Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Greatly Improved Fatigue Symptoms When Treated with Oxygen-Ozone Autohemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Recruitment
2.2. Inclusion and Exclusion Criteria
2.3. Sample Size
2.4. Patients’ Evaluation of the Fatigue Symptomatology
2.5. Patients’ Treatment with Oxygen Ozone Autohemotherapy (O2-O3-AHT)
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deumer, U.S.; Varesi, A.; Floris, V.; Savioli, G.; Mantovani, E.; López-Carrasco, P.; Rosati, G.M.; Prasad, S.; Ricevuti, G. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J. Clin. Med. 2021, 10, 4786. [Google Scholar] [CrossRef]
- Barhorst, E.E.; Boruch, A.E.; Cook, D.B.; Lindheimer, J.B. Pain-related post-exertional malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Fibromyalgia: A systematic review and three-level meta-analysis. Pain Med. 2021, pnab308. [Google Scholar] [CrossRef]
- Noor, N.; Urits, I.; Degueure, A.; Rando, L.; Kata, V.; Cornett, E.M.; Kaye, A.D.; Imani, F.; Narimani-Zamanabadi, M.; Varrassi, G.; et al. A Comprehensive Update of the Current Understanding of Chronic Fatigue Syndrome. Anesthesiol. Pain Med. 2021, 11, e113629. [Google Scholar] [CrossRef]
- Lim, E.J.; Son, C.G. Prevalence of Chronic Fatigue Syndrome (CFS) in Korea and Japan: A Meta-Analysis. J. Clin. Med. 2021, 10, 3204. [Google Scholar] [CrossRef]
- Tirelli, U.; Lleshi, A.; Berretta, M.; Spina, M.; Talamini, R.; Giacalone, A. Treatment of 741 Italian patients with chronic fatigue syndrome. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2847–2852. [Google Scholar]
- Tirelli, U.; Cirrito, C.; Pavanello, M.; Del Pup, L.; Lleshi, A.; Berretta, M. Oxygen-ozone therapy as support and palliative therapy in 50 cancer patients with fatigue—A short report. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8030–8033. [Google Scholar] [PubMed]
- Arpino, C.; Carrieri, M.P.; Valesini, G.; Pizzigallo, E.; Rovere, P.; Tirelli, U.; Conti, F.; Dialmi, P.; Barberio, A.; Rusconi, N.; et al. Idiopathic chronic fatigue and chronic fatigue syndrome: A comparison of two case-definitions. Ann. Dell’istituto Super. Di Sanità 1999, 35, 435–441. [Google Scholar]
- Tirelli, U.; Chierichetti, F.; Tavio, M.; Simonelli, C.; Bianchin, G.; Zanco, P.; Ferlin, G. Brain positron emission tomography (PET) in chronic fatigue syndrome: Preliminary data. Am. J. Med. 1998, 105, 54S–58S. [Google Scholar] [CrossRef]
