Fluorescence-Aided Identification Technique (FIT) Improves Tooth Surface Clean-Up after Debonding of Buccal and Lingual Orthodontic Appliances
Abstract
:1. Introduction
2. Materials and Methods
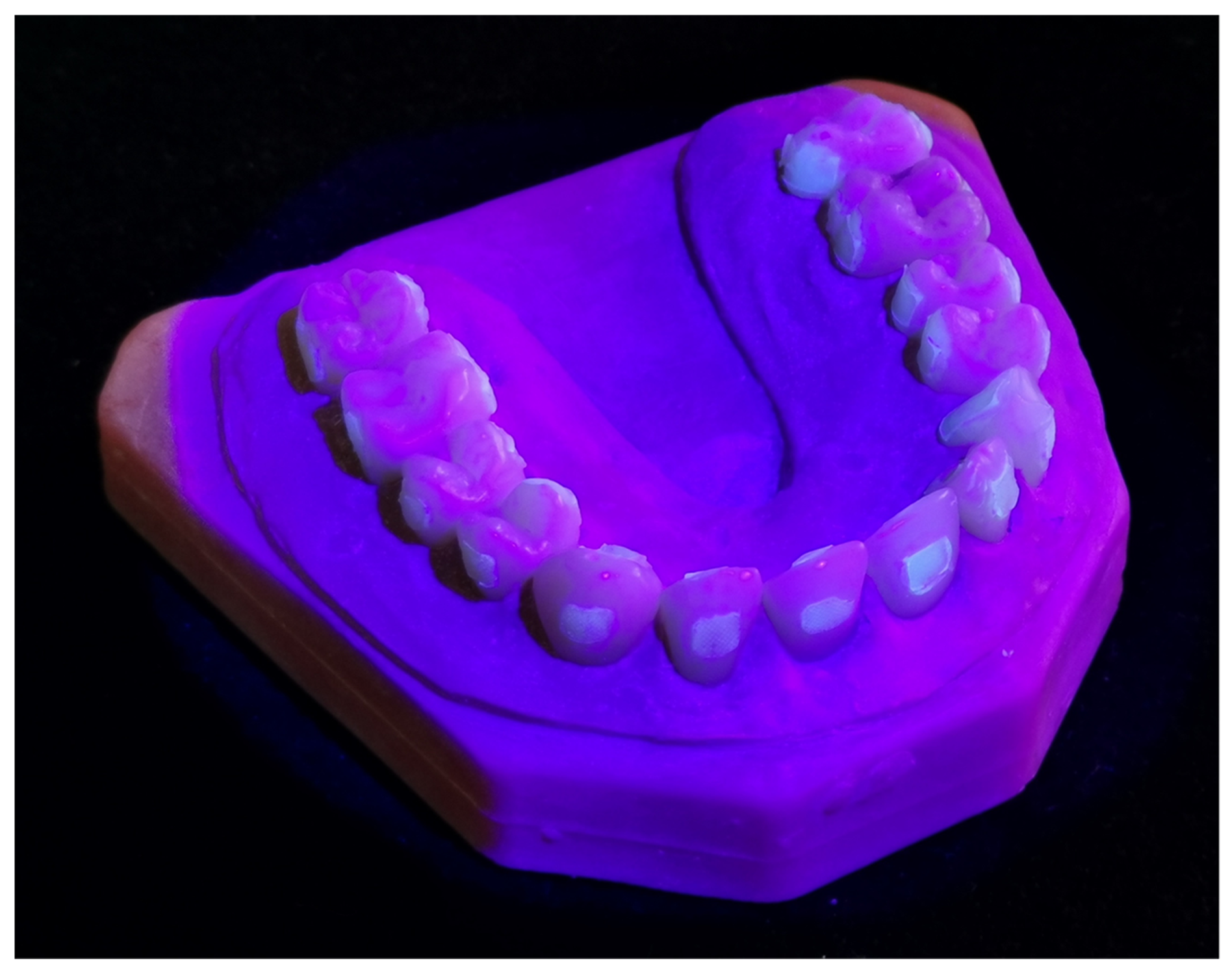
2.1. Tooth Model Preparation
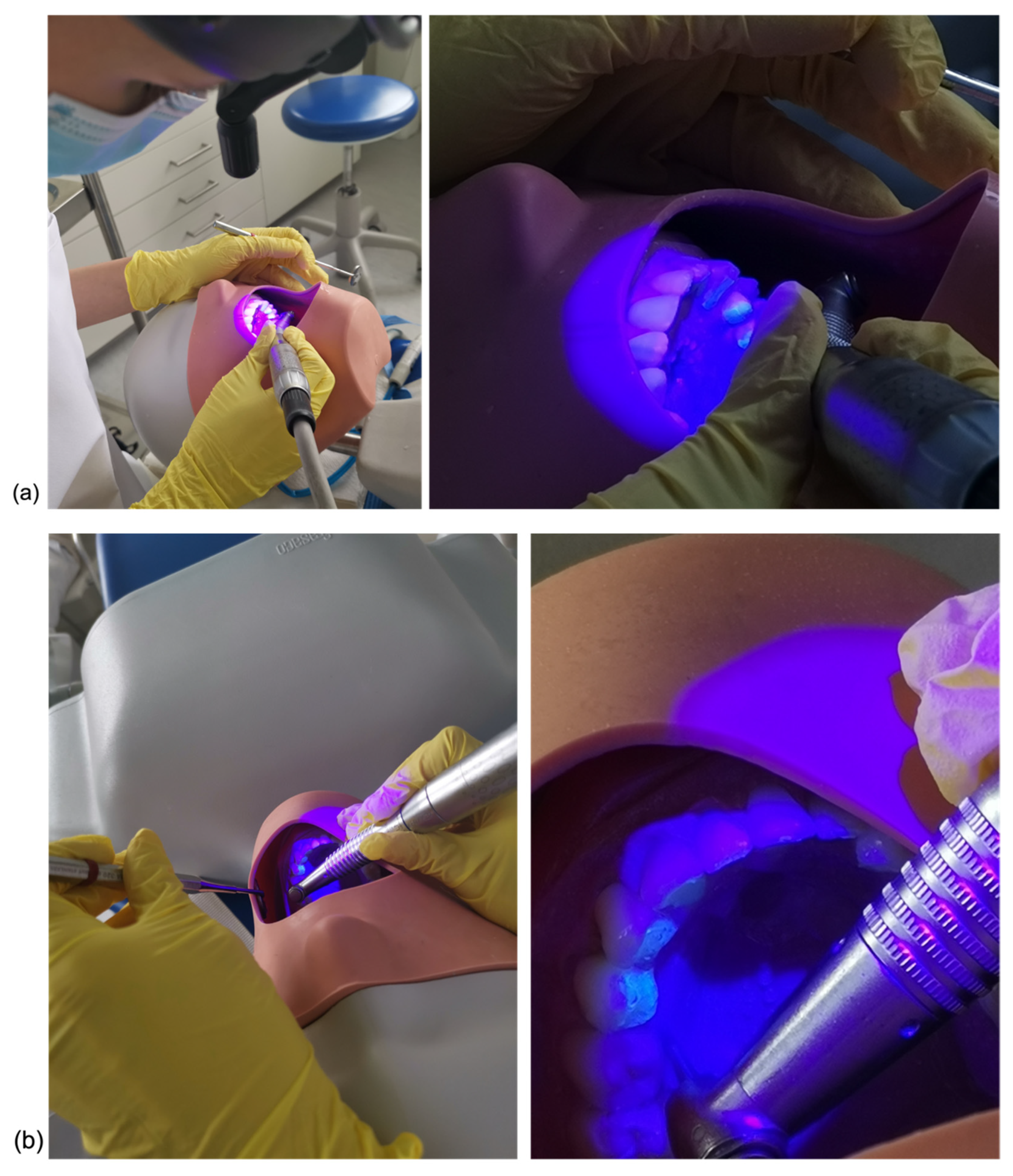
2.2. Surface Clean-Up
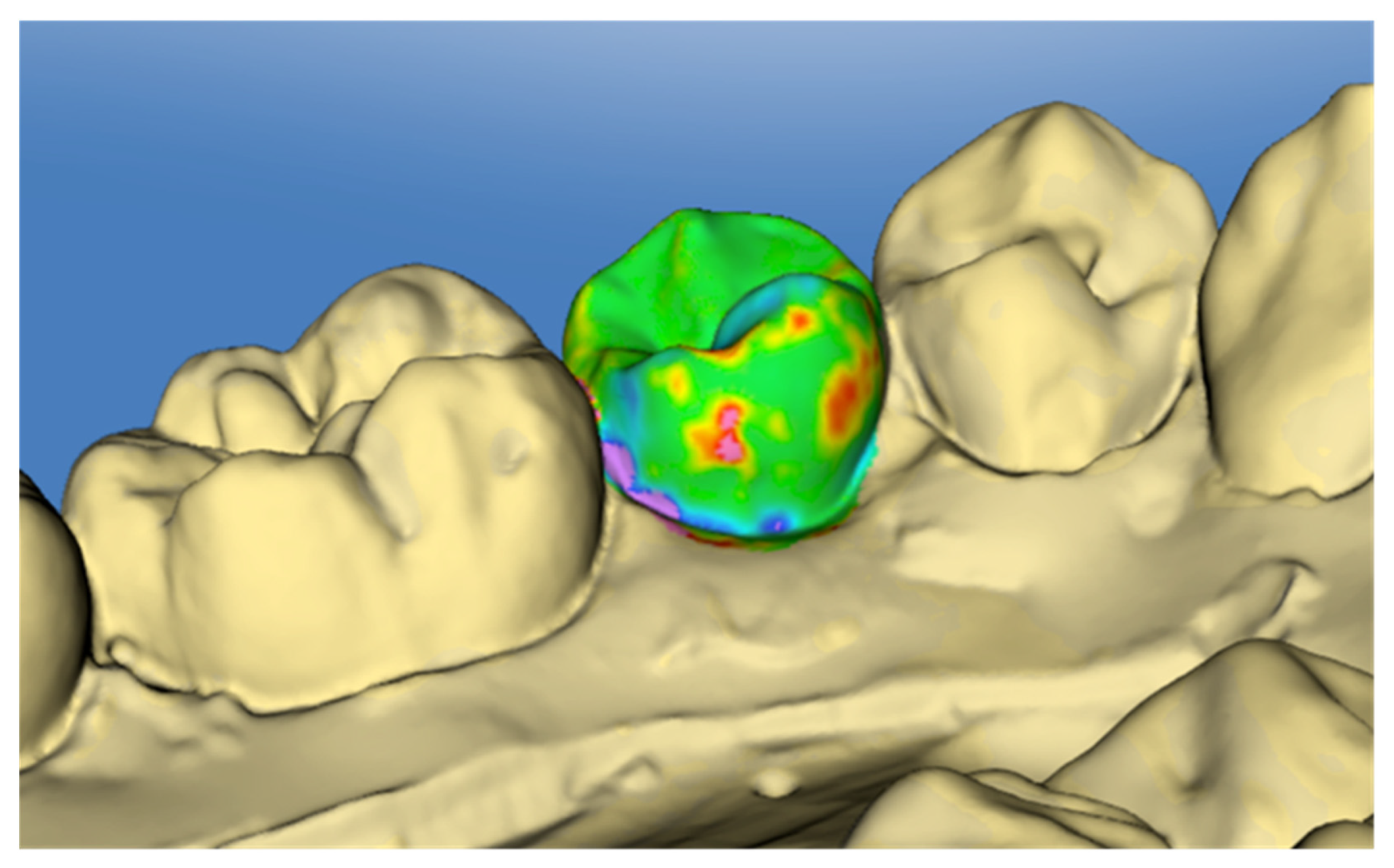
2.3. Surface Analysis
2.4. Statistical Analyses
3. Results
3.1. Error of the Method
3.2. Regression Assumption Testing
3.3. Composite Remnants
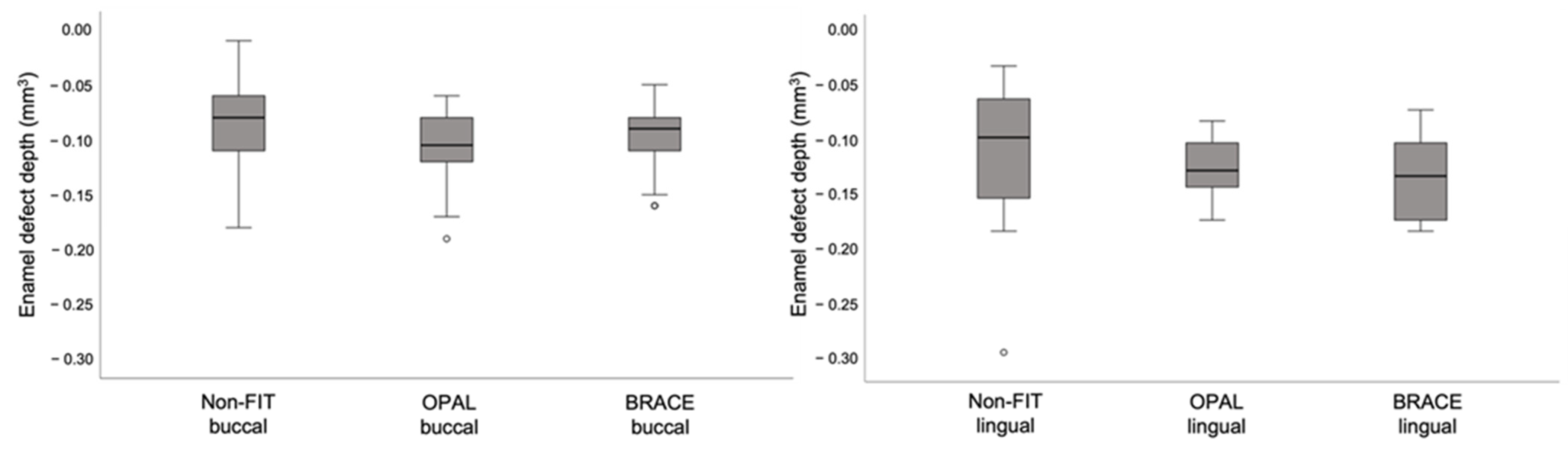
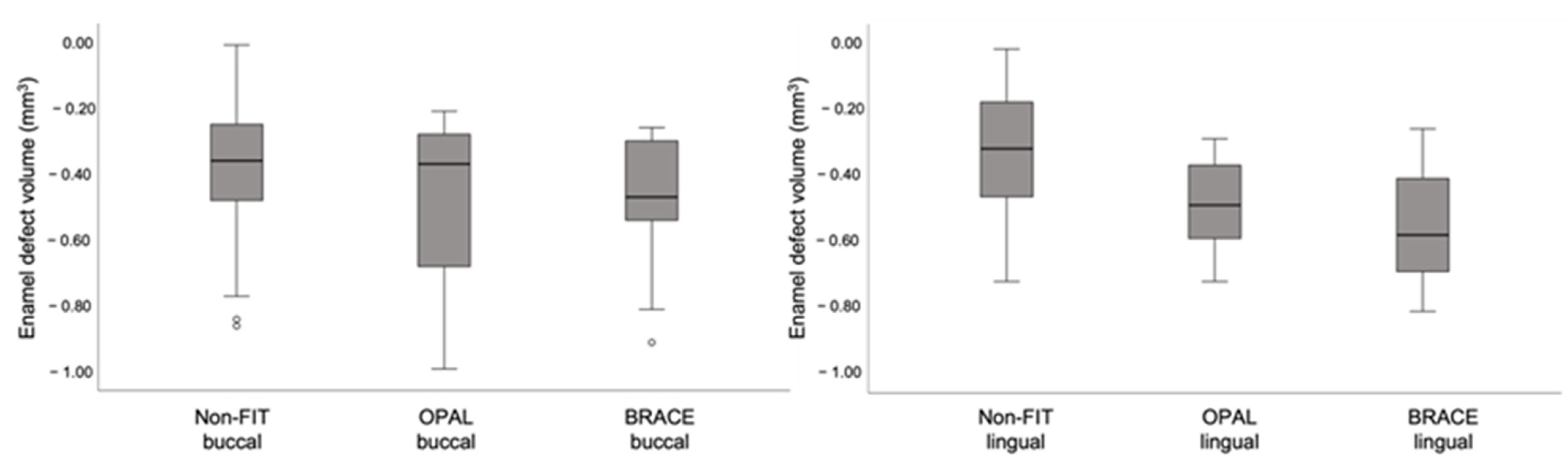
3.4. Enamel Defects
3.5. Clean-Up Time
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, P.M. Enamel Surfaces after Orthodontic Bracket Debonding. Angle Orthod. 1995, 65, 103–110. [Google Scholar] [PubMed]
- Ryf, S.; Flury, S.; Palaniappan, S.; Lussi, A.; van Meerbeek, B.; Zimmerli, B. Enamel Loss and Adhesive Remnants Following Bracket Removal and Various Clean-up Procedures in Vitro. Eur. J. Orthod. 2012, 34, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Eliades, T.; Gioka, C.; Heim, M.; Eliades, G.; Makou, M. Color Stability of Orthodontic Adhesive Resins. Angle Orthod. 2004, 74, 391–393. [Google Scholar]
- Karamouzos, A.; Athanasiou, A.E.; Papadopoulos, M.A.; Kolokithas, G. Tooth-Color Assessment after Orthodontic Treatment: A Prospective Clinical Trial. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 537.e1–537.e8. [Google Scholar] [CrossRef]
- Whittaker, D.K. Structural Variations in the Surface Zone of Human Tooth Enamel Observed by Scanning Electron Microscopy. Arch. Oral Biol. 1982, 27, 383–392. [Google Scholar] [CrossRef]
