The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa
Abstract
1. Introduction
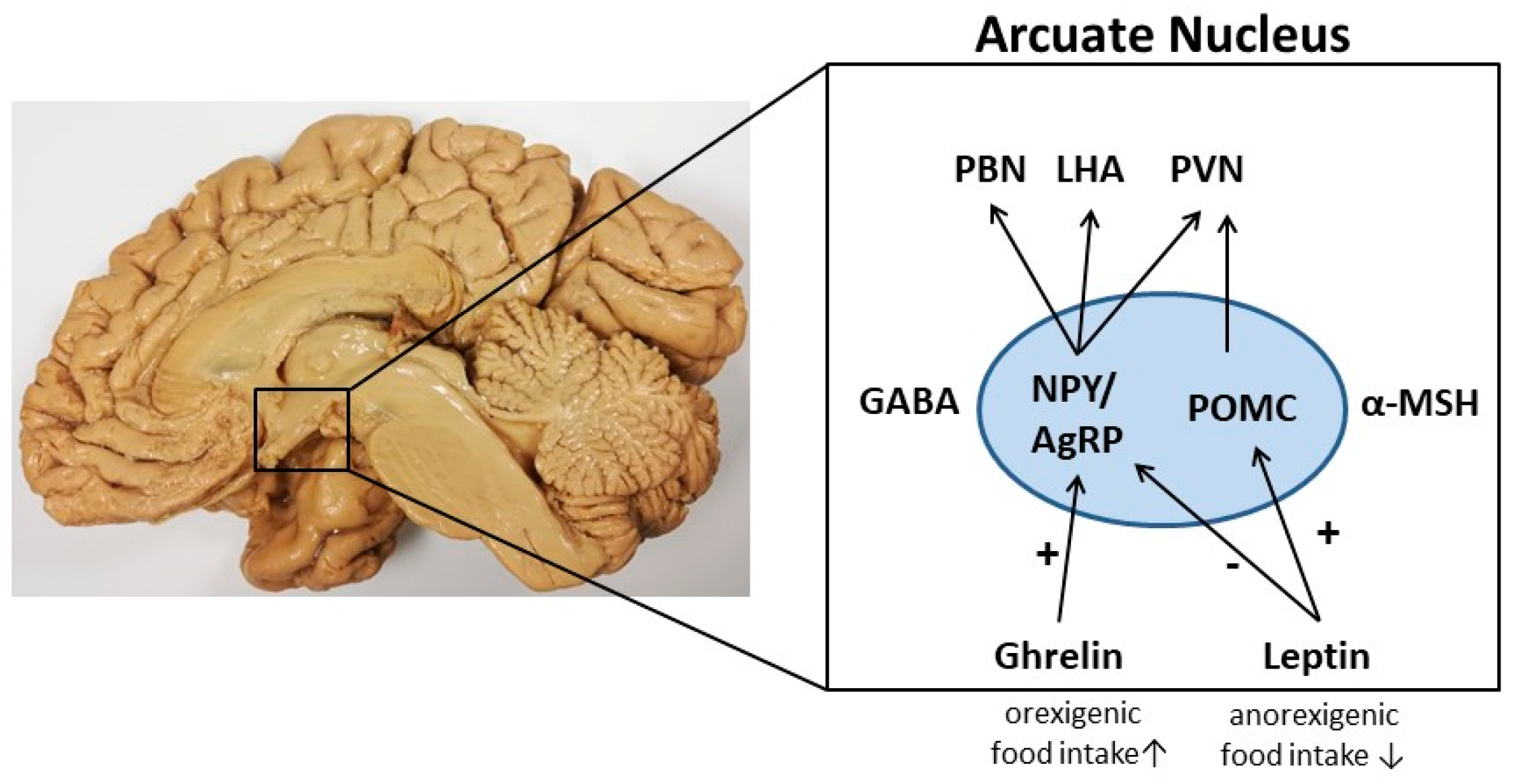
2. Neuronal Control of Appetite in the Hypothalamus
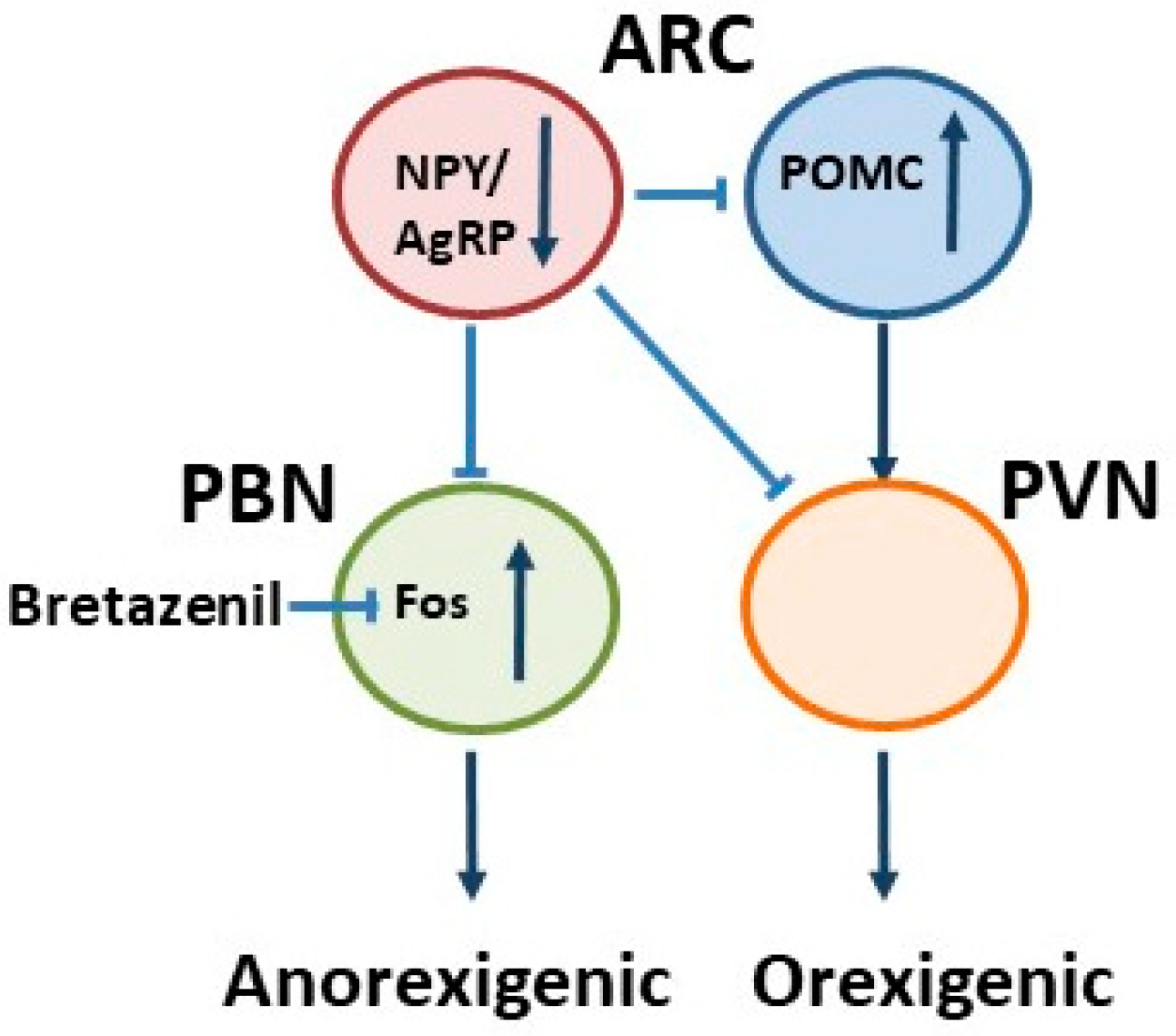
3. Orexigenic Neuropeptide Y (NPY)/Agouti-Related Peptide (AgRP) Neurons
4. Anorexigenic Pro-Opiomelanocortin (POMC) Neurons
5. Astrocyte Pathology
6. Disturbed Gliotransmission as a Regulator of Feeding Behavior
7. Astrocytes in Behavioral Disorders
8. Astrocytes as Targets of Peripheral Food Intake Signals
9. Microglia Pathology
10. Neuroinflammation in Eating Disorders and the Role of Microglia
11. Anorexia Nervosa (AN)
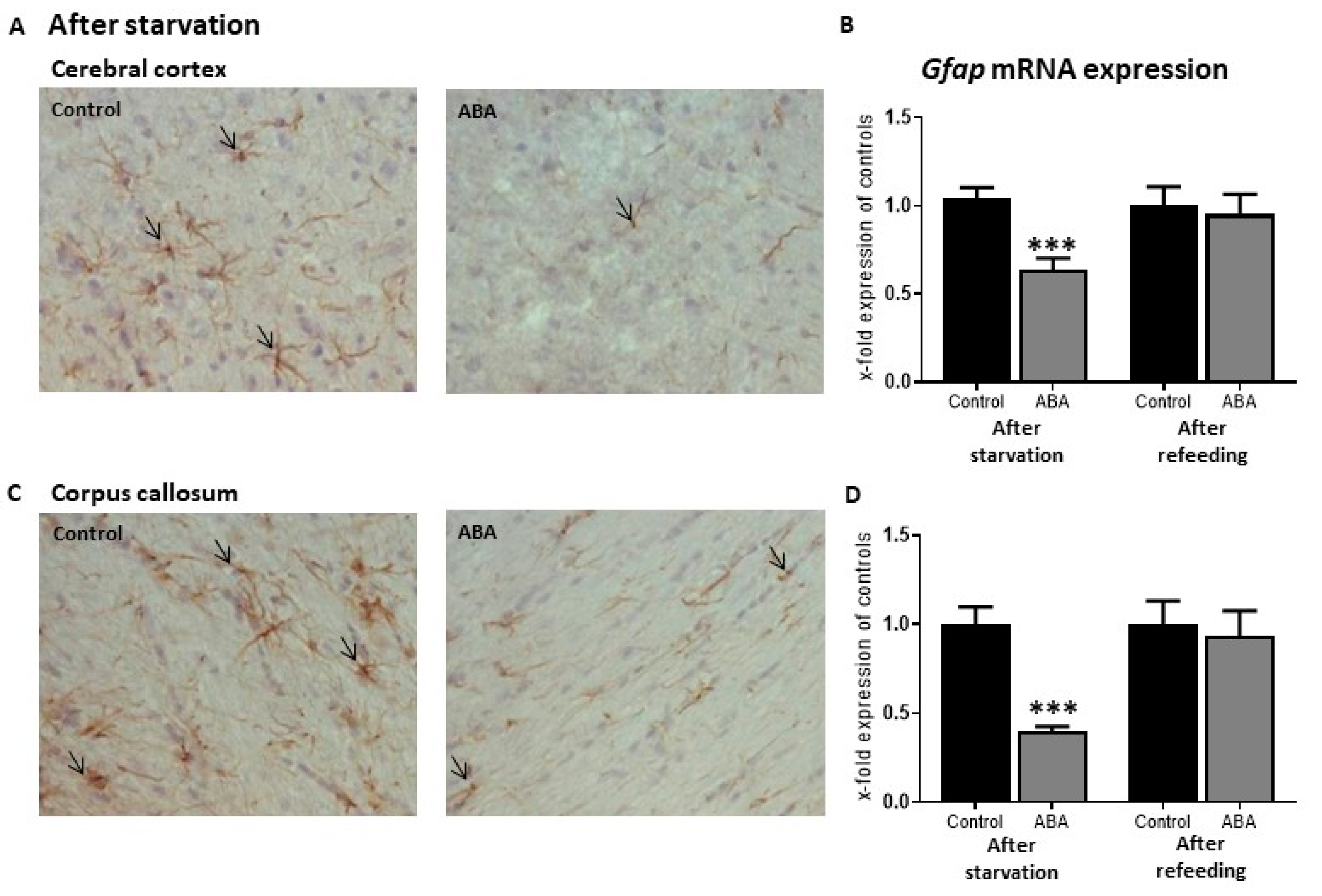
12. Glia Cell Pathology in AN
13. Pathways of Food Intake in ABA
14. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef]
- Risold, P.Y.; Swanson, L.W. Structural evidence for functional domains in the rat hippocampus. Science 1996, 272, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Carus-Cadavieco, M.; Gorbati, M.; Ye, L.; Bender, F.; van der Veldt, S.; Kosse, C.; Börgers, C.; Lee, S.Y.; Ramakrishnan, C.; Hu, Y.; et al. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature 2017, 542, 232–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin function and regulation. Compr. Physiol. 2011, 8, 351–369. [Google Scholar]
- Zhang, F.; Chen, Y.; Heiman, M.; Dimarchi, R. Leptin: Structure, function and biology. Vitam. Horm. 2005, 71, 345–372. [Google Scholar] [CrossRef]
- Sohn, J.-W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Gil-Campos, M.; Aguilera, C.M.; Canete, R.; Gil, A. Ghrelin: A hormone regulating food intake and energy homeostasis. Br. J. Nutr. 2006, 96, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.A.; Cone, R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 2005, 146, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Zimmer, M.R.; Bober, J.; Horvath, T.L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 2015, 160, 1222–1232. [Google Scholar] [CrossRef]
- Betley, J.N.; Xu, S.; Cao, Z.F.H.; Gong, R.; Magnus, C.J.; Yu, Y.; Sternson, S.M. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 2015, 521, 180–185. [Google Scholar] [CrossRef]
- Miletta, M.C.; Iyilikci, O.; Shanabrough, M.; Sestan-Pesa, M.; Cammisa, A.; Zeiss, C.J.; Dietrich, M.O.; Horvath, T.L. AgRP neurons control compulsive exercise and survival in an activity-based anorexia model. Nat. Metab. 2020, 2, 1204–1211. [Google Scholar] [CrossRef]
- Qian, S.; Chen, H.; Weingarth, D.; Trumbauer, M.E.; Novi, D.E.; Guan, X.; Yu, H.; Shen, Z.; Feng, Y.; Frazier, E.; et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 2002, 22, 5027–5035. [Google Scholar] [CrossRef]
- Suyama, S.; Yada, T. New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 2018, 68, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Howell, M.P.; Palmiter, R.D. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J. Neurosci. 2008, 28, 9218–9226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Boyle, M.P.; Palmiter, R.D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 2009, 137, 1225–1234. [Google Scholar] [CrossRef]
- Tong, Q.; Ye, C.P.; Jones, J.E.; Elmquist, J.K.; Lowell, B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008, 11, 998–1000. [Google Scholar] [CrossRef]
