Comparison of Robotic and Conventional Unicompartmental Knee Arthroplasty Outcomes in Patients with Osteoarthritis: A Retrospective Cohort Study
Abstract
:1. Introduction
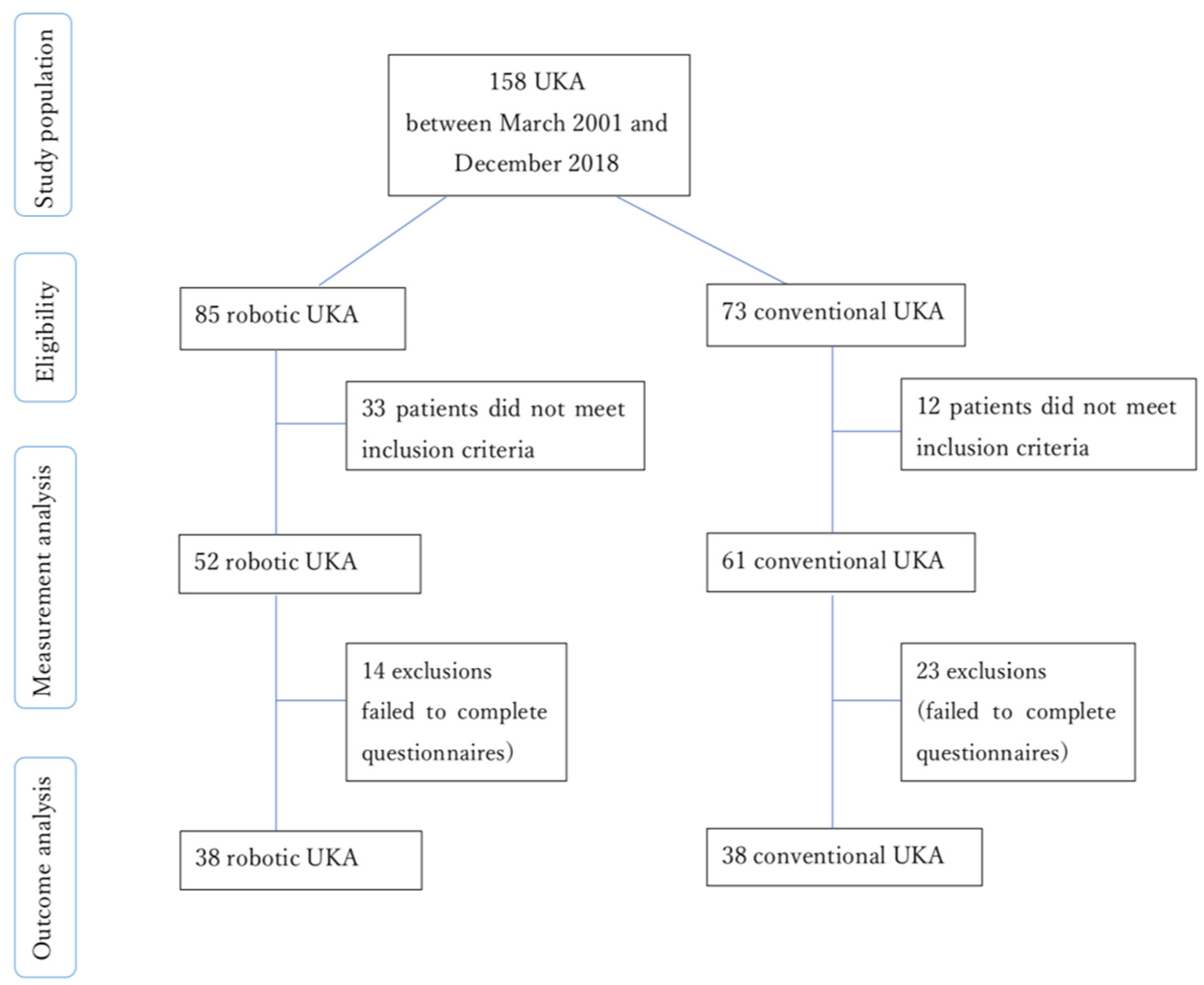
2. Materials and Methods
3. Results
Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johal, S.; Nakano, N.; Baxter, M.; Hujazi, I.; Pandit, H.; Khanduja, V. Unicompartmental Knee Arthroplasty: The Past, Current Controversies, and Future Perspectives. J. Knee Surg. 2018, 31, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, H.; Ba, Z.; Bo, C.; Li, K. Robotic arm-assisted vs conventional unicompartmental knee arthroplasty: A meta-analysis of the effects on clinical outcomes. Medicine 2019, 98, e16968. [Google Scholar] [CrossRef]
- Santoso, M.B.; Wu, L. Unicompartmental knee arthroplasty, is it superior to high tibial osteotomy in treating unicompartmental osteoarthritis? A meta-analysis and systemic review. J. Orthop. Surg. Res. 2017, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Conditt, M.A.; Roche, M.W. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J. Bone Jt. Surg. Ser. A 2009, 91 (Suppl. 1), 63–68. [Google Scholar] [CrossRef]
- Bell, S.W.; Anthony, I.; Jones, B.; MacLean, A.; Rowe, P.; Blyth, M. Improved accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty. J. Bone Jt. Surg. 2016, 98, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Mittal, A.; Meshram, P.; Kim, W.H.; Choi, S.M. Evidence-based surgical technique for medial unicompartmental knee arthroplasty. Knee Surg. Relat. Res. 2021, 33, 1–14. [Google Scholar] [CrossRef]
- Kayani, B.; Haddad, F.S. Robotic unicompartmental knee arthroplasty. Bone Jt. Res. 2019, 8, 228–231. [Google Scholar] [CrossRef]
- Lonner, J.H.; Klement, M.R. Robotic-assisted medial unicompartmental knee arthroplasty: Options and outcomes. J. Am. Acad. Orthop. Surg. 2019, 27, E207–E214. [Google Scholar] [CrossRef]