- Brosh, T.; Strouthou, S.; Sarne, O. Effects of Buccal versus Lingual Surfaces, Enamel Conditioning Procedures and Storage Duration on Brackets Debonding Characteristics. J. Dent. 2005, 33, 99–105. [Google Scholar] [CrossRef]
- Janiszewska-Olszowska, J.; Szatkiewicz, T.; Tomkowski, R.; Tandecka, K.; Grocholewicz, K. Effect of Orthodontic Debonding and Adhesive Removal on the Enamel–Current Knowledge and Future Perspectives—A Systematic Review. Med. Sci. Monit. 2014, 20, 1991–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfondrini, M.F.; Scribante, A.; Fraticelli, D.; Roncallo, S.; Gandini, P. Epidemiological Survey of Different Clinical Techniques of Orthodontic Bracket Debonding and Enamel Polishing. J. Orthod. Sci. 2015, 4, 123–127. [Google Scholar] [CrossRef]
- Eichenberger, M.; Iliadi, A.; Koletsi, D.; Eliades, G.; Verna, C.; Eliades, T. Enamel Surface Roughness after Lingual Bracket Debonding: An In Vitro Study. Materials 2019, 12, 4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meller, C.; Connert, T.; Löst, C.; ElAyouti, A. Reliability of a Fluorescence-Aided Identification Technique (FIT) for Detecting Tooth-Colored Restorations: An Ex Vivo Comparative Study. Clin. Oral Investig. 2017, 21, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Stadler, O.; Dettwiler, C.; Meller, C.; Dalstra, M.; Verna, C.; Connert, T. Evaluation of a Fluorescence-Aided Identification Technique (FIT) to Assist Clean-up after Orthodontic Bracket Debonding. Angle Orthod. 2019, 89, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dettwiler, C.; Meller, C.; Eggmann, F.; Saccardin, F.; Kühl, S.; Filippi, A.; Krastl, G.; Weiger, R.; Connert, T. Evaluation of a Fluorescence-aided Identification Technique (FIT) for Removal of Composite Bonded Trauma Splints. Dent. Traumatol. 2018, 34, 353–359. [Google Scholar] [CrossRef]
