Analysis of the Association between Galectin-3 Concentration in Tears and the Severity of Dry Eye Disease: A Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Diagnosis of Dry Eye Disease (DED)
2.3. Evaluation Factors
2.4. Sample Collection
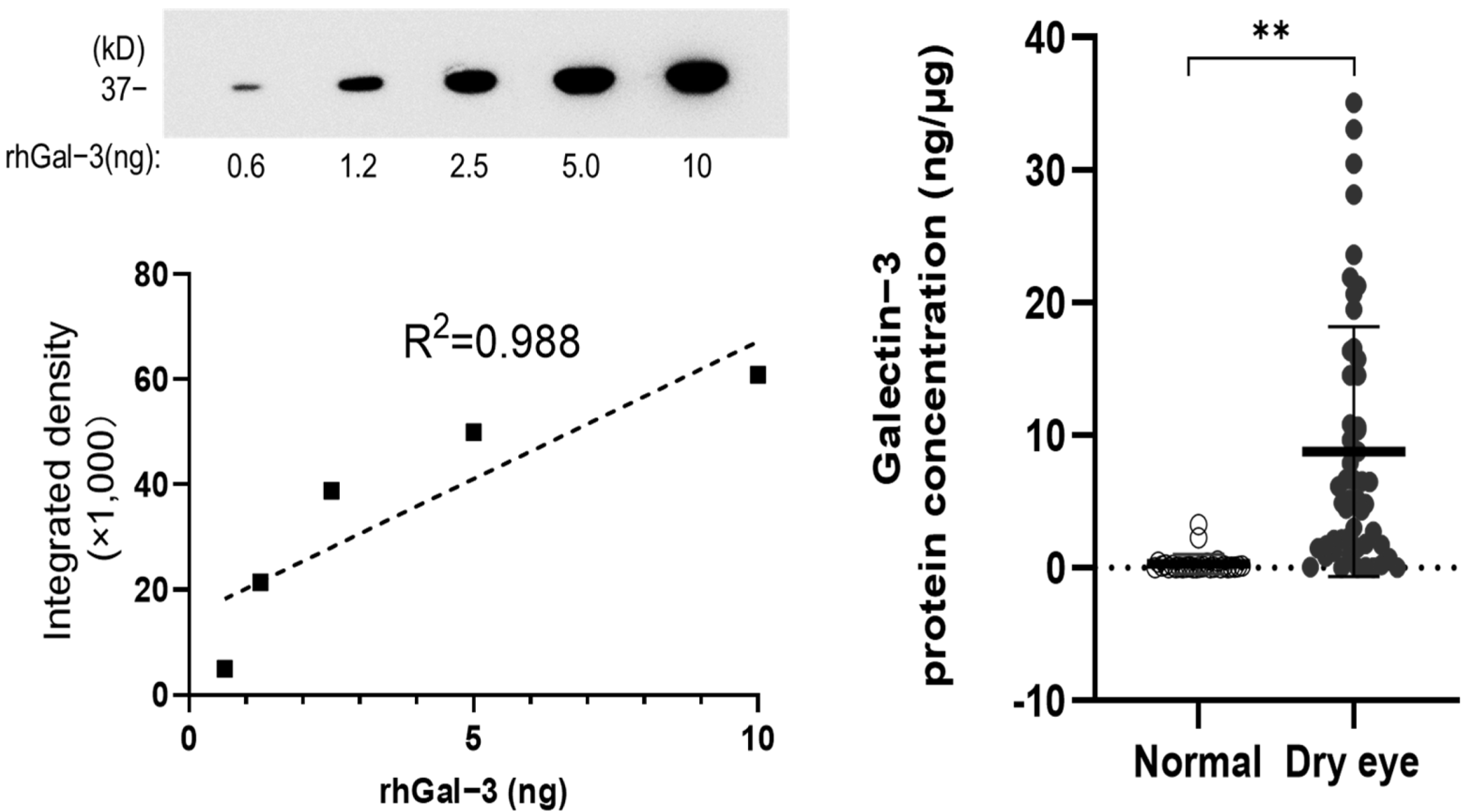
2.5. Analysis of Gal-3 Protein Concentration
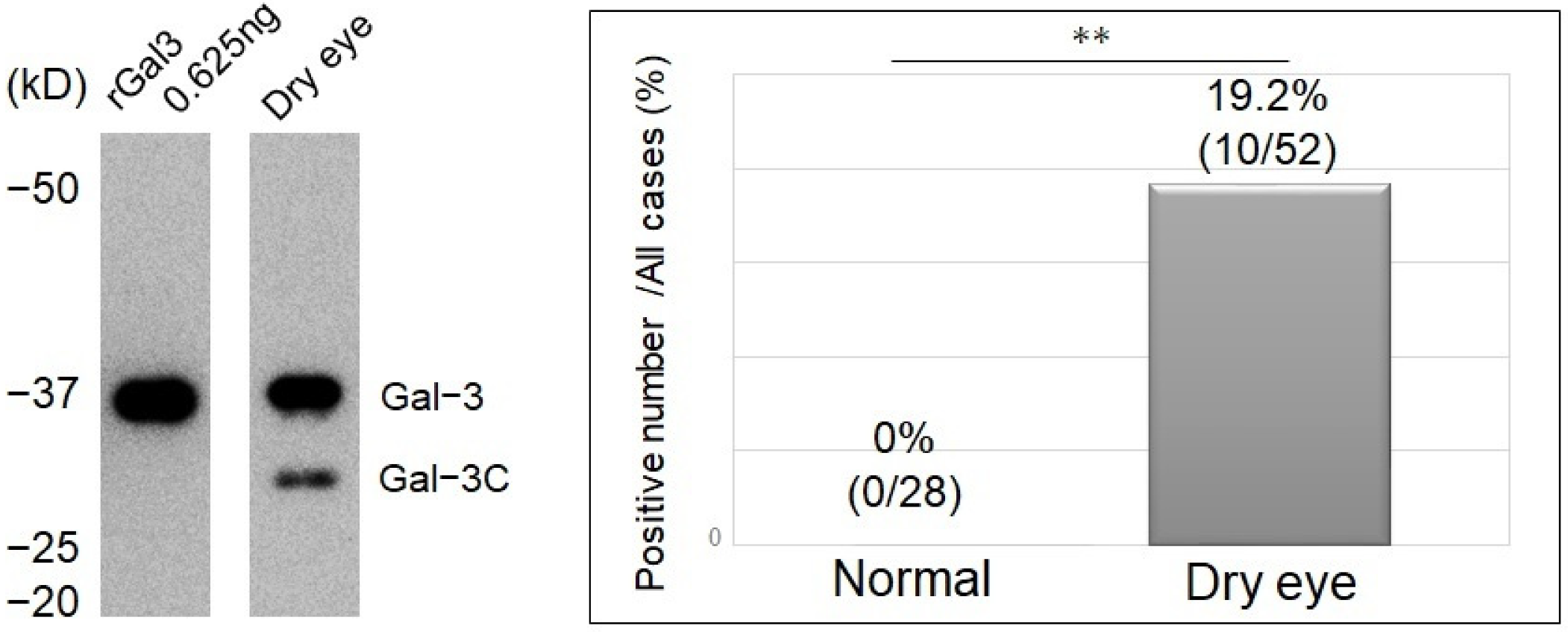
2.6. Analysis of the Detection Rate of Gal-3C
2.7. Statistical Analysis
3. Results
3.1. Clinical Evaluation of Dry Eye and Control Groups
3.2. Gal-3 Protein Concentration in the Tear Fluid of the Dry Eye (DE) and Non-DE Groups
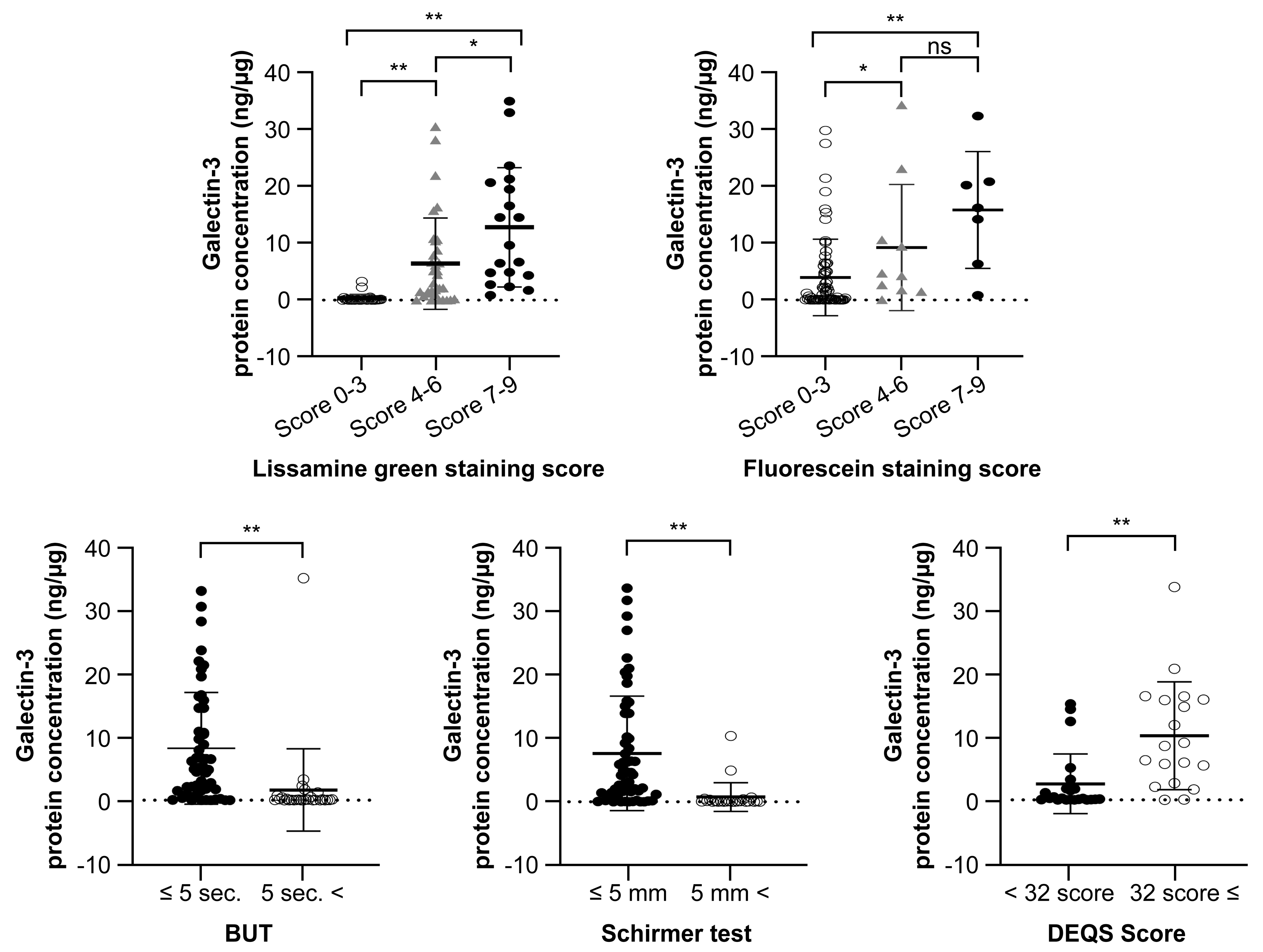
3.3. Gal-3 Protein Concentration and the Classification of DED Severity
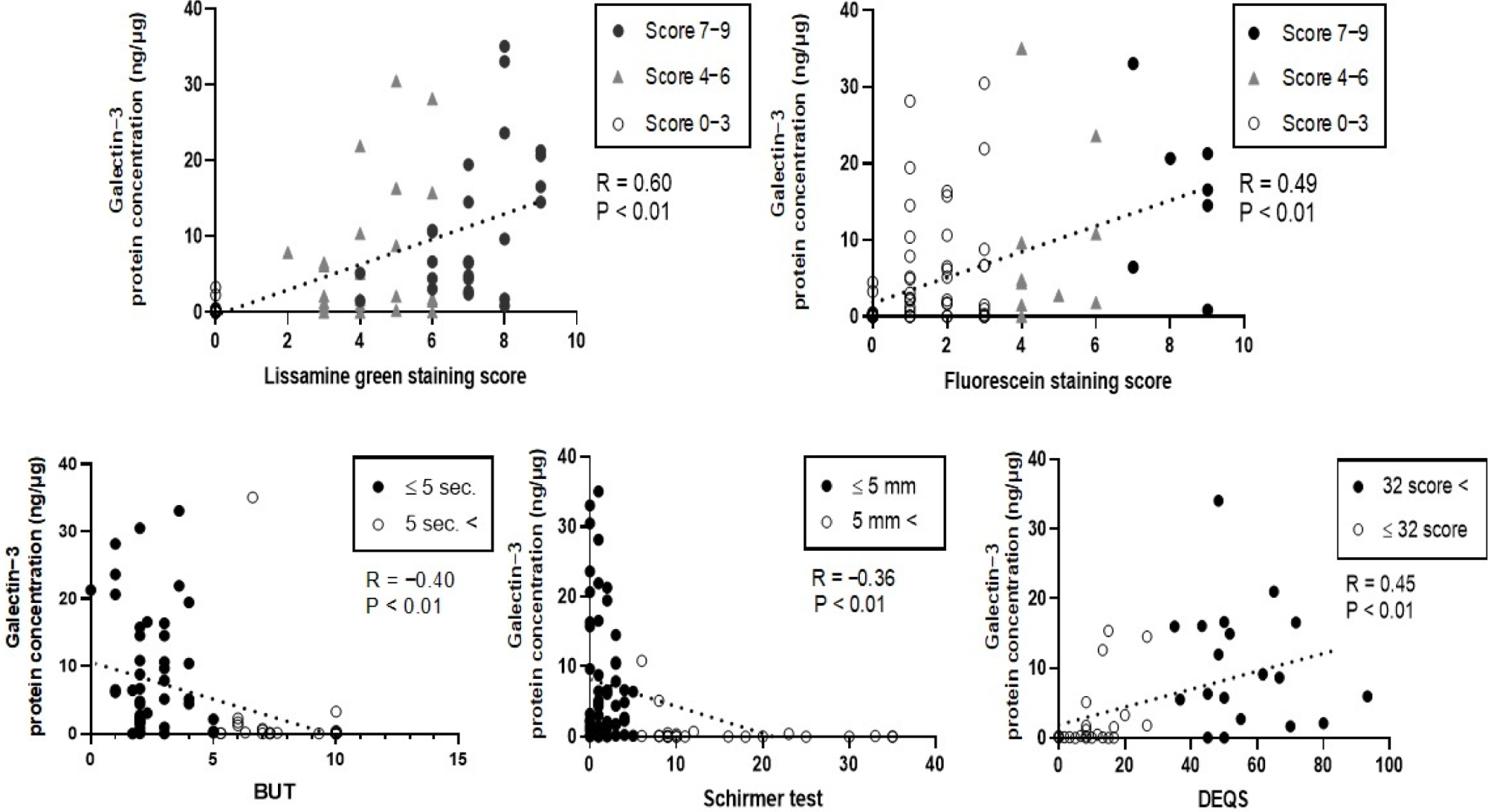
3.4. Correlation between Gal-3 Protein Concentration and the Severity of Clinically Evaluated Factors
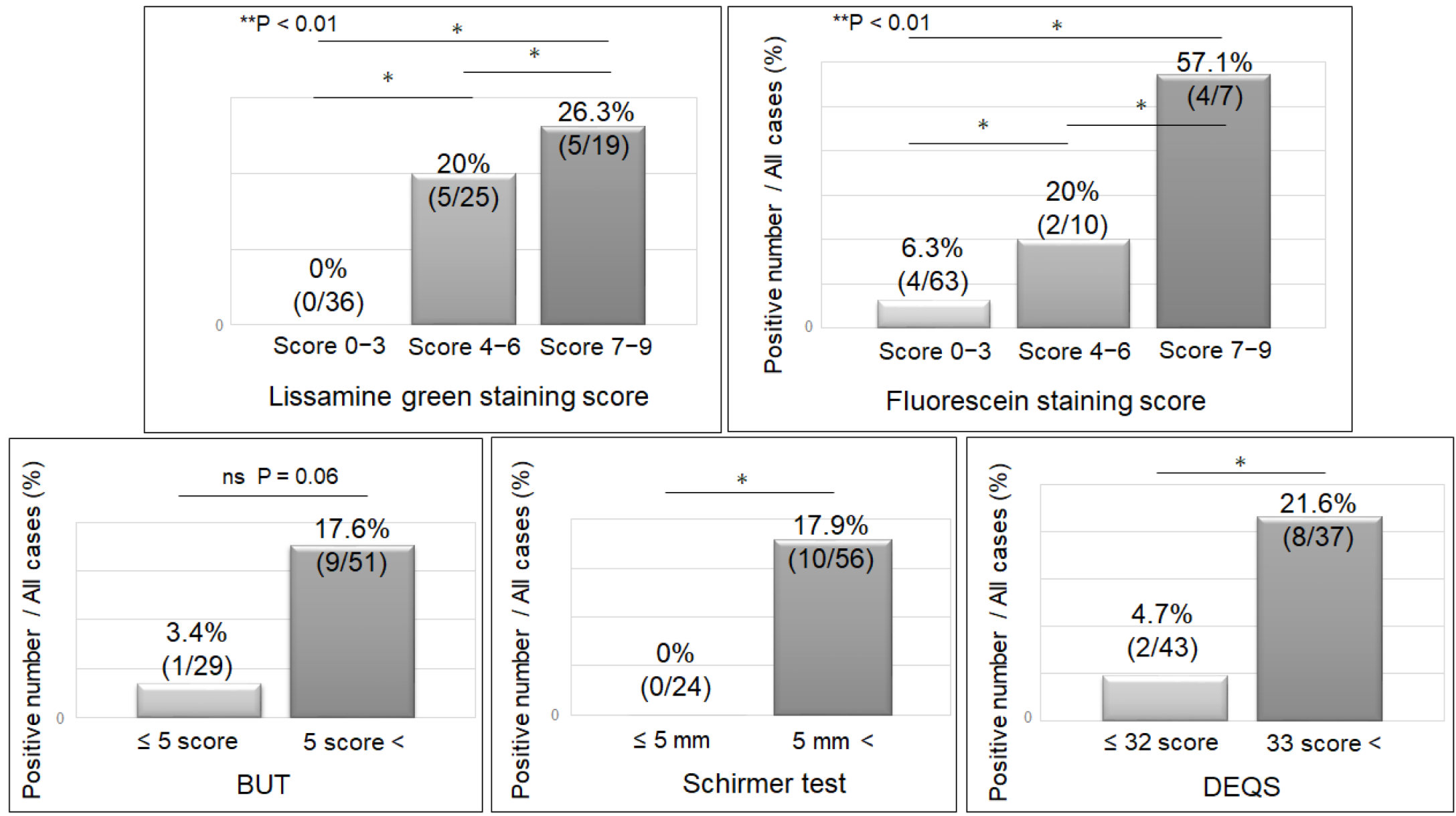
3.5. Detection Rate of Cleavage of Gal-3 and the Severity of Clinically Evaluated Factors
3.6. Characteristics of Gal-3 Cleavage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Dana, R.; Buring, J.E.; Sullivan, D.A. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Mauris, J.; Argüeso, P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: From allergy to infectious diseases. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Yokoi, N.; Georgiev, G.; Kato, H.; Komuro, A.; Sonomura, Y.; Sotozono, C.; Tsubota, K.; Kinoshita, S. Classification of Fluorescein Breakup Patterns: A Novel Method of Differential Diagnosis for Dry Eye. Am. J. Ophthalmol. 2017, 180, 72–85. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, B.; Gipson, I.K. Membrane-tethered mucins have multiple functions on the ocular surface. Exp. Eye Res. 2010, 90, 655–663. [Google Scholar] [CrossRef]
- Gipson, I.K.; Argüeso, P. Role of Mucins in the Function of the Corneal and Conjunctival Epithelia. Int. Rev. Cytol. 2003, 231, 1–49. [Google Scholar] [CrossRef]