- Aoki, C.; Sabaliauskas, N.; Chowdhury, T.; Min, J.Y.; Colacino, A.R.; Laurino, K.; Barbarich-Marsteller, N.C. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse 2012, 66, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.C.; Kuo, T.W.; Knight, Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 2015, 160, 829–841. [Google Scholar] [CrossRef]
- Zemel, M.B.; Shi, H. Pro-opiomelanocortin (POMC) deficiency and peripheral melanocortins in obesity. Nutr. Rev. 2000, 58, 177–180. [Google Scholar] [CrossRef]
- Garcia-Caceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14. [Google Scholar] [CrossRef]
- Barres, B.A. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef]
- Fields, R.D.; Woo, D.H.; Basser, P.J. Glial regulation of the neuronal connectome through local and long-distant communication. Neuron 2015, 86, 374–386. [Google Scholar] [CrossRef]
- Hostenbach, S.; Cambron, M.; D’haeseleer, M.; Kooijman, R.; De Keyser, J. Astrocyte loss and astrogliosis in neuroinflammatory disorders. Neurosci. Lett. 2014, 565, 39–41. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Seifert, G.; Schilling, K.; Steinhäuser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Levin, B.E.; Magnan, C.; Dunn-Meynell, A.; Le Foll, C. Metabolic sensing and the brain: Who, what, where, and how? Endocrinology 2011, 152, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Leloup, C.; Allard, C.; Carneiro, L.; Fioramonti, X.; Collins, S.; Pénicaud, L. Glucose and hypothalamic astrocytes: More than a fueling role? Neuroscience 2016, 323, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, L.; Labouèbe, G.; Thorens, B. Brain glucose sensing in homeostatic and hedonic regulation. Trends Endocrinol. Metab. 2015, 26, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.P.; Hirasawa, M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: Implications for brain energetics during arousal. J. Neurosci. 2010, 30, 8061–8070. [Google Scholar] [CrossRef] [PubMed]
- Frintrop, L.; Liesbrock, J.; Paulukat, L.; Johann, S.; Kas, M.J.; Tolba, R.; Heussen, N.; Neulen, J.; Konrad, K.; Herpertz-Dahlmann, B.; et al. Reduced astrocyte density underlying brain volume reduction in activity-based anorexia rats. World J. Biol. Psychiatry 2018, 19, 225–235. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Leunissen, C.; Kempermann, J.; Etdoger, S.; Kas, M.J.; Tolba, R.; Heussen, N.; Neulen, J.; et al. The reduction of astrocytes and brain volume loss in anorexia nervosa-the impact of starvation and refeeding in a rodent model. Transl. Psychiatry 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-mediated astrocyte-neuron signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef]
- Parpura, V.; Haydon, P.G. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 8629–8634. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, P.; Volterra, A. A neuron-glia signalling network in the active brain. Curr. Opin. Neurobiol. 2001, 11, 387–394. [Google Scholar] [CrossRef]
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond Black-and-White. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 14–25. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, B.E.; Berglund, K.; Oh, S.J.; Park, H.; Shin, H.S.; Augustine, G.J.; Lee, C.J. Channel-mediated tonic GABA release from glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef]
