Extended Trochanteric Osteotomy with Intermediate Resection Arthroplasty Is Safe for Use in Two-Stage Revision Total Hip Arthroplasty for Infection
Abstract
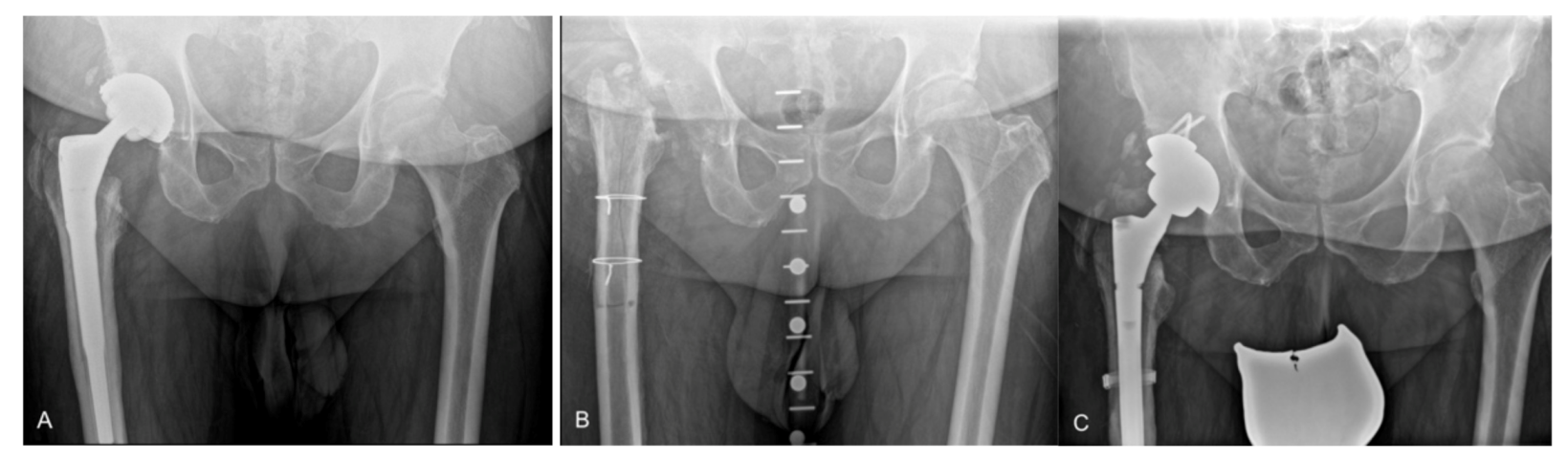
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnosis of PJI
2.3. Surgical Technique
2.4. Antimicrobial Treatment
2.5. Radiographic Analysis
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Demographics
3.2. Microbiology and Reinfection
3.3. Aseptic Revision and Other Complications
3.4. Radiographic and Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamath, A.F.; Ong, K.L.; Lau, E.; Chan, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J.; Bozic, K.J. Quantifying the Burden of Revision Total Joint Arthroplasty for Periprosthetic Infection. J. Arthroplast. 2015, 30, 1492–1497. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Farley, K.X.; Guild, G.N.; Bradbury, T.L. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2030. J. Arthroplast. 2020, 35, S79–S85. [Google Scholar] [CrossRef]
- Tsukayama, D.T.; Estrada, R.; Gustilo, R.B. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Jt. Surg. Am. 1996, 78, 512–523. [Google Scholar] [CrossRef]
- Masri, B.A.; Panagiotopoulos, K.P.; Greidanus, N.V.; Garbuz, D.S.; Duncan, C.P. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J. Arthroplast. 2007, 22, 72–78. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Raja, S.; Khan, M.A.; Haddad, F.S. A multidisciplinary team approach to twostage revision for the infected hip replacement: A minimum five-year follow-up study. Bone Jt. J. 2014, 96-B, 1312–1318. [Google Scholar] [CrossRef]
- Whitehouse, M.R.; Parry, M.C.; Konan, S.; Duncan, C.P. Deep infection after hip arthroplasty: Staying current with change. Bone Jt. J. 2016, 98-B, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllopoulos, G.K.; Memtsoudis, S.G.; Zhang, W.; Ma, Y.; Sculco, T.P.; Poultsides, L.A. Periprosthetic Infection Recurrence After 2-Stage Exchange Arthroplasty: Failure or Fate? J. Arthroplast. 2017, 32, 526–531. [Google Scholar] [CrossRef]
- Akgün, D.; Müller, M.; Perka, C.; Winkler, T. High cure rate of periprosthetic hip joint infection with multidisciplinary team approach using standardized two-stage exchange. J. Orthop. Surg. Res. 2019, 14, 78. [Google Scholar] [CrossRef]
- Petis, S.M.; Abdel, M.P.; Perry, K.I.; Mabry, T.M.; Hanssen, A.D.; Berry, D.J. Long-Term Results of a 2-Stage Exchange Protocol for Periprosthetic Joint Infection Following Total Hip Arthroplasty in 164 Hips. J. Bone Jt. Surg. Am. 2019, 101, 74–84. [Google Scholar] [CrossRef]
- Wagner, H. Revision prosthesis for the hip joint in severe bone loss. Orthopade 1987, 16, 295–300. [Google Scholar]
- Younger, T.I.; Bradford, M.S.; Magnus, R.E.; Paprosky, W.G. Extended proximal femoral osteotomy: A new technique for femoral revision arthroplasty. J. Arthroplasty 1995, 10, 329–338. [Google Scholar] [CrossRef]
- Levine, B.R.; Della Valle, C.J.; Lewis, P.; Berger, R.A.; Sporer, S.M.; Paprosky, W. Extended Trochanteric Osteotomy for the Treatment of Vancouver B2/B3 Periprosthetic Fractures of the Femur. J. Arthroplast. 2008, 23, 527–533. [Google Scholar] [CrossRef]