- Van der List, J.P.; Chawla, H.; Pearle, A.D. Robotic-Assisted Knee Arthroplasty: An Overview. Available online: www.amjorthopedics.com (accessed on 8 June 2021).
- Matsuda, S.; Miura, H.; Nagamine, R.; Mawatari, T.; Tokunaga, M.; Nabeyama, R.; Iwamoto, Y. Anatomical analysis of the femoral condyle in normal and osteoarthritic knees. J. Orthop. Res. 2004, 22, 104–109. [Google Scholar] [CrossRef]
- Cherian, J.J.; Kapadia, B.H.; Banerjee, S.; Jauregui, J.J.; Issa, K.; Mont, M.A. Mechanical, anatomical, and kinematic axis in TKA: Concepts and practical applications. Curr. Rev. Musculoskelet. Med. 2014, 7, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batailler, C.; White, N.; Ranaldi, F.M.; Neyret, P.; Servien, E.; Lustig, S. Improved implant position and lower revision rate with robotic-assisted unicompartmental knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1232–1240. [Google Scholar] [CrossRef]
- Dunbar, N.J.; Roche, M.W.; Park, B.H.; Branch, S.H.; Conditt, M.A.; Banks, S.A. Accuracy of Dynamic Tactile-Guided Unicompartmental Knee Arthroplasty. J. Arthroplast. 2012, 27, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.; Henckel, J.; Gomes, P.; Harris, S.; Jakopec, M.; Rodriguez, F.; Barrett, A.; Davies, B. Hands-on robotic unicompartmental knee replacement. A prospective, randomised controlled study of the Acrobot system. J. Bone Jt. Surg. Ser. B 2006, 88, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Negrín, R.; Duboy, J.; Iñiguez, M.; Reyes, N.O.; Barahona, M.; Ferrer, G.; Infante, C.; Jabes, N. Robotic-assisted vs conventional surgery in medial unicompartmental knee arthroplasty: A clinical and radiological study. Knee Surg. Relat. Res. 2021, 33, 1–7. [Google Scholar] [CrossRef]