- Pannasch, U.; Freche, D.; Dallérac, G.; Ghézali, G.; Escartin, C.; Ezan, P.; Cohen-Salmon, M.; Benchenane, K.; Abudara, V.; Dufour, A.; et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat. Neurosci. 2014, 17, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Storck, T.; Schulte, S.; Hofmann, K.; Stoffel, W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. USA 1992, 89, 10955–10959. [Google Scholar] [CrossRef]
- Pines, G.; Danbolt, N.C.; Bjørås, M.; Zhang, Y.; Bendahan, A.; Eide, L.; Koepsell, H.; Storm-Mathisen, J.; Seeberg, E.; Kanner, B.I. Cloning and expression of a rat brain L-glutamate transporter. Nature 1992, 360, 464–467. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef]
- D’Ascenzo, M.; Fellin, T.; Terunuma, M.; Revilla-Sanchez, R.; Meaney, D.F.; Auberson, Y.P.; Moss, S.J.; Haydon, P.G. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2007, 104, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Bisaga, A.; Danysz, W.; Foltin, R.W. Antagonism of glutamatergic NMDA and mGluR5 receptors decreases consumption of food in baboon model of binge-eating disorder. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2008, 18, 794–802. [Google Scholar] [CrossRef]
- Bradbury, M.J.; Campbell, U.; Giracello, D.; Chapman, D.; King, C.; Tehrani, L.; Cosford, N.D.; Anderson, J.; Varney, M.A.; Strack, A.M. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J. Pharmacol. Exp. Ther. 2005, 313, 395–402. [Google Scholar] [CrossRef]
- Ballester-Rosado, C.J.; Albright, M.J.; Wu, C.S.; Liao, C.C.; Zhu, J.; Xu, J.; Lee, L.J.; Lu, H.C. mGluR5 in cortical excitatory neurons exerts both cell-autonomous and -nonautonomous influences on cortical somatosensory circuit formation. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 16896–16909. [Google Scholar] [CrossRef]
- Mottarlini, F.; Bottan, G.; Tarenzi, B.; Colciago, A.; Fumagalli, F.; Caffino, L. Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats. Nutrients 2020, 12, 3661. [Google Scholar] [CrossRef]
- Bilash, O.M.; Actor-Engel, H.S.; Sherpa, A.D.; Chen, Y.W.; Aoki, C. Suppression of food restriction-evoked hyperactivity in activity-based anorexia animal model through glutamate transporters GLT-1 at excitatory synapses in the hippocampus. Synapse 2021, 75, e22197. [Google Scholar] [CrossRef]
- Sardinha, V.M.; Guerra-Gomes, S.; Caetano, I.; Tavares, G.; Martins, M.; Reis, J.S.; Correia, J.S.; Teixeira-Castro, A.; Pinto, L.; Sousa, N.; et al. Astrocytic signaling supports hippocampal-prefrontal theta synchronization and cognitive function. Glia 2017, 65, 1944–1960. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martin, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef]
- Banasr, M.; Duman, R.S. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatry 2008, 64, 863–870. [Google Scholar] [CrossRef]
- Leng, L.; Zhuang, K.; Liu, Z.; Huang, C.; Gao, Y.; Chen, G.; Lin, H.; Hu, Y.; Wu, D.; Shi, M.; et al. Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation. Neuron 2018, 100, 551–563.e7. [Google Scholar] [CrossRef]
- Rajkowska, G.; Stockmeier, C.A. Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef]