- Mardones, R.; Gonzalez, C.; Cabanela, M.E.; Trousdale, R.T.; Berry, D.J. Extended femoral osteotomy for revision of hip arthroplasty: Results and complications. J. Arthroplast. 2005, 20, 79–83. [Google Scholar] [CrossRef]
- Lakstein, D.; Kosashvili, Y.; Backstein, D.; Safir, O.; Gross, A.E. Modified Extended Trochanteric Osteotomy with Preservation of Posterior Structures. HIP Int. 2010, 20, 102–108. [Google Scholar] [CrossRef]
- Morshed, S.; Huffman, G.R.; Ries, M.D. Extended trochanteric osteotomy for 2-stage revision of infected total hip arthroplasty. J. Arthroplast. 2005, 20, 294–301. [Google Scholar] [CrossRef]
- Petrie, M.J.; Harrison, T.P.; Buckley, S.C.; Gordon, A.; Kerry, R.M.; Hamer, A.J. Stay Short or Go Long? Can a Standard Cemented Femoral Prosthesis Be Used at Second-Stage Total Hip Arthroplasty Revision for Infection Following an Extended Trochanteric Osteotomy? J. Arthroplast. 2017, 32, 2226–2230. [Google Scholar] [CrossRef]
- Fink, B.; Oremek, D. The Transfemoral Approach for Removal of Well-Fixed Femoral Stems in 2-Stage Septic Hip Revision. J. Arthroplast. 2016, 31, 1065–1071. [Google Scholar] [CrossRef]
- Levine, B.R.; Della Valle, C.J.; Hamming, M.; Sporer, S.M.; Berger, R.A.; Paprosky, W.G. Use of the Extended Trochanteric Osteotomy in Treating Prosthetic Hip Infection. J. Arthroplast. 2009, 24, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, Z.; Shen, B.; Yang, J.; Kang, P.; Pei, F. The Use of Extended Trochanteric Osteotomy in 2-Stage Reconstruction of the Hip for Infection. J. Arthroplast. 2019, 34, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, C.; De Geest, T.; Ackerman, P.; Audenaert, E. Preformed gentamicin spacers in two-stage revision hip arthroplasty: Functional results and complications. Int. Orthop. 2011, 35, 1471–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faschingbauer, M.; Reichel, H.; Bieger, R.; Kappe, T. Mechanical complications with one hundred and thirty eight (antibiotic-laden) cement spacers in the treatment of periprosthetic infection after total hip arthroplasty. Int. Orthop. 2015, 39, 989–994. [Google Scholar] [CrossRef]
- Gomez, M.M.; Tan, T.L.; Manrique, J.; Deirmengian, G.K.; Parvizi, J. The Fate of Spacers in the Treatment of Periprosthetic Joint Infection. J. Bone Jt. Surg Am. 2015, 97, 1495–1502. [Google Scholar] [CrossRef] [Green Version]
- Erivan, R.; Lecointe, T.; Villatte, G.; Mulliez, A.; Descamps, S.; Boisgard, S. Complications with cement spacers in 2-stage treatment of periprosthetic joint infection on total hip replacement. Orthop. Traumatol. Surg. Res. 2018, 104, 333–339. [Google Scholar] [CrossRef]
- Garceau, S.; Warschawski, Y.; Sanders, E.; Gross, A.; Safir, O.; Kuzyk, P. Impact of Hip Antibiotic Spacer Dislocation on Final Implant Position and Outcomes. J. Arthroplast. 2019, 34, 2107–2110. [Google Scholar] [CrossRef]
- Jones, C.W.; Selemon, N.; Nocon, A.; Bostrom, M.; Westrich, G.; Sculco, P.K. The Influence of Spacer Design on the Rate of Complications in Two-Stage Revision Hip Arthroplasty. J. Arthroplast. 2019, 34, 1201–1206. [Google Scholar] [CrossRef]
- Lancaster, A.J.; Carlson, V.R.; Pelt, C.E.; Anderson, L.A.; Peters, C.L.; Gililland, J.M. High Rates of Spacer Fracture in the Setting of Extended Trochanteric Osteotomy with a Specific Thin-Core Articulating Antibiotic Hip Spacer. J. Arthroplast. 2021, 36, 2178–2183. [Google Scholar] [CrossRef]