- Dettwiler, C.; Eggmann, F.; Matthisson, L.; Meller, C.; Weiger, R.; Connert, T. Fluorescence-aided Composite Removal in Directly Restored Permanent Posterior Teeth. Oper. Dent. 2020, 45, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Meller, C.; Klein, C. Fluorescence of Composite Resins: A Comparison among Properties of Commercial Shades. Dent. Mater. J. 2015, 34, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farronato, M.; Farronato, D.; Inchingolo, F.; Grassi, L.; Lanteri, V.; Maspero, C. Evaluation of Dental Surface after De-Bonding Orthodontic Bracket Bonded with a Novel Fluorescent Composite: In Vitro Comparative Study. Appl. Sci. 2021, 11, 6354. [Google Scholar] [CrossRef]
- Ribeiro, A.A.; Almeida, L.F.; Martins, L.P.; Martins, R.P. Assessing Adhesive Remnant Removal and Enamel Damage with Ultraviolet Light: An in-Vitro Study. Am. J. Orthod. Dentofac. 2017, 151, 292–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schott, T.C.; Meller, C. A New Fluorescence-Aided Identification Technique (FIT)for Optimal Removal of Resin-Based Bracket Bonding Remnants after Orthodontic Debracketing. Quintessence Int. 2018, 49, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Bush, P.J.; Warunek, S., Jr.; Al-Jewair, T. An in Vitro Comparison of Ultraviolet versus White Light in the Detection of Adhesive Remnants during Orthodontic Debonding. Angle Orthod. 2019, 89, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Nettey-Marbell, A.; Cook, A.; Pimenta, L.A.F.; Leonard, R.; Ritter, A.V. Using Extracted Teeth for Research The Effect of Storage Medium and Sterilization on Dentin Bond Strengths. J. Am. Dent. Assoc. 2007, 138, 1599–1603. [Google Scholar] [CrossRef]