- Hilkens, J.; Ligtenberg, M.J.; Vos, H.L.; Litvinov, S.V. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem. Sci. 1992, 17, 359–363. [Google Scholar] [CrossRef]
- Bramwell, M.; Wiseman, G.; Shotton, D. Electron-microscopic studies of the CA antigen, epitectin. J. Cell Sci. 1986, 86, 249–261. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. Structure and Function of the Cell Surface (Tethered) Mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Gipson, I.K.; Spurr-Michaud, S.; Tisdale, A. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Exp. Eye Res. 2015, 145, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wei, B.; Wen, T.; Johansson, M.E.; Liu, X.; Bradford, E.; Thomsson, K.A.; McGee, S.; Mansour, L.; Tong, M.; et al. Loss of intestinal core 1–derived O-glycans causes spontaneous colitis in mice. J. Clin. Investig. 2011, 121, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.-G. O-Glycosylation of the Mucin Type. Biol. Chem. 2001, 382, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K. Distribution of mucins at the ocular surface. Exp. Eye Res. 2004, 78, 379–388. [Google Scholar] [CrossRef]
- Argüeso, P. Glycobiology of the ocular surface: Mucins and lectins. Jpn. J. Ophthalmol. 2013, 57, 150–155. [Google Scholar] [CrossRef][Green Version]
- Yokoi, N.; Georgiev, G. Tear-film-oriented diagnosis for dry eye. Jpn. J. Ophthalmol. 2019, 63, 127–136. [Google Scholar] [CrossRef]
- Inatomi, T.; Spurr-Michaud, S.; Tisdale, A.S.; Gipson, I.K. Human corneal and conjunctival epithelia express MUC1 mucin. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1818–1827. [Google Scholar]