- Charlton, W.P.H.; Hozack, W.J.; Teloken, M.A.; Rao, R.; Bissett, G.A. Complications associated with reimplantation after Girdlestone arthroplasty. Clin. Orthop Relat Res. 2003, 407, 119–126. [Google Scholar] [CrossRef]
- Carli, A.V.; Bhimani, S.; Yang, X.; de Mesy Bentley, K.L.; Ross, F.P.; Bostrom, M.P.G. Vancomycin-Loaded Polymethylmethacrylate Spacers Fail to Eradicate Periprosthetic Joint Infection in a Clinically Representative Mouse Model. J. Bone Jt. Surg. Am. 2018, 100, e76. [Google Scholar] [CrossRef]
- Marczak, D.; Synder, M.; Sibinski, M.; Polguj, M.; Dudka, J.; Kowalczewski, J. Two stage revision hip arthroplasty in periprosthetic joint infection. Comparison study: With or without the use of a spacer. Int. Orthop. 2017, 41, 2253–2258. [Google Scholar]
- Gramlich, Y.; Hagebusch, P.; Faul, P.; Klug, A.; Walter, G.; Hoffmann, R. Two-stage hip revision arthroplasty for periprosthetic joint infection without the use of spacer or cemented implants. Int. Orthop. 2019, 43, 2457–2466. [Google Scholar] [CrossRef]
- Hipfl, C.; Carganico, T.; Leopold, V.; Perka, C.; Müller, M.; Hardt, S. Two-Stage Revision Total Hip Arthroplasty Without Spacer Placement: A Viable Option to Manage Infection in Patients with Severe Bone Loss or Abductor Deficiency. J. Arthroplast. 2021, 36, 2575–2585. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip. Pelvis. 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Ochsner, P.E.; Borens, O.; Bodler, P.M. Schweizerische Gesellschaft für Orthopädie und Traumatologie. Infections of the Musculoskeletal System—Basic Principles, Prevention, Diagnosis and Treatment, 1st ed.; Swiss Orthopaedics: Grandvaux, Switzerland, 2014. [Google Scholar]
- Portillo, M.E.; Salvadó, M.; Trampuz, A.; Plasencia, V.; Rodriguez-Villasante, M.; Sorli, L.; Puig, L.; Horcajada, J.P. Sonication versus vortexing of implants for diagnosis of prosthetic joint infection. J. Clin. Microbiol. 2013, 51, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Krenn, V.; Morawietz, L.; Perino, G.; Kienapfel, H.; Ascherl, R.; Hassenpflug, G.J.; Thomsen, M.; Thomas, P.; Huber, M.; Kendoff, D.; et al. Revised histopathological consensus classification of joint implant related pathology. Pathol. Res. Pract. 2014, 210, 779–786. [Google Scholar] [CrossRef]
- Janz, V.; Bartek, B.; Wassilew, G.I.; Stuhlert, M.; Perka, C.F.; Winkler, T. Validation of Synovial Aspiration in Girdlestone Hips for Detection of Infection Persistence in Patients Undergoing 2-Stage Revision Total Hip Arthroplasty. J. Arthroplast. 2016, 31, 684–687. [Google Scholar] [CrossRef]
- Paprosky, W.G.; Paprosky, W.G.; Perona, P.G.; Perona, P.G.; Lawrence, J.M.; Lawrence, J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J. Arthroplast. 1994, 9, 33–44. [Google Scholar] [CrossRef]
- Valle, C.J.D.; Paprosky, W.G. Classification and an algorithmic approach to the reconstruction of femoral deficiency in revision total hip arthroplasty. J. Bone Jt. Surg. Am. 2003, 85-A (Suppl. 4), 1–6. [Google Scholar] [CrossRef]