- Hines, D.J.; Schmitt, L.I.; Hines, R.M.; Moss, S.J.; Haydon, P.G. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatry 2013, 3, e212. [Google Scholar] [CrossRef]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Huang, W.; Jaspan, J.B.; Maness, L.M. Leptin enters the brain by a saturable system independent of insulin. Peptides 1996, 17, 305–311. [Google Scholar] [CrossRef]
- Kim, J.G.; Suyama, S.; Koch, M.; Jin, S.; Argente-Arizon, P.; Argente, J.; Liu, Z.W.; Zimmer, M.R.; Jeong, J.K.; Szigeti-Buck, K.; et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 2014, 17, 908–910. [Google Scholar] [CrossRef]
- Wang, Y.; Hsuchou, H.; He, Y.; Kastin, A.J.; Pan, W. Role of Astrocytes in Leptin Signaling. J. Mol. Neurosci. 2015, 56, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, S.; Li, Z.; Li, S.; Xia, M.; Verkhratsky, A.; Li, B. Leptin Increases Expression of 5-HT(2B) Receptors in Astrocytes Thus Enhancing Action of Fluoxetine on the Depressive Behavior Induced by Sleep Deprivation. Front. Psychiatry 2018, 9, 734. [Google Scholar] [CrossRef]
- Naranjo, V.; Contreras, A.; Merino, B.; Plaza, A.; Lorenzo, M.P.; García-Cáceres, C.; García, A.; Chowen, J.A.; Ruiz-Gayo, M.; Del Olmo, N.; et al. Specific Deletion of the Astrocyte Leptin Receptor Induces Changes in Hippocampus Glutamate Metabolism, Synaptic Transmission and Plasticity. Neuroscience 2020, 447, 182–190. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. The Effects of Leptin on Glial Cells in Neurological Diseases. Front. Neurosci. 2019, 13, 828. [Google Scholar] [CrossRef]
- Frago, L.M.; Chowen, J.A. Involvement of Astrocytes in Mediating the Central Effects of Ghrelin. Int. J. Mol. Sci. 2017, 18, 536. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Y.; Yang, Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep. 2015, 11, 798–807. [Google Scholar] [CrossRef]
- García-Cáceres, C.; Quarta, C.; Varela, L.; Gao, Y.; Gruber, T.; Legutko, B.; Jastroch, M.; Johansson, P.; Ninkovic, J.; Yi, C.-X. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 2016, 166, 867–880. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Holmes, F.E.; Beall, C.; Pickering, A.E.; Ellacott, K.L. Regulation of food intake by astrocytes in the brainstem dorsal vagal complex. Glia 2020, 68, 1241–1254. [Google Scholar] [CrossRef]
- Schipper, H.M. Gomori-positive astrocytes: Biological properties and implications for neurologic and neuroendocrine disorders. Glia 1991, 4, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Young, J.K.; McKenzie, J.C. GLUT2 immunoreactivity in Gomori-positive astrocytes of the hypothalamus. J. Histochem. Cytochem. 2004, 52, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Jung, S.; Priller, J. Microglia biology: One century of evolving concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M.È. Microglia across the lifespan: From origin to function in brain development, plasticity and cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic mediators of synapse development and plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol. 2014, 49, 1422–1434. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-astrocyte crosstalk: An intimate molecular conversation. Neuroscience 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017, 26, 185–197.e3. [Google Scholar] [CrossRef]
