Axillary Lymphadenopathy on Ultrasound after COVID-19 Vaccination and Its Influencing Factors: A Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Ultrasound Technique
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faermann, R.; Nissan, N.; Halshtok-Neiman, O.; Shalmon, A.; Gotlieb, M.; Yagil, Y.; Samoocha, D.; Friedman, E.; Sklair-Levy, M. COVID-19 vaccination induced lymphadenopathy in a specialized breast imaging clinic in Israel: Analysis of 163 cases. Acad. Radiol. 2021, 28, 1191–1197. [Google Scholar] [CrossRef]
- Keshavarz, P.; Yazdanpanah, F.; Rafiee, F.; Mizandari, M. Lymphadenopathy following COVID-19 vaccination: Imaging findings review. Acad. Radiol. 2021, 28, 1058–1071. [Google Scholar] [CrossRef]
- Edmonds, C.E.; Zuckerman, S.P.; Conant, E.F. Management of unilateral axillary lymphadenopathy detected on breast MRI in the Era of COVID-19 vaccination. Am. J. Roentgenol. 2021, 217, 831–834. [Google Scholar] [CrossRef]
- Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine. US Centers for Disease Control and Prevention Web Site. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html#18-local-reactions (accessed on 20 November 2021).
- Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Pfizer-BioNTech COVID-19 Vaccine. US Centers for Disease Control and Prevention Web Site. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html#18-local-reactions (accessed on 20 November 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Information for UK Recipients on COVID 19 Vaccine AstraZeneca-Regulation 174 Information for UK Recipients. NHS Web Site. Available online: https://www.cntw.nhs.uk/resource-library/information-for-uk-recipients-on-covid-19-vaccine-astrazeneca-regulation-174-information-for-uk-recipients (accessed on 20 November 2021).
- Robinson, K.A.; Maimone, S.; Gococo-Benore, D.A.; Li, Z.; Advani, P.P.; Chumsri, S. Incidence of axillary adenopathy in breast imaging after COVID-19 vaccination. JAMA. Oncol. 2021, 7, 1395–1397. [Google Scholar] [CrossRef]
- Özütemiz, C.; Krystosek, L.A.; Church, A.L.; Chauhan, A.; Ellermann, J.M.; Domingo-Musibay, E.; Steinberger, D. Lymphadenopathy in COVID-19 vaccine recipients: Diagnostic dilemma in oncologic patients. Radiology 2021, 300, E296–E300. [Google Scholar] [CrossRef]
- Mehta, N.; Sales, R.M.; Babagbemi, K.; Levy, A.D.; McGrath, A.L.; Drotman, M.; Dodelzon, K. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin. Imaging 2021, 75, 12–15. [Google Scholar] [CrossRef]
- Cocco, G.; Delli Pizzi, A.; Fabiani, S.; Cocco, N.; Boccatonda, A.; Frisone, A.; Scarano, S.; Shiavone, C. Lymphadenopathy after the anti-COVID-19 Vaccine: Multiparametric ultrasound findings. Biology 2021, 10, 652. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Galdiero, R.; Picone, C.; Izzo, F.; D’Aniello, R.; Miele, V.; Grassi, R.; Grassi, R.; et al. Lymphadenopathy after BNT162b2 Covid-19 vaccine: Preliminary ultrasound findings. Biology 2021, 10, 214. [Google Scholar] [CrossRef]
- Tu, W.; Gierada, D.S.; Joe, B.N. COVID-19 vaccination-related lymphadenopathy: What to be aware of. Radiol. Imaging Cancer 2021, 3, e210038. [Google Scholar] [CrossRef]