- Argüeso, P.; Gipson, I.K. Epithelial Mucins of the Ocular Surface: Structure, Biosynthesis and Function. Exp. Eye Res. 2001, 73, 281–289. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Liu, Z.; Monroy, D.; Li, D.Q.; Carvajal, M.E.; Price-Schiavi, S.A.; Idris, N.; Solomon, A.; Perez, A.; Carraway, K.L. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1316–1326. [Google Scholar]
- Argueso, P.; Spurr-Michaud, S.; Russo, C.L.; Tisdale, A.; Gipson, I.K. MUC16 Mucin Is Expressed by the Human Ocular Surface Epithelia and Carries the H185 Carbohydrate Epitope. Investig. Opthalmol. Vis. Sci. 2003, 44, 2487–2495. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.Z.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Lab. Cold Spring Harbor: New York, NY, USA, 2009. [Google Scholar]
- Gong, H.C.; Honjo, Y.; Nangia-Makker, P.; Hogan, V.; Mazurak, N.; Bresalier, R.; Raz, A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999, 59, 6239–6245. [Google Scholar] [PubMed]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, B.; Feeney, J.; Burdett, I.D.J.; Bawumia, S.; Barboni, E.A.M.; Hughes, R.C. NMR Solution Studies of Hamster Galectin-3 and Electron Microscopic Visualization of Surface-Adsorbed Complexes: Evidence for Interactions between the N- and C-Terminal Domains. Biochemistry 2001, 40, 4859–4866. [Google Scholar] [CrossRef]
- Argüeso, P.; Guzman-Aranguez, A.; Mantelli, F.; Cao, Z.; Ricciuto, J.; Panjwani, N. Association of Cell Surface Mucins with Galectin-3 Contributes to the Ocular Surface Epithelial Barrier. J. Biol. Chem. 2009, 284, 23037–23045. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.M.; Cooper, D.N.W.; Leffler, H.; Barondes, S.H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 1993, 32, 260–267. [Google Scholar] [CrossRef]
- Ochieng, J.; Leite-Browning, M.L.; Warfield, P. Regulation of Cellular Adhesion to Extracellular Matrix Proteins by Galectin-3. Biochem. Biophys. Res. Commun. 1998, 246, 788–791. [Google Scholar] [CrossRef]