- Mobarak, E.H.; El-Badrawy, W.; Pashley, D.H.; Jamjoom, H. Effect of Pretest Storage Conditions of Extracted Teeth on Their Dentin Bond Strengths. J. Prosthet. Dent. 2010, 104, 92–97. [Google Scholar] [CrossRef]
- Mehl, A.; Gloger, W.; Kunzelmann, K.-H.; Hickel, R. A New Optical 3-D Device for the Detection of Wear. J. Dent. Res. 1997, 76, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kanavakis, G. Comparison of Shear Bond Strength and Bonding Time of a Novel Flash-Free Bonding System. Angle Orthod. 2016, 86, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kanavakis, G.; Finkelman, M.D.; Lee, M. Microleakage under Ceramic Flash-Free Orthodontic Brackets after Thermal Cycling. Angle Orthod. 2016, 86, 905–908. [Google Scholar] [CrossRef] [Green Version]
- Pompei-Reynolds, R.C.; Kanavakis, G. Interlot Variations of Transition Temperature Range and Force Delivery in Copper-Nickel-Titanium Orthodontic Wires. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Nicolay, O.F.; Cangialosi, T.J. Pseudoelasticity and Thermoelasticity of Nickel-Titanium Alloys: A Clinically Oriented Review. Part I: Temperature Transitional Ranges. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, G.; Santoro, M.; Nicolay, O.F.; Cangialosi, T.J. Pseudoelasticity and Thermoelasticity of Nickel-Titanium Alloys: A Clinically Oriented Review. Part II: Deactivation Forces. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Gil, F.J.; Gil, F.J.; Planell, J.A.; Planell, J.A. Effect of Copper Addition on the Superelastic Behavior of Ni-Ti Shape Memory Alloys for Orthodontic Applications. J. Biomed. Mater. Res. 1999, 48, 682–688. [Google Scholar] [CrossRef]