- Becker, A.S.; Perez-Johnston, R.; Chikarmane, S.A.; Chen, M.M.; Homsi, M.E.; Feigin, K.N.; Gallagher, K.M.; Hanna, E.Y.; Hicks, M.; Ilica, A.T.; et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: Radiology scientific expert panel. Radiology 2021, 300, E323–E327. [Google Scholar] [CrossRef]
- Youn, H.; Hong, K.J. Non-invasive molecular imaging of immune cell dynamics for vaccine research. Clin. Exp. Vaccine Res. 2019, 8, 89–93. [Google Scholar] [CrossRef]
- Burger, I.A.; Husmann, L.; Hany, T.F.; Schmid, D.T.; Schaefer, N.G. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin. Nucl. Med. 2011, 36, 848–853. [Google Scholar] [CrossRef]
- Studdiford, J.; Lamb, K.; Horvath, K.; Altshuler, M.; Stonehouse, A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy 2008, 28, 1194–1197. [Google Scholar] [CrossRef]
- Casey, C.G.; Iskander, J.K.; Roper, M.H.; Mast, E.E.; Wen, X.J.; Török, T.J.; Chapman, L.E.; Swerdlow, E.L.; Morgan, J.; Heffelfinger, J.D.; et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA 2005, 294, 2734–2743. [Google Scholar] [CrossRef] [Green Version]
- Marais, B.J.; Wright, C.A.; Schaaf, H.S.; Gie, R.P.; Hesseling, A.C.; Enarson, D.A.; Beyers, N. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from tuberculosis-endemic area. Pediatr. Infect. Dis. J. 2006, 25, 142–146. [Google Scholar] [CrossRef]
- Dorfman, R.F.; Herweg, J.C. Live, attenuated measles virus vaccine. Inguinal lymphadenopathy complicating administration. JAMA 1966, 198, 320–321. [Google Scholar] [CrossRef]
- Ukey, R.; Bruiners, N.; Mishra, H.; Mishra, P.K.; McCloskey, D.; Onyuka, A.; Chen, F.; Pinter, A.; Weiskopf, D.; Sette, A.; et al. Dichotomy between the humoral and cellular responses elicited by mRNA and adenoviral vector vaccines against SARS-CoV-2. medRxiv 2021, in press. [Google Scholar]
- Society of Breast Imaging. SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination; Society of Breast Imaging: Reston, VA, USA, 2021. [Google Scholar]
- Schiaffino, S.; Pinker, K.; Magni, V.; Cozzi, A.; Athanasiou, A.; Baltzer, P.A.; Herrero, J.C.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; et al. Axillary lymphadenopathy at the time of COVID-19 vaccination: Ten recommendations from the European Society of Breast Imaging (EUSOBI). Insights Imaging 2021, 12, 119. [Google Scholar] [CrossRef]
- Eshet, Y.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Eifer, M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology 2021, 300, E345–E347. [Google Scholar] [CrossRef]
- Richards, N.E.; Keshavarz, B.; Workman, L.J.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA. Netw. Open 2021, 4, e2124331. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT 162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Igual-Rouilleault, A.C.; Soriano, I.; Quan, P.L.; Fernández-Montero, A.; Elizalde, A.; Pina, L. Unilateral axillary adenopathy induced by COVID-19 vaccine: US follow-up evaluation. Eur. Radiol. 2021, in press. [Google Scholar] [CrossRef]


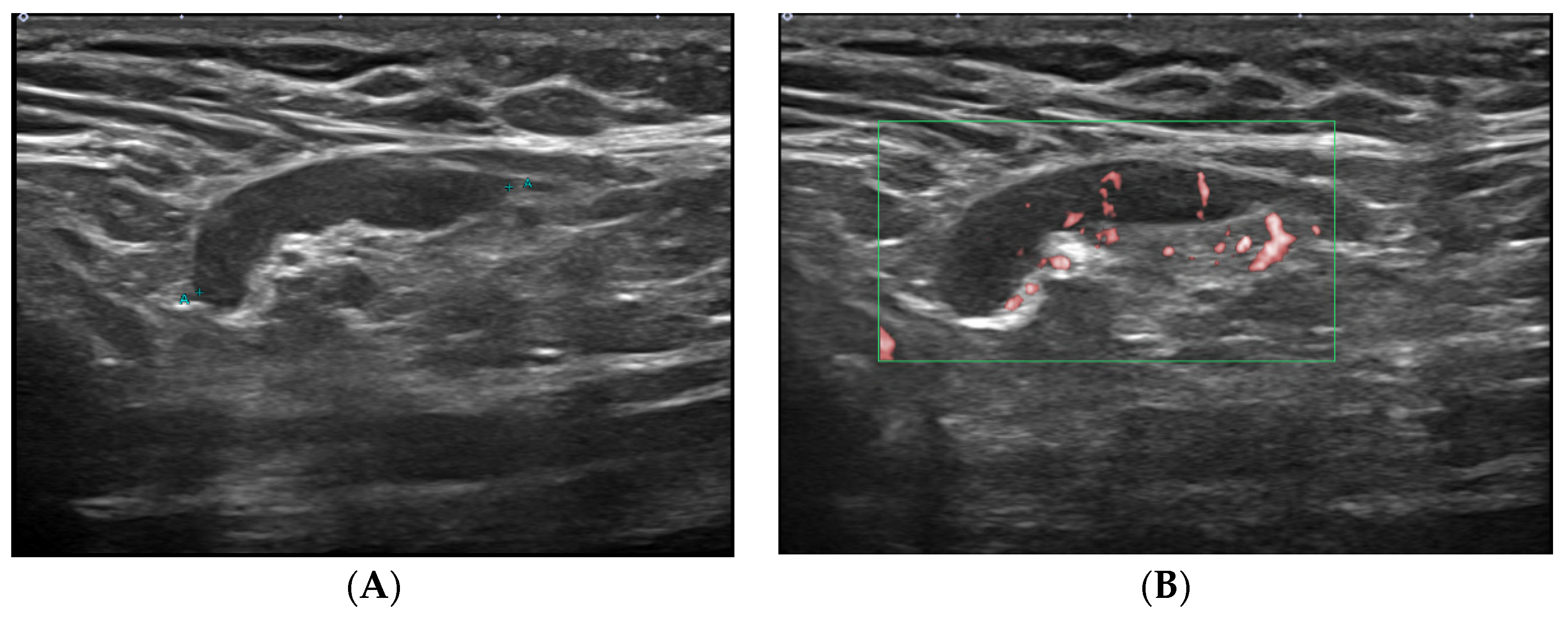