- Shekhar, M.P.; Nangia-Makker, P.; Tait, L.; Miller, F.; Raz, A. Alterations in Galectin-3 Expression and Distribution Correlate with Breast Cancer Progression: Functional Analysis of Galectin-3 in Breast Epithelial-Endothelial Interactions. Am. J. Pathol. 2004, 165, 1931–1941. [Google Scholar] [CrossRef]
- Lagana, A.; Goetz, J.G.; Cheung, P.; Raz, A.; Dennis, J.W.; Nabi, I.R. Galectin Binding to Mgat5-Modified N-Glycans Regulates Fibronectin Matrix Remodeling in Tumor Cells. Mol. Cell. Biol. 2006, 26, 3181–3193. [Google Scholar] [CrossRef]
- Partridge, E.A.; Le Roy, C.; Di Guglielmo, G.M.; Pawling, J.; Cheung, P.; Granovsky, M.; Nabi, I.R.; Wrana, J.L.; Dennis, J.W. Regulation of Cytokine Receptors by Golgi N-Glycan Processing and Endocytosis. Science 2004, 306, 120–124. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Ricciuto, J.; Tisdale, A.; Gipson, I.K.; Mantelli, F.; Argüeso, P. Antiadhesive Character of Mucin O-glycans at the Apical Surface of Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2008, 49, 197–203. [Google Scholar] [CrossRef]
- Ricciuto, J.; Heimer, S.R.; Gilmore, M.S.; Argüeso, P. Cell Surface O-Glycans Limit Staphylococcus aureus Adherence to Corneal Epithelial Cells. Infect. Immun. 2008, 76, 5215–5220. [Google Scholar] [CrossRef] [PubMed]
- Argüeso, P.; Tisdale, A.; Spurr-Michaud, S.; Sumiyoshi, M.; Gipson, I.K. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Investig. Opthalmol. Vis. Sci. 2006, 47, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Danjo, Y.; Watanabe, H.; Tisdale, A.S.; George, M.; Tsumura, T.; Abelson, M.B.; Gipson, I.K. Alteration of mucin in human conjunctival epithelia in dry eye. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2602–2609. [Google Scholar]