- Güth, J.-F.; Keul, C.; Stimmelmayr, M.; Beuer, F.; Edelhoff, D. Accuracy of Digital Models Obtained by Direct and Indirect Data Capturing. Clin. Oral Investig. 2012, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Ender, A.; Zimmermann, M.; Attin, T.; Mehl, A. In Vivo Precision of Conventional and Digital Methods for Obtaining Quadrant Dental Impressions. Clin. Oral Investig. 2016, 20, 1495–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.; Koller, C.; Rumetsch, M.; Ender, A.; Mehl, A. Precision of Guided Scanning Procedures for Full-Arch Digital Impressions in Vivo Präzision von Guided-Scanning-Verfahren Bei Digitalen Gesamtkieferabformungen in Vivo. J. Orofac. Orthop. 2017, 78, 466–471. [Google Scholar] [CrossRef]
- Lee, J.J.; Jeong, I.D.; Park, J.Y.; Jeon, J.H.; Kim, J.H.; Kim, W.C. Accuracy of Single-Abutment Digital Cast Obtained Using Intraoral and Cast Scanners. J. Prosthet. Dent. 2017, 117, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Vasilakos, G.; Schilling, R.; Halazonetis, D.; Gkantidis, N. Assessment of Different Techniques for 3D Superimposition of Serial Digital Maxillary Dental Casts on Palatal Structures. Sci. Rep. 2017, 7, 5838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, K. New Orthodontic Treatment with Lingual Bracket Mushroom Arch Wire Appliance. Am. J. Orthod. 1979, 76, 657–675. [Google Scholar] [CrossRef]