- Mofidi, A.; Plate, J.F.; Lu, B.; Conditt, M.A.; Lang, J.E.; Poehling, G.G.; Jinnah, R.H. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1918–1925. [Google Scholar] [CrossRef]
- Khan, H.; Dhillon, K.; Mahapatra, P.; Popat, R.; Zakieh, O.; Kim, W.J.; Nathwani, D. Blood loss and transfusion risk in robotic-assisted knee arthroplasty: A retrospective analysis. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2308. [Google Scholar] [CrossRef]
- Gilmour, A.; MacLean, A.D.; Rowe, P.J.; Banger, M.S.; Donnelly, I.; Jones, B.G.; Blyth, M.J. Robotic-Arm–Assisted vs Conventional Unicompartmental Knee Arthroplasty. The 2-Year Clinical Outcomes of a Randomized Controlled Trial. J. Arthroplast. 2018, 33, S109–S115. [Google Scholar] [CrossRef] [Green Version]
- Blyth, M.J.G.; Anthony, I.; Rowe, P.; Banger, M.S.; MacLean, A.; Jones, B. Robotic arm-assisted versus conventional unicompartmental knee arthroplasty: Exploratory secondary analysis of a randomised controlled trial. Bone Jt. Res. 2017, 6, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.C.; Kusuma, S.K.; Palmer, R.M.; Harris, K.B. Robotic guidance does not improve component position or short-term outcome in medial unicompartmental knee arthroplasty. J. Arthroplast. 2014, 29, 1784–1789. [Google Scholar] [CrossRef]
- Lonner, J.H.; Kerr, G.J. Low rate of iatrogenic complications during unicompartmental knee arthroplasty with two semiautonomous robotic systems. Knee 2019, 26, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, G.; Batailler, C.; Lording, T.; Servien, E.; Lustig, S. Is robotic-assisted unicompartmental knee arthroplasty a safe procedure? A case control study. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Battenberg, A.K.; Netravali, N.A.; Lonner, J.H. A novel handheld robotic-assisted system for unicompartmental knee arthroplasty: Surgical technique and early survivorship. J. Robot. Surg. 2020, 14, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinarelli, A.; Pesce, V.; Campagna, C.; Maccagnano, G.; Moretti, B. Painful knee prosthesis: CT scan to assess patellar angle and implant malrotation. Muscles Ligaments Tendons J. 2016, 6, 461–466. [Google Scholar] [CrossRef] [PubMed]
| Conventional (n = 61) | Robotic (n = 52) | p Value | |
|---|---|---|---|
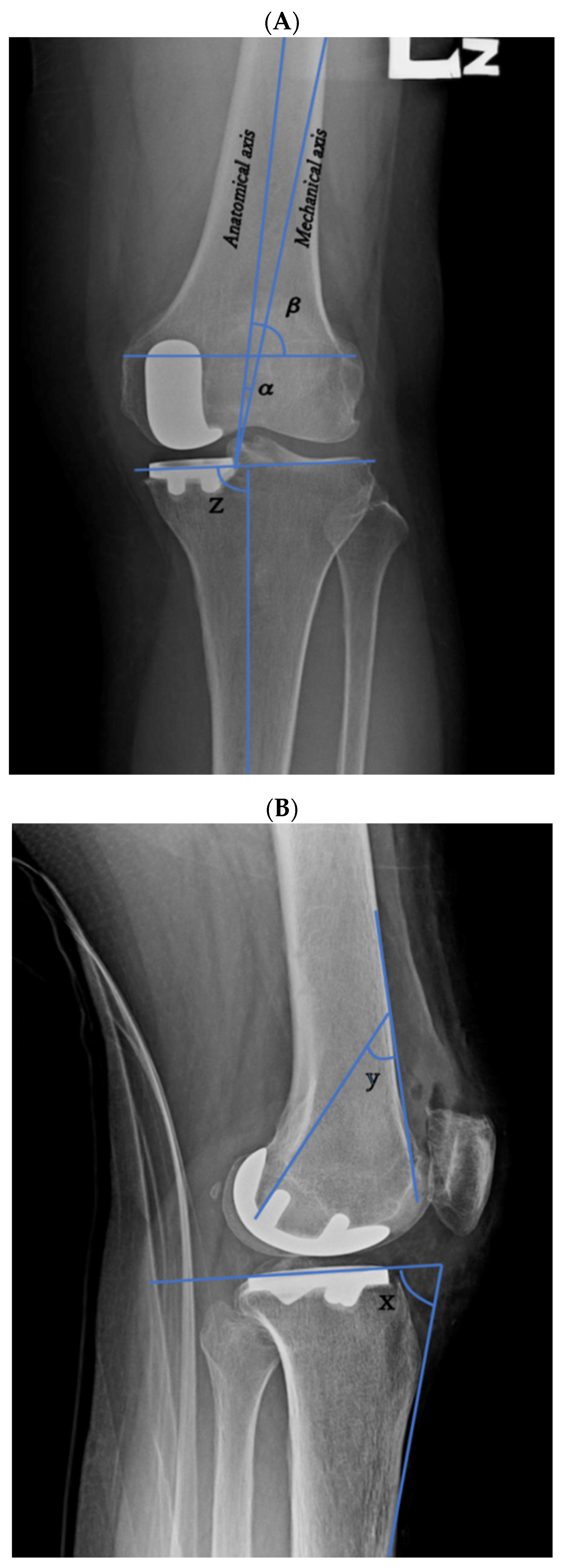
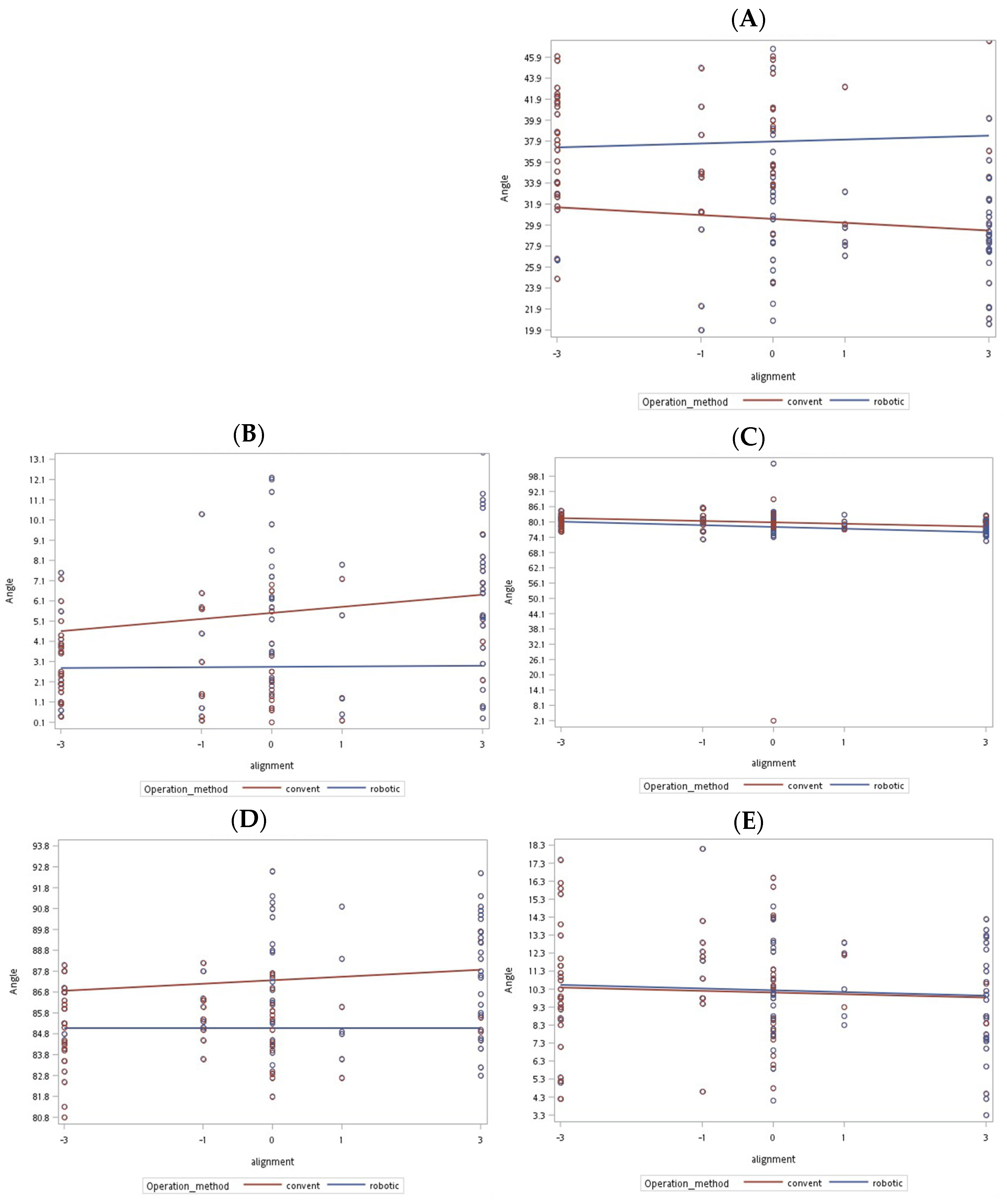
| Preanatomical axis angle (mean [SD]) | 3.26° ± 2.53° | 3.57° ± 2.49° | 0.519 |
| Postanatomical axis angle (mean [SD]) | 5.87° ± 3.5° | 2.81° ± 2.06° | <0.001 |
| Prefemoral condyle angle (mean [SD]) | 81.21° ± 2.41° | 81.12° ± 3.64° | 0.88 |
| Postfemoral condyle angle (mean [SD]) | 79.28° ± 4.19° | 79.15° ± 11.22° | 0.933 |
| Femoral implant angle (mean [SD]) | 30.04° ± 5.65° | 37.59° ± 5.4° | <0.001 |
| Pretibial axis angle (mean [SD]) | 86.39° ± 2.93° | 86.11° ± 2.56° | 0.598 |
| Posttibial axis angle (mean [SD]) | 87.57° ± 2.71° | 85.07° ± 1.76° | <0.001 |
| Pretibial slope angle (mean [SD]) | 12.48° ± 3.8° | 11.35° ± 3.19° | 0.093 |
| Posttibial slope (mean [SD]) | 10° ± 3.19° | 10.38° ± 3.32° | 0.535 |
| Tibial eminence saved (%) | 22 (36.1%) | 14 (26.9%) | 0.403 |
| Implant alignment aligned (%) | 11 (18%) | 30 (57.7%) | <0.001 |
| Overhang or normal (%) | <0.001 | ||
| Overhang | 27 (44.3%) | 2 (3.8%) | |
| Slight overhang | 5 (8.2%) | 2 (3.8%) | |
| Normal | 21 (34.4%) | 15 (28.8%) | |
| Normal, femoral implant abnormal | 1 (1.6%) | 0 | |
| Normal, gap not clear | 1 (1.6%) | 0 | |
| Slight underhang | 3 (4.9%) | 8 (15.4%) | |
| Underhang | 3 (4.9%) | 25 (48.1%) |
| Conventional (n = 61) | Robotic (n = 52) | p Value | |
|---|---|---|---|