- Montoya, J.G.; Holmes, T.H.; Anderson, J.N.; Maecker, H.T.; Rosenberg-Hasson, Y.; Valencia, I.J.; Chu, L.; Younger, J.W.; Tato, C.M.; Davis, M.M. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc. Natl. Acad. Sci. USA 2017, 114, E7150–E7158. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, U.; Marotta, G.; Improta, S.; Pinto, A. Immunological abnormalities in patients with chronic fatigue syndrome. Scand. J. Immunol. 1994, 40, 601–608. [Google Scholar] [CrossRef]
- Tirelli, V.; Pinto, A.; Marotta, G.; Crovato, M.; Quaia, M.; De Paoli, P.; Galligioni, E.; Santini, G. Clinical and immunologic study of 205 patients with chronic fatigue syndrome: A case series from Italy. Arch. Intern. Med. 1993, 153, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Estévez-López, F.; Mudie, K.; Wang-Steverding, X.; Bakken, I.J.; Ivanovs, A.; Castro-Marrero, J.; Nacul, L.; Alegre, J.; Zalewski, P.; Słomko, J.; et al. Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology. J. Clin. Med. 2020, 9, 1557. [Google Scholar] [CrossRef]
- Vincent, A.; Brimmer, D.J.; Whipple, M.O.; Jones, J.F.; Boneva, R.; Lahr, B.D.; Maloney, E.; St Sauver, J.L.; Reeves, W.C. Prevalence, incidence, and classification of chronic fatigue syndrome in Olmsted County, Minnesota, as estimated using the Rochester Epidemiology Project. Mayo Clin. Proc. 2012, 87, 1145–1152. [Google Scholar] [CrossRef]
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Capelli, E.; Lorusso, L.; Ghitti, M.; Venturini, L.; Cusa, C.; Ricevuti, G. Chronic fatigue syndrome: Features of a population of patients from northern Italy. Int. J. Immunopathol. Pharmacol. 2015, 28, 53–59. [Google Scholar] [CrossRef]
- Spazzapan, S.; Bearz, A.; Tirelli, U. Fatigue in cancer patients receiving chemotherapy. Anal. Publ. Stud. Ann. Oncol. 2004, 15, 1576. [Google Scholar]
- Solomon, L.; Reeves, W.C. Factors influencing the diagnosis of chronic fatigue syndrome. Arch. Intern. Med. 2004, 164, 2241–2245. [Google Scholar] [CrossRef]
- Brenna, E.; Araja, D.; Pheby, D.F.H. Comparative Survey of People with ME/CFS in Italy, Latvia, and the UK: A Report on Behalf of the Socioeconomics Working Group of the European ME/CFS Research Network (EUROMENE). Medicina 2021, 57, 300. [Google Scholar] [CrossRef]
- Kennedy, G.; Abbot, N.C.; Spence, V.; Underwood, C.; Belch, J.J. The specificity of the CDC-1994 criteria for chronic fatigue syndrome: Comparison of health status in three groups of patients who fulfill the criteria. Ann. Epidemiol. 2004, 14, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kuratsune, H. Diagnosis and Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Brain Nerve 2018, 70, 11–18. [Google Scholar] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Bruun Wyller, V.; Bjørneklett, A.; Brubakk, O.; Festvåg, L.; Follestad, I.; Malt, U.; Malterud, K.; Nyland, H.; Rambøl, H.; Stubhaug, B.; et al. Diagnosis and Treatment of Chronic Fatigue Syndrome/Myalgic Encephalopathy (CFS/ME) [Internet]; Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 09-2006; Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH): Oslo, Norway, 2006. [Google Scholar]