- Uchino, Y.; Mauris, J.; Woodward, A.M.; Dieckow, J.; Amparo, F.; Dana, R.; Mantelli, F.; Argüeso, P. Alteration of Galectin-3 in Tears of Patients With Dry Eye Disease. Am. J. Ophthalmol. 2015, 159, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Sakane, Y.; Yamaguchi, M.; Yokoi, N.; Uchino, M.; Dogru, M.; Oishi, T.; Ohashi, Y.; Ohashi, Y. Development and Validation of the Dry Eye–Related Quality-of-Life Score Questionnaire. JAMA Ophthalmol. 2013, 131, 1331–1338. [Google Scholar] [CrossRef]
- Argüeso, P.; Gipson, I.K. Assessing Mucin Expression and Function in Human Ocular Surface Epithelia In Vivo and In Vitro. Methods Mol. Biol. 2011, 842, 313–325. [Google Scholar] [CrossRef]
- Ferrero, D.M.; Moeller, L.M.; Osakada, T.; Horio, N.; Li, Q.; Roy, D.S.; Cichy, A.; Spehr, M.; Touhara, K.; Liberles, S.D. A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nat. Cell Biol. 2013, 502, 368–371. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Balan, V.; Raz, A. Regulation of Tumor Progression by Extracellular Galectin-3. Cancer Microenviron. 2008, 1, 43–51. [Google Scholar] [CrossRef]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 181–189. [Google Scholar] [CrossRef]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef]
- Miyazaki, J.; Hokari, R.; Kato, S.; Tsuzuki, Y.; Kawaguchi, A.; Nagao, S.; Itoh, K.; Miura, S. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol. Rep. 2002, 9, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, J.; Yang, G.; Yu, N.; Huang, Z.; Xu, H.; Li, J.; Qiu, J.; Zeng, X.; Chen, S.; et al. Posttranscriptional regulation of Galectin-3 by miR-128 contributes to colorectal cancer progression. Oncotarget 2017, 8, 15242–15251. [Google Scholar] [CrossRef] [PubMed]
- Song, S. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS ONE 2012, 7, e42699. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E. Prognostication of clinical outcomes in diabetes mellitus: Emerging role of cardiac biomarkers. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, T.; Goulis, I.; Ntogramatzi, F.; Athanasiadou, Z.; Vagdatli, E.; Akriviadis, E.; Cholongitas, E. Galectin-3 is associated with glomerular filtration rate and outcome in patients with stable decompensated cirrhosis. Dig. Liver Dis. 2019, 51, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J. Reflections on the tears. Eye 1997, 11, 583–602. [Google Scholar] [CrossRef]
- Korb, D.R.; Herman, J.P.; Finnemore, V.M.; Exford, J.M.; Blackie, C.A. An Evaluation of the Efficacy of Fluorescein, Rose Bengal, Lissamine Green, and a New Dye Mixture for Ocular Surface Staining. Eye Contact Lens Sci. Clin. Pract. 2008, 34, 61–64. [Google Scholar] [CrossRef]
- Bron, A.; Argüeso, P.; Irkec, M.; Bright, F. Clinical staining of the ocular surface: Mechanisms and interpretations. Prog. Retin. Eye Res. 2015, 44, 36–61. [Google Scholar] [CrossRef]
- Manning, F.J.; Wehrly, S.R.; Foulks, G.N. Patient Tolerance and Ocular Surface Staining Characteristics of Lissamine Green versus Rose Bengal. Ophthalmology 1995, 102, 1953–1957. [Google Scholar] [CrossRef]
- Argüeso, P.; Mauris, J.; Uchino, Y. Galectin-3 as a regulator of the epithelial junction: Implications to wound repair and cancer. Tissue Barriers 2015, 3, e1026505. [Google Scholar] [CrossRef] [PubMed]
- Komuro, A.; Yokoi, N.; Takehisa, Y.; Kinoshita, S. Association of tear lipid layer interference patterns with superficial punctate keratopathy. Adv. Exp. Med. Biol. 1998, 438, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Uchino, Y.; Dogru, M.; Kawashima, M.; Yokoi, N.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Dry Eye Disease and Work Productivity Loss in Visual Display Users: The Osaka Study. Am. J. Ophthalmol. 2014, 157, 294–300. [Google Scholar] [CrossRef]