| Characteristics | No. of Patients (n = 413) |
|---|---|
| Mean age (years) * | 48 ± 12 (17–80) |
| Sex (Women/Men) | 403:10 (98:2) |
| Mean interval from vaccination (days) * | 26 ± 18 (1–82) |
| Site | |
| Left arm | 389 (94) |
| Right arm | 24 (6) |
| Vaccine brand | |
| Pfizer | 330 (80) |
| AstraZeneca | 64 (15) |
| Moderna | 19 (5) |
| Vaccine type | |
| mRNA | 349 (85) |
| Vector | 64 (15) |
| Dose | |
| 1st | 156 (38) |
| 2nd | 257 (62) |
| Axillary symptom | |
| Yes | 8 (2) |
| No | 405 (98) |
| Axillary lymphadenopathy | |
| Yes | 202 (49) |
| No | 211 (51) |
| Characteristics | Lymphadenopathy (n = 202) | Non-lymphadenopathy (n = 211) | p Value |
|---|---|---|---|
| Mean age (years) * | 44 ± 11 (17–79) | 52 ± 10 (24–80) | <0.001 |
| Age group | <0.001 | ||
| ≤40 years | 67 (33) | 28 (13) | |
| >40 years | 135 (67) | 183 (87) | |
| Sex | 0.106 | ||
| Women | 200 (99) | 203 (96) | |
| Men | 2 (1) | 8 (4) | |
| Mean interval (days) * | 20 ± 14 (2–80) | 32 ± 19 (1–82) | <0.001 |
| Interval group | <0.001 | ||
| 1–14 days | 77 (38) | 44 (21) | |
| 15–28 days | 82 (41) | 49 (23) | |
| 29–42 days | 29 (14) | 63 (30) | |
| ≥43 days | 14 (7) | 55 (26) | |
| Site | 0.341 | ||
| Left arm | 188 (93) | 201 (95) | |
| Right arm | 14 (7) | 10 (5) | |
| Vaccine brand | <0.001 | ||
| Pfizer | 182 (90) | 148 (70) | |
| AstraZeneca | 5 (3) | 59 (28) | |
| Moderna | 15 (7) | 4 (2) | |
| Vaccine type | <0.001 | ||
| mRNA | 197 (98) | 152 (72) | |
| Vector | 5 (2) | 59 (28) | |
| Dose | <0.001 | ||
| 1st | 98 (48) | 58 (28) | |
| 2nd | 104 (52) | 153 (73) | |
| Axillary symptom | 0.003 | ||
| Yes | 8 (4) | 0 (0) | |
| No | 194 (96) | 211 (100) |
| Variables | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Age group | ||
| ≤40 years | 2.2 (1.3–3.8) | 0.006 |
| Interval group | ||
| 1–14 days | 4.3 (2.0–9.4) | <0.001 |
| 15–28 days | 4.3 (2.0–9.2) | <0.001 |
| 29–42 days | 1.5 (0.7–3.4) | 0.327 |
| Vaccine type | ||
| mRNA | 6.7 (2.5–17.9) | <0.001 |
| Dose | ||
| 1st | 1.6 (1.0–2.5) | 0.045 |
| Axillary symptom | ||
| No | 0 | >0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Lee, J.Y.; Yi, S.Y. Axillary Lymphadenopathy on Ultrasound after COVID-19 Vaccination and Its Influencing Factors: A Single-Center Study. J. Clin. Med. 2022, 11, 238. https://doi.org/10.3390/jcm11010238
Park JY, Lee JY, Yi SY. Axillary Lymphadenopathy on Ultrasound after COVID-19 Vaccination and Its Influencing Factors: A Single-Center Study. Journal of Clinical Medicine. 2022; 11(1):238. https://doi.org/10.3390/jcm11010238
Chicago/Turabian StylePark, Ji Yeon, Ji Young Lee, and Seong Yoon Yi. 2022. "Axillary Lymphadenopathy on Ultrasound after COVID-19 Vaccination and Its Influencing Factors: A Single-Center Study" Journal of Clinical Medicine 11, no. 1: 238. https://doi.org/10.3390/jcm11010238
APA StylePark, J. Y., Lee, J. Y., & Yi, S. Y. (2022). Axillary Lymphadenopathy on Ultrasound after COVID-19 Vaccination and Its Influencing Factors: A Single-Center Study. Journal of Clinical Medicine, 11(1), 238. https://doi.org/10.3390/jcm11010238