- André, C.; Guzman-Quevedo, O.; Rey, C.; Rémus-Borel, J.; Clark, S.; Castellanos-Jankiewicz, A.; Ladeveze, E.; Leste-Lasserre, T.; Nadjar, A.; Abrous, D.N. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes 2017, 66, 908–919. [Google Scholar] [CrossRef]
- Gao, Y.; Ottaway, N.; Schriever, S.C.; Legutko, B.; García-Cáceres, C.; de la Fuente, E.; Mergen, C.; Bour, S.; Thaler, J.P.; Seeley, R.J. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 2014, 62, 17–25. [Google Scholar] [CrossRef]
- Jin, S.; Kim, J.G.; Park, J.W.; Koch, M.; Horvath, T.L.; Lee, B.J. Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis. Sci. Rep. 2016, 6, 1–13. [Google Scholar]
- Gao, Y.; Vidal-Itriago, A.; Milanova, I.; Korpel, N.L.; Kalsbeek, M.J.; Tom, R.Z.; Kalsbeek, A.; Hofmann, S.M.; Yi, C.-X. Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Mol. Metab. 2018, 7, 155–160. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B. Adolescent eating disorders: Update on definitions, symptomatology, epidemiology, and comorbidity. Child Adolesc. Psychiatr. Clin. 2015, 24, 177–196. [Google Scholar] [CrossRef]
- Klump, K.L. Puberty as a critical risk period for eating disorders: A review of human and animal studies. Horm. Behav. 2013, 64, 399–410. [Google Scholar] [CrossRef]
- Gonzalez, A.; Clarke, S.; Kohn, M. Eating disorders in adolescents. Aust. Fam. Physician 2007, 36, 614. [Google Scholar]
- Seitz, J.; Bühren, K.; von Polier, G.G.; Heussen, N.; Herpertz-Dahlmann, B.; Konrad, K. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. Z. Kinder Jugendpsychiatrie Psychother. 2014, 42, 7–18. [Google Scholar] [CrossRef]
- McCormick, L.M.; Keel, P.K.; Brumm, M.C.; Bowers, W.; Swayze, V.; Andersen, A.; Andreasen, N. Implications of starvation-induced change in right dorsal anterior cingulate volume in anorexia nervosa. Int. J. Eat. Disord. 2008, 41, 602–610. [Google Scholar] [CrossRef]
- Buehren, K.; Konrad, K.; Schaefer, K.; Kratzsch, J.; Kahraman-Lanzerath, B.; Lente, C.; Herpertz-Dahlmann, B. Association between neuroendocrinological parameters and learning and memory functions in adolescent anorexia nervosa before and after weight recovery. J. Neural Transm. 2011, 118, 963–968. [Google Scholar] [CrossRef]
- Castro-Fornieles, J.; Caldú, X.; Andrés-Perpiñá, S.; Lázaro, L.; Bargalló, N.; Falcón, C.; Plana, M.T.; Junqué, C. A cross-sectional and follow-up functional MRI study with a working memory task in adolescent anorexia nervosa. Neuropsychologia 2010, 48, 4111–4116. [Google Scholar] [CrossRef]
- Seitz, J.; Walter, M.; Mainz, V.; Herpertz-Dahlmann, B.; Konrad, K.; von Polier, G. Brain volume reduction predicts weight development in adolescent patients with anorexia nervosa. J. Psychiatr. Res. 2015, 68, 228–237. [Google Scholar] [CrossRef]
- Joos, A.; Klöppel, S.; Hartmann, A.; Glauche, V.; Tüscher, O.; Perlov, E.; Saum, B.; Freyer, T.; Zeeck, A.; van Elst, L.T. Voxel-based morphometry in eating disorders: Correlation of psychopathology with grey matter volume. Psychiatry Res. Neuroimaging 2010, 182, 146–151. [Google Scholar] [CrossRef]