- Chew-Graham, C.; Dowrick, C.; Wearden, A.; Richardson, V.; Peters, S. Making the diagnosis of Chronic Fatigue Syndrome/Myalgic Encephalitis in primary care: A qualitative study. BMC Fam. Pract. 2010, 11, 16. [Google Scholar] [CrossRef]
- Son, C.G. Differential diagnosis between “chronic fatigue” and “chronic fatigue syndrome”. Integr. Med. Res. 2019, 8, 89–91. [Google Scholar] [CrossRef]
- Nelsen, D.A., Jr. Differential diagnosis for chronic fatigue syndrome. Am. Fam. Physician 2003, 67, 252. [Google Scholar]
- Craig, T.; Kakumanu, S. Chronic fatigue syndrome: Evaluation and treatment. Am. Fam. Physician 2002, 65, 1083–1089. [Google Scholar]
- Tirelli, U.; Cirrito, C.; Pavanello, M. Ozone therapy is an effective therapy in chronic fatigue syndrome: Result of an Italian study in 65 patients. Ozon Ther. 2018, 3, 27–30. [Google Scholar] [CrossRef]
- Morelli, L.; Bramani, S.C.; Morelli, F.C. Oxygen-ozone therapy in meningoencephalitis and chronic fatigue syndrome. Treat. Field Compet. Sports Case Rep. Ozone Ther. 2019, 4, 20–23. [Google Scholar]
- Borrelli, E.; Bocci, V. A novel therapeutic option for Chronic Fatigue Syndrome and Fibromyalgia. Riv. Ital. Ossig.-Ozonoterap. 2002, 1, 149–153. [Google Scholar]
- Viebahn-Haensler, R.; León Fernández, O.S. Ozone in Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Maes, M. Environmental, Neuro-immune, and Neuro-oxidative Stress Interactions in Chronic Fatigue Syndrome. Mol. Neurobiol. 2020, 57, 4598–4607. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Valdenassi, L.; Simonetti, V.; Bertossi, D.; Ricevuti, G.; Franzini, M.; Pandolfi, S. Insights on the mechanisms of action of ozone in the medical therapy against COVID-19. Int. Immunopharmacol. 2021, 96, 107777. [Google Scholar] [CrossRef]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Shaw, E.J. Diagnosis and management of chronic fatigue syndrome or myalgic encephalomyelitis (or encephalopathy): Summary of NICE guidance. BMJ 2007, 335, 446–448. [Google Scholar] [CrossRef]
- Neuberger, G.B. Measures of fatigue Arthritis and Rheumatisms. Arthritis Care Res. 2003, 48, S175–S183. [Google Scholar] [CrossRef]
- Tirelli, U.; Franzini, M.; Valdenassi, L.; Pisconti, S.; Taibi, R.; Torrisi, C.; Pandolfi, S.; Chirumbolo, S. Fatigue in post-acute sequelae of SARS-CoV2 (PASC) treated with oxygen-ozone autohemotherapy-preliminary results on 100 patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5871–5875. [Google Scholar] [PubMed]
- lvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar]
- Anderson, G.; Maes, M. Mitochondria and immunity in chronic fatigue syndrome. Prog. Neuro.-Psychopharmacol. Biol. Psychiatry 2020, 103, 109976. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N.; Kubera, M.; Ringel, K. Evidence for inflammation and activation of cell-mediated immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Increased interleukin-1, tumor necrosis factor-α, PMN-elastase, lysozyme and neopterin. J. Affect Disord. 2012, 136, 933–939. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N.; Ringel, K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: Inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother. Psychosom. 2012, 81, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Brenu, E.W.; Huth, T.K.; Hardcastle, S.L.; Fuller, K.; Kaur, M.; Johnston, S.; Ramos, S.B.; Staines, D.R.; Marshall-Gradisnik, S.M. Role of adaptive and innate immune cells in chronic fatigue syndrome/myalgic encephalomyelitis. Int. Immunol. 2014, 26, 233–242. [Google Scholar] [CrossRef]
- Wei, M.; Tu, L.; Liang, Y.H.; Liu, J.; Gong, Y.J.; Zhang, J.H.; Zhang, Y.H. Effects of ozone exposure on percentage of CD4(+)CD25(high)Foxp(3+) regulatory T cells and mRNA expression of Foxp3 in asthmatic rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2013, 31, 693–696. [Google Scholar]
- Broderick, G.; Fuite, J.; Kreitz, A.; Vernon, S.D.; Klimas, N.; Fletcher, M.A. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav. Immun. 2010, 24, 1209–1217. [Google Scholar] [CrossRef]
- Che, L.; Jin, Y.; Zhang, C.; Lai, T.; Zhou, H.; Xia, L.; Tian, B.; Zhao, Y.; Liu, J.; Wu, Y.; et al. Ozone-induced IL-17A and neutrophilic airway inflammation is orchestrated by the caspase-1-IL-1 cascade. Sci. Rep. 2016, 6, 18680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Saito, Y.; Yoshida, Y.; Sekine, A.; Noguchi, N.; Niki, E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J. Biol. Chem. 2005, 280, 41921–41927. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, H.; Li, Q.; Li, Y.; Hong, A. Thioredoxin induces Tregs to generate an immunotolerant tumor microenvironment in metastatic melanoma. Oncoimmunology 2015, 4, e1027471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meeus, M.; Van Eupen, I.; Hondequin, J.; De Hauwere, L.; Kos, D.; Nijs, J. Nitric oxide concentrations are normal and unrelated to activity level in chronic fatigue syndrome: A case-control study. In Vivo 2010, 24, 865–869. [Google Scholar]