| Age (years) (mean ± SD) | 69.36 ± 9.14 | 68.52 ± 9.78 | 0.638 |
| Sex (%) | 0.521 | ||
| Male | 9 (14.8%) | 11 (21.2%) | |
| Female | 52 (85.2%) | 41 (78.8%) | |
| ASA = 2 (%) | 50 (81.97%) | 48 (92.31%) | 0.106 |
| Operation site (%) | 0.427 | ||
| Left | 30 (49.2%) | 26 (50%) | |
| Right | 31 (50.8%) | 26 (50%) | |
| Medial or lateral = medial (%) | 61 (100%) | 52 (100%) | NA |
| Kellgren–Lawrence grade (mean [SD]) | 2.25 ± 0.43 | 2.77 ± 0.67 | <0.001 |
| Surgery Time (min) (mean [SD]) | 43.85 ± 6.08 | 60.87 ± 11.41 | <0.001 |
| Blood loss (ml) | 201.2 ± 58.44 | 257.71 ± 100.85 | <0.001 |
| Pre-OP Hb (gm/dL) (mean [SD]) | 12.96 ± 1.05 | 12.86 ± 1.23 | 0.687 |
| Post-OP Hb (gm/dL) (mean [SD]) | 11.23 ± 0.8 | NM | NA |
| Complication | 0 | 1 (1.9%) | 0.936 |
| Femoral implant size (%) (Femoral implant size: 1–6 indicates size of Stryker implant used in RUKA; A–E indicates size of Zimmer implant used in CUKA) | |||
| 1 | 0 | 9 (17.3%) | |
| 2 | 0 | 13 (25%) | |
| 3 | 0 | 19 (36.5%) | |
| 4 | 0 | 7 (13.5%) | |
| 5 | 0 | 2 (3.8%) | |
| 6 | 0 | 2 (3.8%) | |
| A | 1 (1.6%) | 0 | |
| B | 10 (16.4%) | 0 | |
| C | 32 (52.5%) | 0 | |
| D | 15 (24.6%) | 0 | |
| E | 3 (4.9%) | 0 | |
| Tibial implant size (mean [SD]) | 2.54 ± 0.74 | 2.94 ± 1.43 | 0.059 |
| CUKA (n = 38) | RUKA (n = 38) | p Value | CUKA (n = 38) | RUKA( n = 38) | p Value | CUKA (n = 38) | RUKA (n = 38) | p Value | CUKA (n = 38) | RUKA (n = 38) | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Op | 6 m Post-OP | 1 yr Post-OP | 2 yr Post-OP | |||||||||
| Duration of pain (months) (mean [SD]) | 2.65 ± 1.04 | 2.66 ± 0.73 | 0.963 | |||||||||
| NRS (mean [SD]) | 6.82 ± 1.31 | 6.79 ± 2.09 | 0.948 | 1.39 ± 1.79 | 1.76 ± 1.6 | 0.348 | 1.39 ± 1.79 | 1.82 ± 1.57 | 0.28 | 1.39 ± 1.79 | 1.82 ± 1.57 | 0.28 |