- Harris, W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J. Bone Jt. Surg. Am. 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Q.; Kuo, F.C.; Goswami, K.; Tan, T.L.; Parvizi, J. The Presence of Sinus Tract Adversely Affects the Outcome of Treatment of Periprosthetic Joint Infections. J. Arthroplast. 2019, 34, 1227–1232.e2. [Google Scholar] [CrossRef]
- Akgün, D.; Müller, M.; Perka, C.; Winkler, T. An often-unrecognized entity as cause of recurrent infection after successfully treated two-stage exchange arthroplasty: Hematogenous infection. Arch. Orthop. Trauma Surg. 2018, 138, 1199–1206. [Google Scholar] [CrossRef]
- Herzwurm, P.J.; Walsh, J.; Pettine, K.A.; Ebert, F.R. Prophylactic cerclage: A method of preventing femur fracture in uncemented total hip arthroplasty. Orthopedics 1992, 15, 143–146. [Google Scholar] [CrossRef]
- McAlister, I.P.; Perry, K.I.; Mara, K.C.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Two-Stage Revision of Total Hip Arthroplasty for Infection Is Associated with a High Rate of Dislocation. J. Bone Jt. Surg. Am. 2019, 101, 322–329. [Google Scholar] [CrossRef]
| Variable | ETO (n = 32) | Non-ETO (n = 64) | p Value |
|---|---|---|---|
| Cementation in earlier prosthesis | 4 (13%) | 18 (28%) | 0.086 |
| Time from index THA (years) | 7.8 ± 6.7 | 7.9 ± 8.3 | 0.932 |
| Paprosky bone loss, femoral | 0.001 | ||
| 1 | 5 (16%) | 29 (45%) | |
| 2 | 12 (38%) | 26 (41%) | |
| 3A | 9 (28%) | 4 (6%) | |
| 3B | 6 (19%) | 3 (5%) | |
| 4 | 0 (0%) | 2 (3%) | |
| Paprosky bone loss, acetabular | 0.639 | ||
| 1 | 2 (6%) | 8 (13%) | |
| 2A | 6 (19%) | 14(22%) | |
| 2B | 3 (9%) | 7 (11%) | |
| 2C | 12 (38%) | 25 (39%) | |
| 3A | 3 (9%) | 6 (9%) | |
| 3B | 5 (16%) | 3 (5%) | |
| Pelvic discontinuity | 1 (3%) | 1 (2%) | |
| Duration of first-stage surgery (minutes) | 192.8 ± 58.6 | 143.3 ± 51.6 | 0.000 |
| ETO length (mm) | 162 (98–238) | - | - |
| Reimplanted components at second-stage | |||
| Femoral | |||
| Extensively porous-coated | 13 (41%) | 51 (80%) | 0.000 |
| Modular, fluted tapered | 19 (59%) | 9 (14%) | |
| Cemented | 0 (0%) | 4 (6%) | |
| Acetabular | 0.953 | ||
| Modular, porous-coated | 3 (9%) | 8 (13%) | |
| Highly porous Trabecular metal | 20 (63%) | 40 (63%) | |
| Antiprotrusio cage | 5 (16%) | 8 (13%) | |
| Cemented | 4 (13%) | 8 (13%) | |
| Dual-mobility articulation | 5 (16%) | 10 (16%) | 1.000 |
| Duration of second-stage surgery (minutes) | 169.3 ± 47.9 | 145.3 ± 57.7 | 0.046 |
| Isolated Microorganism * | ETO (n = 32) | Non-ETO (n = 64) |
|---|---|---|
| Gram-positive bacteria | ||
| Coagulase-negative Staphylococcus (sensitive) | 12 | 34 |
| Coagulase-negative Staphylococcus (resistant) | 1 | 0 |
| Methicillin-sensitive Staphylococcus aureus | 4 | 11 |
| Methicillin-resistant Staphylococcus aureus | 0 | 3 |
| Cutibacterium spp. | 3 | 5 |
| Staphylococcus lugdunensis | 1 | 1 |
| Viridans group Streptococcus | 1 | 2 |
| Entercoccus faecalis | 2 | 3 |
| Enterococcus faecium | 0 | 3 |
| Peptostreptococcus micros | 1 | 2 |
| Finegoldia magna | 3 | 1 |
| Corynebacterium spp. | 0 | 2 |
| Actinomyces spp. | 0 | 2 |
| Peptoniphilus spp. | 1 | 2 |
| Cellulomonas | 0 | 1 |
| Gram-negative bacteria | ||
| Escherichia coli | 1 | 4 |
| Roseomonas mucosa | 0 | 1 |
| Polymicrobial | 5 | 25 |
| Negative culture | 3 | 7 |
| Variable | ETO (n = 32) | Non-ETO (n = 64) | p Value |