- Robinson, M.; Gray, S.R.; Watson, M.S.; Kennedy, G.; Hill, A.; Belch, J.J.; Nimmo, M.A. Plasma IL-6, its soluble receptors and F2-isoprostanes at rest and during exercise in chronic fatigue syndrome. Scand. J. Med. Sci. Sports 2010, 20, 282–290. [Google Scholar] [CrossRef]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10 (Suppl. S1), S10–S23. [Google Scholar] [CrossRef] [PubMed]
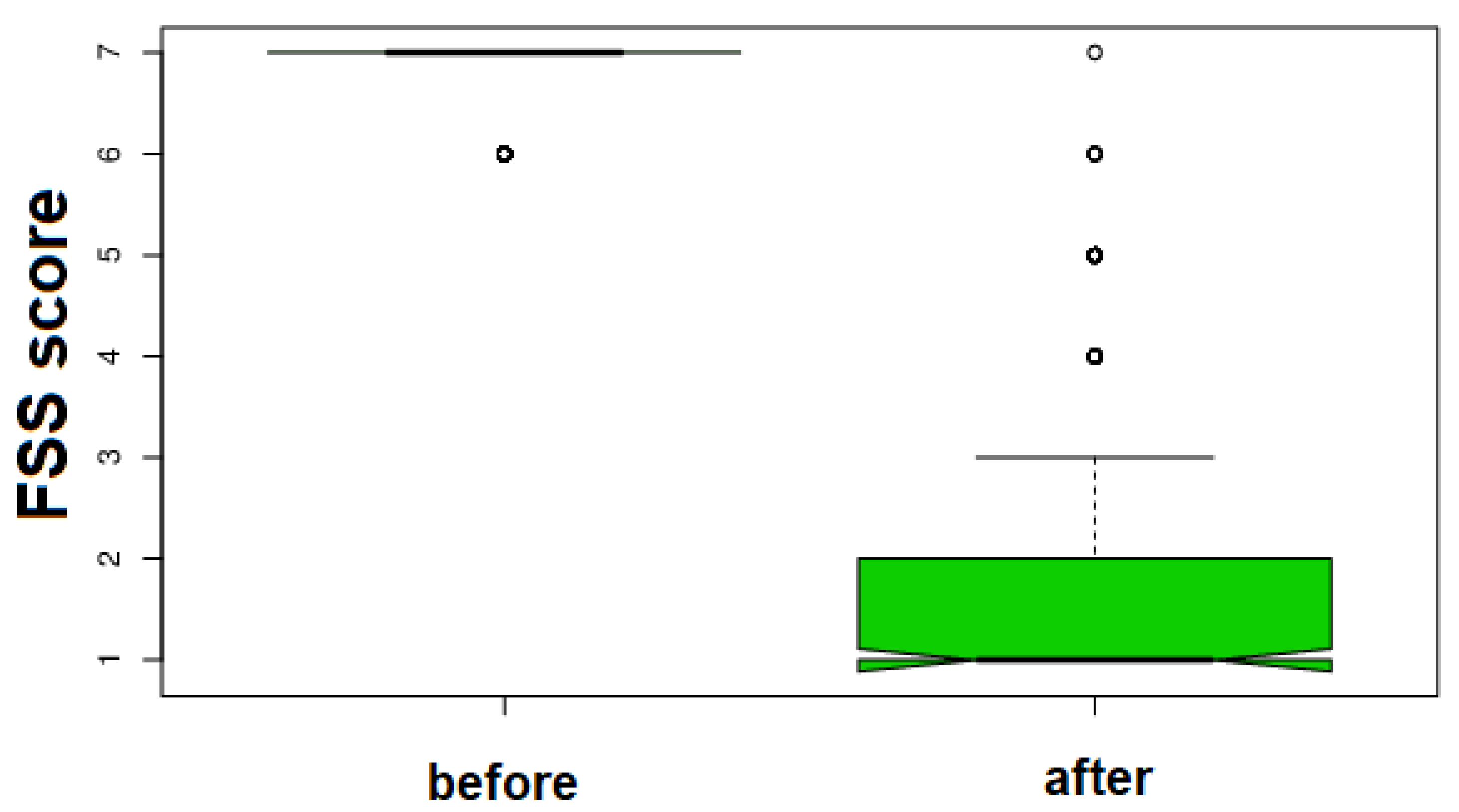
| Before Treatment | After Treatment | KW Test Delta % Rate | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||||
| 6.825 ± 0.381 | 2.085 ± 1.503 | H = 293.6672 Δ% = 69.45 0.34 | <0.0001 | ||||||||
| Male patients | |||||||||||
| 6.725 ± 0.450 | 2.725 ± 1.781 | H = 54.4956 Δ% = 59.48 0.86 | <0.0001 | ||||||||
| Female patients | |||||||||||
| 6.878 ± 0.329 | 1.748 ± 1.211 | H =195.388 Δ% = 74.58 0.57 | <0.0001 | ||||||||
| Statistical and FFS score data | |||||||||||
| Mean age (all): 33.085 ± 13.503 SD | Mean age (males): 37.406 ± 13.958 SD | Mean age (females): 30.809 ± 12.730 SD | |||||||||
| 7 to 7 = 1 | 7 to 6 = 4 | 7 to 5 = 17 | 7 to 4 = 9 | 7 to 3 = 5 | 7 to 2 = 42 | 7 to 1 = 87 | |||||
| 6 to 6 = 0 | 6 to 5 = 6 | 6 to 4 = 3 | 6 to 3 = 0 | 6 to 2 = 11 | 6 to 1 = 15 | 5 to 1 = 0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirelli, U.; Franzini, M.; Valdenassi, L.; Pandolfi, S.; Berretta, M.; Ricevuti, G.; Chirumbolo, S. Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Greatly Improved Fatigue Symptoms When Treated with Oxygen-Ozone Autohemotherapy. J. Clin. Med. 2022, 11, 29. https://doi.org/10.3390/jcm11010029
Tirelli U, Franzini M, Valdenassi L, Pandolfi S, Berretta M, Ricevuti G, Chirumbolo S. Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Greatly Improved Fatigue Symptoms When Treated with Oxygen-Ozone Autohemotherapy. Journal of Clinical Medicine. 2022; 11(1):29. https://doi.org/10.3390/jcm11010029
Chicago/Turabian StyleTirelli, Umberto, Marianno Franzini, Luigi Valdenassi, Sergio Pandolfi, Massimiliano Berretta, Giovanni Ricevuti, and Salvatore Chirumbolo. 2022. "Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Greatly Improved Fatigue Symptoms When Treated with Oxygen-Ozone Autohemotherapy" Journal of Clinical Medicine 11, no. 1: 29. https://doi.org/10.3390/jcm11010029
APA StyleTirelli, U., Franzini, M., Valdenassi, L., Pandolfi, S., Berretta, M., Ricevuti, G., & Chirumbolo, S. (2022). Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Greatly Improved Fatigue Symptoms When Treated with Oxygen-Ozone Autohemotherapy. Journal of Clinical Medicine, 11(1), 29. https://doi.org/10.3390/jcm11010029