- Vieira, A.C.; Pinto, R.A.; Chevitarese, O.; Almeida, M.A. Polishing after Debracketing: Its Influence upon Enamel Surface. J. Clin. Pediatric Dent. 1993, 18, 7–11. [Google Scholar]
- Hong, Y.H.; Lew, K.K.K. Quantitative and Qualitative Assessment of Enamel Surface Following Five Composite Removal Methods after Bracket Debonding. Eur. J. Orthod. 1995, 17, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Polychronis, G.; Christou, P.; Mavragani, M.; Halazonetis, D.J. Geometric Morphometric 3D Shape Analysis and Covariation of Human Mandibular and Maxillary First Molars. Am. J. Phys. Anthropol. 2013, 152, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Schuler, F.S.; van Waes, H. SEM-Evaluation of Enamel Surfaces after Removal of Fixed Orthodontic Appliances. Am. J. Dent. 2003, 16, 390–394. [Google Scholar] [PubMed]
- Fjeld, M.; Øgaard, B. Scanning Electron Microscopic Evaluation of Enamel Surfaces Exposed to 3 Orthodontic Bonding Systems. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Toledano, M.; Garcia-Godoy, F. Bracket Bonding with 15- or 60-Second Etching and Adhesive Remaining on Enamel after Debonding. Angle Orthod. 1999, 69, 45–48. [Google Scholar]
- Osorio, R.; Toledano, M.; García-Godoy, F. Enamel Surface Morphology after Bracket Debonding. ASDC J. Dent. Child. 1998, 65, 313–317. [Google Scholar] [PubMed]
- Oliver, R.G.; Griffiths, J. Different Techniques of Residual Composite Removal Following Debonding—Time Taken and Surface Enamel Appearance. Br. J. Orthod. 2016, 19, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Baumann, D.F.; Brauchli, L.; van Waes, H. The influence of dental loupes on the quality of adhesive removal in orthodontic debonding. J. Orofac. Orthop. 