|---|---|---|---|
| Age at first-stage (years) | 71.3 ± 10.5 | 70.9 ± 7.3 | - |
| Sex (M:F) | 11:21 | 22:42 | - |
| BMI (kg/m2) | 27.1 ± 5.2 | 29.3 ± 5.4 | 0.076 |
| Systemic host grade | 0.291 | ||
| A | 9 (28%) | 10 (16%) | |
| B | 17 (53%) | 36 (56%) | |
| C | 6 (19%) | 18 (28%) | |
| Local extremity grade | 0.147 | ||
| II | 23 (72%) | 54 (84%) | |
| III | 9 (28%) | 10 (16%) | |
| Sinus tract present | 8 (25%) | 8 (13%) | 0.121 |
| Microbiology at first-stage | |||
| Difficult-to-treat * | 6 (19%) | 9 (14%) | 0.551 |
| Negative cultures | 3 (9%) | 7 (11%) | 0.813 |
| Positive cultures at second-stage | 3 (9%) | 6 (9%) | 1.000 |
| Weeks between stages | 8.8 ± 5.4 | 9.1 ± 4.3 | 0.712 |
| Follow-up (months) | 66.1 ± 20.0 | 65.5 ± 17.4 | 0.882 |
| ETO (n = 32) | Non-ETO (n = 64) | p Value | |
|---|---|---|---|
| Radiographic results | |||
| ETO fragment fracture | 4 (13%) | 5 (8%) * | 0.458 |
| ETO migration (>5 mm) | 1 (3%) | - | |
| Union of ETO | 31 (97%) | - | |
| Femoral stem subsidence (>5 mm) | 4 (13%) | 6 (9%) | 0.637 |
| Reinfection | |||
| Interim re-debridement for infection persistence | 0 (0%) | 9 (14%) | 0.026 |
| Reinfection after reimplantation | 4 (13%) | 6 (9%) | 0.365 |
| Other complications | |||
| Traumatic femoral fracture in the interim period | 1 (3%) | 1 (2%) | 0.613 |
| Early superficial wound complication after first-stage | 2 (6%) | 4 (6%) | 1.000 |
| Early superficial wound complication after reimplantation | 1 (3%) | 7 (11%) | 0.192 |
| Hip instability after reimplantation | 4 (13%) | 8 (13%) | 0.978 |
| Periprosthetic femoral fracture after reimplantation | 0 (0%) | 3 (5%) | 0.213 |
| Cup loosening | 0 (0%) | 1 (2%) | 0.477 |
| Stem loosening | 1 (3%) | 1 (2%) | 0.613 |
| Functional outcome | |||
| Preoperative mHHS before first-stage | 37.7 ± 17.1 | 37.3 ± 12.2 | 0.904 |
| Postoperative mHHS at final follow-up | 65.9 ± 15.7 | 67.4 ± 15.2 | 0.700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardt, S.; Leopold, V.J.; Khakzad, T.; Pumberger, M.; Perka, C.; Hipfl, C. Extended Trochanteric Osteotomy with Intermediate Resection Arthroplasty Is Safe for Use in Two-Stage Revision Total Hip Arthroplasty for Infection. J. Clin. Med. 2022, 11, 36. https://doi.org/10.3390/jcm11010036
Hardt S, Leopold VJ, Khakzad T, Pumberger M, Perka C, Hipfl C. Extended Trochanteric Osteotomy with Intermediate Resection Arthroplasty Is Safe for Use in Two-Stage Revision Total Hip Arthroplasty for Infection. Journal of Clinical Medicine. 2022; 11(1):36. https://doi.org/10.3390/jcm11010036
Chicago/Turabian StyleHardt, Sebastian, Vincent Justus Leopold, Thilo Khakzad, Matthias Pumberger, Carsten Perka, and Christian Hipfl. 2022. "Extended Trochanteric Osteotomy with Intermediate Resection Arthroplasty Is Safe for Use in Two-Stage Revision Total Hip Arthroplasty for Infection" Journal of Clinical Medicine 11, no. 1: 36. https://doi.org/10.3390/jcm11010036
APA StyleHardt, S., Leopold, V. J., Khakzad, T., Pumberger, M., Perka, C., & Hipfl, C. (2022). Extended Trochanteric Osteotomy with Intermediate Resection Arthroplasty Is Safe for Use in Two-Stage Revision Total Hip Arthroplasty for Infection. Journal of Clinical Medicine, 11(1), 36. https://doi.org/10.3390/jcm11010036