- Nickel, K.; Joos, A.; Tebartz van Elst, L.; Matthis, J.; Holovics, L.; Endres, D.; Zeeck, A.; Hartmann, A.; Tuscher, O.; Maier, S. Recovery of cortical volume and thickness after remission from acute anorexia nervosa. Int. J. Eat. Disord. 2018, 51, 1056–1069. [Google Scholar] [CrossRef]
- King, J.A.; Geisler, D.; Ritschel, F.; Boehm, I.; Seidel, M.; Roschinski, B.; Soltwedel, L.; Zwipp, J.; Pfuhl, G.; Marxen, M.; et al. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol. Psychiatry 2015, 77, 624–632. [Google Scholar] [CrossRef]
- Castro-Fornieles, J.; Bargallo, N.; Lazaro, L.; Andres, S.; Falcon, C.; Plana, M.T.; Junque, C. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J. Psychiatry Res. 2009, 43, 331–340. [Google Scholar] [CrossRef]
- Seitz, J.; Herpertz-Dahlmann, B.; Konrad, K. Brain morphological changes in adolescent and adult patients with anorexia nervosa. J. Neural Transm. 2016, 123, 949–959. [Google Scholar] [CrossRef]
- Hellerhoff, I.; King, J.A.; Tam, F.I.; Pauligk, S.; Seidel, M.; Geisler, D.; Bahnsen, K.; Kretschmann, N.; Akgün, K.; Roessner, V. Differential longitudinal changes of neuronal and glial damage markers in anorexia nervosa after partial weight restoration. Transl. Psychiatry 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Martin, F. Pathology of neurological & psychiatric aspects of various deficiency manifestations with digestive & neuro-endocrine disorders: Study of the changes of the central nervous system in 2 cases of anorexia in young girls (so-called mental anorexia). Acta Neurol. Psychiatr. Belg. 1958, 58, 816. [Google Scholar]
- Neumärker, K.J. Mortality and sudden death in anorexia nervosa. Int. J. Eat. Disord. 1997, 21, 205–212. [Google Scholar] [CrossRef]
- Umeda, K.; Kawakami, I.; Ikeda, K.; Tanei, Z.I.; Matsubara, T.; Murayama, S.; Murahashi, Y.; Niizato, K.; Oshima, K.; Iritani, S. Case report of anorexia nervosa showing periventricular gliosis at autopsy. Neuropathology 2021, 41, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cragg, B. The development of cortical synapses during starvation in the rat. Brain J. Neurol. 1972, 95, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Chowdhury, T.G.; Wable, G.S.; Chen, Y.-W. Synaptic changes in the hippocampus of adolescent female rodents associated with resilience to anxiety and suppression of food restriction-evoked hyperactivity in an animal model for anorexia nervosa. Brain Res. 2017, 1654, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.M.; Collica, S.C.; Aston, S.A.; Wiles, L.J.; Weiner, R.C.; Biswas, A.; Bhasin, H.; Sabir, A.I.; Goodman, E.J.; Purbey, R.; et al. Adolescent female rats prone to the activity based anorexia (ABA) paradigm have altered hedonic responses and cortical astrocyte density compared to resistant animals. Appetite 2021, 168, 105666. [Google Scholar] [CrossRef]
- Reyes-Haro, D.; Labrada-Moncada, F.E.; Miledi, R.; Martinez-Torres, A. Dehydration-Induced Anorexia Reduces Astrocyte Density in the Rat Corpus Callosum. Neural Plast. 2015, 2015, 474917. [Google Scholar] [CrossRef]
- Ragu-Varman, D.; Macedo-Mendoza, M.; Labrada-Moncada, F.E.; Reyes-Ortega, P.; Morales, T.; Martinez-Torres, A.; Reyes-Haro, D. Anorexia increases microglial density and cytokine expression in the hippocampus of young female rats. Behav. Brain Res. 2019, 363, 118–125. [Google Scholar] [CrossRef]