| Health score (0–100) (mean [SD]) | 65.68 ± 7.56 | 71.68 ± 10.36 | 0.006 | 77.63 ± 7.51 | 79.03 ± 10.31 | 0.502 | 77.6 ± 7.51 | 78.7 ± 10.41 | 0.589 | 77.6 ± 7.51 | 78.7 ± 10.41 | 0.589 |
| WOMAC total (mean [SD]) | 28.08 ± 8.13 | 27.64 ± 13.05 | 0.861 | 11.84 ± 12.36 | 14.44 ± 11.25 | 0.338 | 10.66 ± 11.66 | 14.64 ± 11.08 | 0.122 | 10.49 ± 11.44 | 14.67 ± 11.1 | 0.102 |
| EQ5D5L total (mean [SD]) | 11.89 ± 13.79 | 8.85 ± 2.33 | 0.178 | 5.54 ± 2.09 | 5.9 ± 1.7 | 0.4 | 5.46 ± 2.1 | 6 ± 1.65 | 0.209 | 5.46 ± 2.1 | 6.03 ± 1.63 | 0.186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Fukui, N.; Lin, Y.-K.; Lee, C.-Y.; Chou, S.-H.; Huang, T.-J.; Chen, J.-Y.; Wu, M.-H. Comparison of Robotic and Conventional Unicompartmental Knee Arthroplasty Outcomes in Patients with Osteoarthritis: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 220. https://doi.org/10.3390/jcm11010220
Wu C, Fukui N, Lin Y-K, Lee C-Y, Chou S-H, Huang T-J, Chen J-Y, Wu M-H. Comparison of Robotic and Conventional Unicompartmental Knee Arthroplasty Outcomes in Patients with Osteoarthritis: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(1):220. https://doi.org/10.3390/jcm11010220
Chicago/Turabian StyleWu, Christopher, Nobuei Fukui, Yen-Kuang Lin, Ching-Yu Lee, Shih-Hsiang Chou, Tsung-Jen Huang, Jen-Yuh Chen, and Meng-Huang Wu. 2022. "Comparison of Robotic and Conventional Unicompartmental Knee Arthroplasty Outcomes in Patients with Osteoarthritis: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 1: 220. https://doi.org/10.3390/jcm11010220
APA StyleWu, C., Fukui, N., Lin, Y.-K., Lee, C.-Y., Chou, S.-H., Huang, T.-J., Chen, J.-Y., & Wu, M.-H. (2022). Comparison of Robotic and Conventional Unicompartmental Knee Arthroplasty Outcomes in Patients with Osteoarthritis: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(1), 220. https://doi.org/10.3390/jcm11010220