- Yokoi, N.; Takehisa, Y.; Kinoshita, S. Correlation of Tear Lipid Layer Interference Patterns With the Diagnosis and Severity of Dry Eye. Am. J. Ophthalmol. 1996, 122, 818–824. [Google Scholar] [CrossRef]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.-M.; Tchah, H.-W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Willcox, M.D.; Argüeso, P.; Georgiev, G.; Holopainen, J.M.; Laurie, G.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- King-Smith, P.E.; Fink, B.A.; Hill, R.M.; Koelling, K.W.; Tiffany, J.M. The thickness of the tear film. Curr. Eye Res. 2004, 29, 357–368. [Google Scholar] [CrossRef]
- Ochieng, J.; Fridman, R.; Nangia-Makker, P.; Kleiner, D.; Liotta, L.A.; Stetler-Stevenson, W.G.; Raz, A. Galectin-3 Is a Novel Substrate for Human Matrix Metalloproteinases-2 and -9. Biochemistry 1994, 33, 14109–14114. [Google Scholar] [CrossRef]
- Balan, V.; Nangia-Makker, P.; Schwartz, A.G.; Jung, Y.S.; Tait, L.; Hogan, V.; Raz, T.; Wang, Y.; Yang, Z.Q.; Wu, G.S.; et al. Racial Disparity in Breast Cancer and Functional Germ Line Mutation in Galectin-3 (rs4644): A Pilot Study. Cancer Res. 2008, 68, 10045–10050. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nangia-Makker, P.; Tait, L.; Balan, V.; Hogan, V.; Pienta, K.J.; Raz, A. Regulation of Prostate Cancer Progression by Galectin-3. Am. J. Pathol. 2009, 174, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Viacava, P.; Naccarato, A.G.; Bocci, G.; Fanelli, G.; Aretini, P.; Lonobile, A.; Evangelista, G.; Montruccoli, G.; Bevilacqua, G. Angiogenesis and VEGF expression in pre-invasive lesions of the human breast. J. Pathol. 2004, 204, 140–146. [Google Scholar] [CrossRef] [PubMed]
| n | |
|---|---|
| Primary Sjögren’s syndrome | 19 |
| Secondary Sjögren’s syndrome | 3 |
| Graft versus host disease | 3 |
| Ocular cicatricial pemphigoid | 1 |
| No systemic disease | 2 |
| Total | 28 |
| Ophthalmic Evaluation Factors | Dry Eye Disease | Control | p |
|---|---|---|---|
| LG staining score (0–9 points) | 5.67 ± 1.90 | 0 | <0.01 |
| FL staining score (0–9 points) | 3.31 ± 2.41 | 0.14 ± 0.35 | <0.01 |
