporo-fronto-occipital region. Zhen Ci Yan Jiu = Acupunct. Res. 1990, 15, 92–96. [Google Scholar]
- Yan, H.; Jiang, C.; Liang, W. Clinical application of combined acupuncture-drug anesthesia in cerebral functional area oper-ation. Zhongguo Zhong Xi Yi Jie He Za Zhi 1998, 18, 138. [Google Scholar]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A Simple Method for the Assessment of Palliative Care Patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef]
- Ryff, C.D.; Love, G.D.; Urry, H.L.; Muller, D.; Rosenkranz, M.A.; Friedman, E.M.; Davidson, R.J.; Singer, B. Psychological Well-Being and Ill-Being: Do They Have Distinct or Mirrored Biological Correlates? Psychother. Psychosom. 2006, 75, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Lien, K.; Zeng, L.; Zhang, L.; Nguyen, J.; Di Giovanni, J.; Popovic, M.; Jamani, R.; Cramarossa, G.; Culleton, S.; Chow, E. Predictive Factors for Well-being in Advanced Cancer Patients Referred for Palliative Radiotherapy. Clin. Oncol. 2012, 24, 443–451. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J. Pain Symptom Manag. 2016, 53, 630–643. [Google Scholar] [CrossRef] [Green Version]
- Disease, M.D.S.T.F.O.R.S.F.P. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef]
- Asmussen, S.; Maybauer, D.M.; Chen, J.D.; Fraser, J.F.; Toon, M.H.; Przkora, R.; Jennings, K.; Maybauer, M. Effects of Acupuncture in Anesthesia for Craniotomy: A Meta-Analysis. J. Neurosurg. Anesthesiol. 2017, 29, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-Q.; Li, P.-C.; Zhou, L.; Tang, W.-F.; Li, N. Acupuncture at the P6 Acupoint to Prevent Postoperative Pain after Craniotomy: A Randomized, Placebo-Controlled Study. Evidence-Based Complement. Altern. Med. 2021, 2021, 6619855. [Google Scholar] [CrossRef]
- Tobias, K.; Rosenfeld, B.; Pessin, H.; Breitbart, W. Measuring sickness behavior in the context of pancreatic cancer. Med. Hypotheses 2015, 84, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezdicek, O.; Michalec, J.; Nikolai, T.; Havránková, P.; Roth, J.; Jech, R.; Růžička, E. Clinical Validity of the Mattis Dementia Rating Scale in Differentiating Mild Cognitive Impairment in Parkinson’s Disease and Normative Data. Dement. Geriatr. Cogn. Disord. 2015, 39, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Kelley, K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007, 21, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, F.J.; Lange, I.V.D.B.-D.; Huygen, F.J.P.M.; Klein, J. Anti-inflammatory actions of acupuncture. Mediat. Inflamm. 2003, 12, 59–69. [Google Scholar] [CrossRef]
- Bai, H.; Xu, S.; Wu, Q.; Xu, S.; Sun, K.; Wu, J.; Xia, X.; Liu, Y.; Zhang, H.; Lu, S. Clinical Events Associated with Acupuncture Intervention for the Treatment of Chronic Inflammation Associated Disorders. Mediat. Inflamm. 2020, 2020, 2675785. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Q.; Li, Y.; Tai, Y.; Xin, X.; Wang, X.; Wang, Q. Transcutaneous electrical acupoint stimulation for prevention of postoperative delirium in geriatric patients with silent lacunar infarction: A preliminary study. Clin. Interv. Aging 2018, ume 13, 2127–2134. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-B.; Cho, S.-Y.; Kwon, S.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Shin, H.S.; Lee, S.H.; Koh, J.S.; et al. Acupuncture attenuates postoperative inflammation in patients after craniotomy. Medicine 2020, 99, e19071. [Google Scholar] [CrossRef]
- Maydych, V. The Interplay Between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Front. Neurosci. 2019, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.A.; Fearing, M.A.; Sternberg, E.J.; Inouye, S.K. The Confusion Assessment Method: A Systematic Review of Current Usage. J. Am. Geriatr. Soc. 2008, 56, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Gao, X.; Chen, Q.; Jiang, X.; Li, Y.; Xu, J.; Qin, G.; Lu, S.; Huang, D. Preoperative Acute Pain Is Associated with Postoperative Delirium. Pain Med. 2020, 22, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, S.; Feng, J. Delirium after Deep Brain Stimulation in Parkinson’s Disease. Park. Dis. 2021, 2021, 8885386. [Google Scholar] [CrossRef]

| Control Group (n = 10) | EA Group (n = 10) | p Values | |
|---|---|---|---|
| Age (years) mean (sd) | 56 (7.1) | 57 (5.7) | 0.40 |
| Gender (women. men) | 5w. 5m | 2w. 8m | 0.33 |
| UPDRS-III score mean (sd) | 22 (11.2) | 22 (13.3) | 0.56 |
| HAMA score (sd) | 7.6 (1.9) | 12.4 (2.4) | 0.009 |
| ESAS total score mean (sd) | 8.6 (8.6) | 9.8 (5.2) | 0.38 |
| ESAS physical subscore mean (sd) | 3.3 (5.6) | 4.7 (3.2) | 0.10 |
| ESAS emotional subscore mean (sd) | 2.7 (2.0) | 2.6 (1.6) | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raoul, S.; Brissot, R.; Lefaucheur, J.-P.; Nguyen, J.-M.; Rouaud, T.; Meas, Y.; Huchet, A.; Razafimahefa, N.; Damier, P.; Nizard, J.; et al. Additional Benefit of Intraoperative Electroacupuncture in Improving Tolerance of Deep Brain Stimulation Surgical Procedure in Parkinsonian Patients. J. Clin. Med. 2022, 11, 2680. https://doi.org/10.3390/jcm11102680
Raoul S, Brissot R, Lefaucheur J-P, Nguyen J-M, Rouaud T, Meas Y, Huchet A, Razafimahefa N, Damier P, Nizard J, et al. Additional Benefit of Intraoperative Electroacupuncture in Improving Tolerance of Deep Brain Stimulation Surgical Procedure in Parkinsonian Patients. Journal of Clinical Medicine. 2022; 11(10):2680. https://doi.org/10.3390/jcm11102680
Chicago/Turabian StyleRaoul, Sylvie, Régine Brissot, Jean-Pascal Lefaucheur, Jean-Michel Nguyen, Tiphaine Rouaud, Yunsan Meas, Alain Huchet, Ndrianaina Razafimahefa, Philippe Damier, Julien Nizard, and et al. 2022. "Additional Benefit of Intraoperative Electroacupuncture in Improving Tolerance of Deep Brain Stimulation Surgical Procedure in Parkinsonian Patients" Journal of Clinical Medicine 11, no. 10: 2680. https://doi.org/10.3390/jcm11102680
APA StyleRaoul, S., Brissot, R., Lefaucheur, J.-P., Nguyen, J.-M., Rouaud, T., Meas, Y., Huchet, A., Razafimahefa, N., Damier, P., Nizard, J., & Nguyen, J.-P. (2022). Additional Benefit of Intraoperative Electroacupuncture in Improving Tolerance of Deep Brain Stimulation Surgical Procedure in Parkinsonian Patients. Journal of Clinical Medicine, 11(10), 2680. https://doi.org/10.3390/jcm11102680