2011, 72, 125–132. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, M.; Bernardi, S.; Di Lauro, D.; Marzo, G.; Macchiarelli, G.; Capogreco, M. Debonding and Clean-Up in Orthodontics: Evaluation of Different Techniques and Micro-Morphological Aspects of the Enamel Surface. Dent. J. 2020, 8, 58. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engeler, O.; Stadler, O.; Horn, S.; Dettwiler, C.; Connert, T.; Verna, C.; Kanavakis, G. Fluorescence-Aided Identification Technique (FIT) Improves Tooth Surface Clean-Up after Debonding of Buccal and Lingual Orthodontic Appliances. J. Clin. Med. 2022, 11, 213. https://doi.org/10.3390/jcm11010213
Engeler O, Stadler O, Horn S, Dettwiler C, Connert T, Verna C, Kanavakis G. Fluorescence-Aided Identification Technique (FIT) Improves Tooth Surface Clean-Up after Debonding of Buccal and Lingual Orthodontic Appliances. Journal of Clinical Medicine. 2022; 11(1):213. https://doi.org/10.3390/jcm11010213
Chicago/Turabian StyleEngeler, Olivia, Oliver Stadler, Simone Horn, Christian Dettwiler, Thomas Connert, Carlalberta Verna, and Georgios Kanavakis. 2022. "Fluorescence-Aided Identification Technique (FIT) Improves Tooth Surface Clean-Up after Debonding of Buccal and Lingual Orthodontic Appliances" Journal of Clinical Medicine 11, no. 1: 213. https://doi.org/10.3390/jcm11010213
APA StyleEngeler, O., Stadler, O., Horn, S., Dettwiler, C., Connert, T., Verna, C., & Kanavakis, G. (2022). Fluorescence-Aided Identification Technique (FIT) Improves Tooth Surface Clean-Up after Debonding of Buccal and Lingual Orthodontic Appliances. Journal of Clinical Medicine, 11(1), 213. https://doi.org/10.3390/jcm11010213