| TBUT (seconds) | 2.64 ± 1.42 | 8.24 ± 1.97 | <0.01 |
| Schirmer test (mm) | 2.02 ± 2.19 | 12.9 ± 10.37 | <0.01 |
| DEQS | 43.58 ± 22.17 | 6.42 ± 4.91 | <0.01 |
| Gal-3C | Positive | Negative | p |
|---|---|---|---|
| LG staining score (0–9 points) | 6.8 ± 1.9 | 5.4 ± 1.78 | <0.05 |
| FL staining score (0–9 points) | 5.5 ± 2.9 | 2.8 ± 1.9 | <0.01 |
| TBUT (seconds) | 2.1 ± 1.5 | 2.8 ± 1.4 | 0.18 |
| Schirmer test (mm) | 1.2 ± 1.2 | 2.2 ± 2.3 | 0.06 |
| DEQS | 50.7 ± 25.2 | 43.0 ± 20.8 | 0.36 |
| Gal-3 protein concentration | 12.7 ± 10.5 | 7.8 ± 8.8 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hata-Mizuno, M.; Uchino, Y.; Uchino, M.; Shimmura, S.; Ogawa, Y.; Tsubota, K.; Negishi, K. Analysis of the Association between Galectin-3 Concentration in Tears and the Severity of Dry Eye Disease: A Case-Control Study. J. Clin. Med. 2022, 11, 66. https://doi.org/10.3390/jcm11010066
Hata-Mizuno M, Uchino Y, Uchino M, Shimmura S, Ogawa Y, Tsubota K, Negishi K. Analysis of the Association between Galectin-3 Concentration in Tears and the Severity of Dry Eye Disease: A Case-Control Study. Journal of Clinical Medicine. 2022; 11(1):66. https://doi.org/10.3390/jcm11010066
Chicago/Turabian StyleHata-Mizuno, Miki, Yuichi Uchino, Miki Uchino, Shigeto Shimmura, Yoko Ogawa, Kazuo Tsubota, and Kazuno Negishi. 2022. "Analysis of the Association between Galectin-3 Concentration in Tears and the Severity of Dry Eye Disease: A Case-Control Study" Journal of Clinical Medicine 11, no. 1: 66. https://doi.org/10.3390/jcm11010066
APA StyleHata-Mizuno, M., Uchino, Y., Uchino, M., Shimmura, S., Ogawa, Y., Tsubota, K., & Negishi, K. (2022). Analysis of the Association between Galectin-3 Concentration in Tears and the Severity of Dry Eye Disease: A Case-Control Study. Journal of Clinical Medicine, 11(1), 66. https://doi.org/10.3390/jcm11010066