- Routtenberg, A.; Kuznesof, A.W. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J. Comp. Physiol. Psychol. 1967, 64, 414. [Google Scholar] [CrossRef]
- Hebebrand, J.; Exner, C.; Hebebrand, K.; Holtkamp, C.; Casper, R.C.; Remschmidt, H.; Herpertz-Dahlmann, B.; Klingenspor, M. Hyperactivity in patients with anorexia nervosa and in semistarved rats: Evidence for a pivotal role of hypoleptinemia. Physiol. Behav. 2003, 79, 25–37. [Google Scholar] [CrossRef]
- Wu, H.; van Kuyck, K.; Tambuyzer, T.; Luyten, L.; Aerts, J.M.; Nuttin, B. Rethinking food anticipatory activity in the activity-based anorexia rat model. Sci. Rep. 2014, 4, 3929. [Google Scholar] [CrossRef]
- Foldi, C.J.; Milton, L.K.; Oldfield, B.J. A focus on reward in anorexia nervosa through the lens of the activity-based anorexia rodent model. J. Neuroendocr. 2017, 29, e12479. [Google Scholar] [CrossRef]
- Milton, L.K.; Oldfield, B.J.; Foldi, C.J. Evaluating anhedonia in the activity-based anorexia (ABA) rat model. Physiol. Behav. 2018, 194, 324–332. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Paulukat, L.; Kas, M.J.; Tolba, R.; Konrad, K.; Herpertz-Dahlmann, B.; Beyer, C.; Seitz, J. Establishment of a chronic activity-based anorexia rat model. J. Neurosci. Methods 2018, 293, 191–198. [Google Scholar] [CrossRef]
- Seitz, J.; Konrad, K.; Herpertz-Dahlmann, B. Extend, Pathomechanism and Clinical Consequences of Brain Volume Changes in Anorexia Nervosa. Curr. Neuropharmacol. 2018, 16, 1164–1173. [Google Scholar] [CrossRef]
- McCarthy, G.F.; Leblond, C.P. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J. Comp. Neurol. 1988, 271, 589–603. [Google Scholar] [CrossRef]
- Barbarich-Marsteller, N.C.; Fornal, C.A.; Takase, L.F.; Bocarsly, M.E.; Arner, C.; Walsh, B.T.; Hoebel, B.G.; Jacobs, B.L. Activity-based anorexia is associated with reduced hippocampal cell proliferation in adolescent female rats. Behav. Brain Res. 2013, 236, 251–257. [Google Scholar] [CrossRef]
- Reyes-Haro, D.; Labrada-Moncada, F.E.; Varman, D.R.; Kruger, J.; Morales, T.; Miledi, R.; Martinez-Torres, A. Anorexia Reduces GFAP+ Cell Density in the Rat Hippocampus. Neural Plast. 2016, 2016, 2426413. [Google Scholar] [CrossRef]
- Belmonte, L.; Achamrah, N.; Nobis, S.; Guerin, C.; Riou, G.; Bole-Feysot, C.; Boyer, O.; Richard, V.; Rego, J.C.; Dechelotte, P.; et al. A role for intestinal TLR4-driven inflammatory response during activity-based anorexia. Sci. Rep. 2016, 6, 35813. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, P.; Ragu-Varman, D.; Rodríguez, V.M.; Reyes-Haro, D. Anorexia induces a microglial associated pro-inflammatory environment and correlates with neurodegeneration in the prefrontal cortex of young female rats. Behav. Brain Res. 2020, 392, 112606. [Google Scholar] [CrossRef] [PubMed]
- Paulukat, L.; Frintrop, L.; Liesbrock, J.; Heussen, N.; Johann, S.; Exner, C.; Martien, K.J.; Tolba, R.; Neulen, J.; Konrad, K. Memory impairment is associated with the loss of regular oestrous cycle and plasma oestradiol levels in an activity-based anorexia animal model. World J. Biol. Psychiatry 2016, 17, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Wable, G.S.; Min, J.-Y.; Chen, Y.-W.; Aoki, C. Anxiety is correlated with running in adolescent female mice undergoing activity-based anorexia. Behav. Neurosci. 2015, 129, 170. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, C.; Voelz, C.; Kogel, V.; Schlosser, A.; Herpertz-Dahlmann, B.; Beyer, C.; Seitz, J.; Trinh, S. Fear and food: Anxiety-like behavior and the susceptibility to weight loss in an activity-based anorexia rat model. Clin. Transl. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rokot, N.T.; Ataka, K.; Iwai, H.; Suzuki, H.; Tachibe, H.; Kairupan, T.S.; Cheng, K.C.; Amitani, H.; Inui, A.; Asakawa, A. Antagonism for NPY signaling reverses cognitive behavior defects induced by activity-based anorexia in mice. Psychoneuroendocrinology 2021, 126, 105133. [Google Scholar] [CrossRef]
- Milton, L.K.; Mirabella, P.N.; Greaves, E.; Spanswick, D.C.; van den Buuse, M.; Oldfield, B.J.; Foldi, C.J. Suppression of Corticostriatal Circuit Activity Improves Cognitive Flexibility and Prevents Body Weight Loss in Activity-Based Anorexia in Rats. Biol. Psychiatry 2021, 90, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kogel, V.; Trinh, S.; Gasterich, N.; Beyer, C.; Seitz, J. Long-Term Glucose Starvation Induces Inflammatory Responses and Phenotype Switch in Primary Cortical Rat Astrocytes. J. Mol. Neurosci. 2021, 71, 2368–2382. [Google Scholar] [CrossRef] [PubMed]
- Scharner, S.; Prinz, P.; Goebel-Stengel, M.; Kobelt, P.; Hofmann, T.; Rose, M.; Stengel, A. Activity-based anorexia reduces body weight without inducing a separate food intake microstructure or activity phenotype in female rats—mediation via an activation of distinct brain nuclei. Front. Neurosci. 2016, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Nergårdh, R.; Ammar, A.; Brodin, U.; Bergström, J.; Scheurink, A.; Södersten, P. Neuropeptide Y facilitates activity-based-anorexia. Psychoneuroendocrinology 2007, 32, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, J.J.; Kas, M.J.; Scheurink, A.J.; van Dijk, G.; Adan, R.A. AgRP (83–132) and SHU9119 differently affect activity-based anorexia. Eur. Neuropsychopharmacol. 2006, 16, 403–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kas, M.; Van Dijk, G.; Scheurink, A.; Adan, R. Agouti-related protein prevents self-starvation. Mol. Psychiatry 2003, 8, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, J.J.; de Rijke, C.E.; Brakkee, J.H.; Kas, M.J.; Adan, R.A. Voluntary access to a warm plate reduces hyperactivity in activity-based anorexia. Physiol. Behav. 2005, 85, 151–157. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frintrop, L.; Trinh, S.; Seitz, J.; Kipp, M. The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa. J. Clin. Med. 2022, 11, 186. https://doi.org/10.3390/jcm11010186
Frintrop L, Trinh S, Seitz J, Kipp M. The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa. Journal of Clinical Medicine. 2022; 11(1):186. https://doi.org/10.3390/jcm11010186
Chicago/Turabian StyleFrintrop, Linda, Stefanie Trinh, Jochen Seitz, and Markus Kipp. 2022. "The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa" Journal of Clinical Medicine 11, no. 1: 186. https://doi.org/10.3390/jcm11010186
APA StyleFrintrop, L., Trinh, S., Seitz, J., & Kipp, M. (2022). The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa. Journal of Clinical Medicine, 11(1), 186. https://doi.org/10.3390/jcm11010186






