A Comparative Study of the Effectiveness of Pharmacopuncture Therapy for Chronic Neck Pain: A Pragmatic, Randomized, Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participant Timeline
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion criteria
- (1)
- Non-specific neck pain for more than 6 months;
- (2)
- Visual analogue scale (VAS) score > 5 for neck pain;
- (3)
- Age 17 to 70 years;
- (4)
- Provision of written informed consent.
2.3.2. Exclusion Criteria
- (1)
- Cancer migration to the spine or spinal fracture;
- (2)
- Progressive or severe neurologic deficits;
- (3)
- Cancer, fibromyalgia, rheumatoid arthritis, or gout;
- (4)
- Stroke, myocardial infarction, kidney disease, dementia, diabetic neuropathy, or epilepsy;
- (5)
- Using steroids, immunosuppressants, or psychotropic medications;
- (6)
- Hemorrhagic disease, severe diabetes, or using anticoagulants;
- (7)
- Use of NSAIDs or pharmacopuncture performed within the past week;
- (8)
- Pregnancy or lactation;
- (9)
- Cervical surgery within the past 3 months;
- (10)
- Participation in another clinical trial within 1 month or planning to participate in another trial during the follow-up period of the present trial;
- (11)
- Failure to provide written informed consent;
- (12)
- Other difficulties participating in the trial according to the investigator’s decision (who cannot read or understand the questionnaire).
2.4. Interventions
2.4.1. Experimental Group: PPT
2.4.2. Control Group: PT
2.5. Discontinuation and Dropout Criteria
- (1)
- If a participant had a disease that was undetected during the pretrial screening that could affect the study outcome;
- (2)
- If the participant or the participant’s legal representative requested study discontinuation, or if the participant withdrew consent for study participation;
- (3)
- If pregnancy was confirmed during the study;
- (4)
- If the administered intervention for neck pain caused problems for the participant;
- (5)
- Other conditions that made study participation unfit according to the decision of the principal investigator.
2.6. Concomitant Treatment
2.7. Outcomes
2.7.1. Primary Outcome
VAS Score for Neck Pain
2.7.2. Secondary Outcomes
Northwick Park Questionnaire
VAS Score for Radiating Arm Pain
Numeric Rating Scale of Neck and Arm Bothersomeness
Neck Disability Index
Patient Global Impression of Change
12-Item Short Form Health Survey Version 2
EuroQoL 5-Dimension 5-Level Instrument
Drug Consumption
2.7.3. Adverse Events
2.7.4. Sample Size Calculation
2.7.5. Enrollment
2.7.6. Randomization and Allocation Concealment
2.7.7. Blinding
2.7.8. Data Collection and Management
2.7.9. Statistical Methods
2.8. Ethics Approval
2.8.1. Informed Consent
2.8.2. Confidentiality
2.9. Ancillary and Post-Trial Care
3. Results
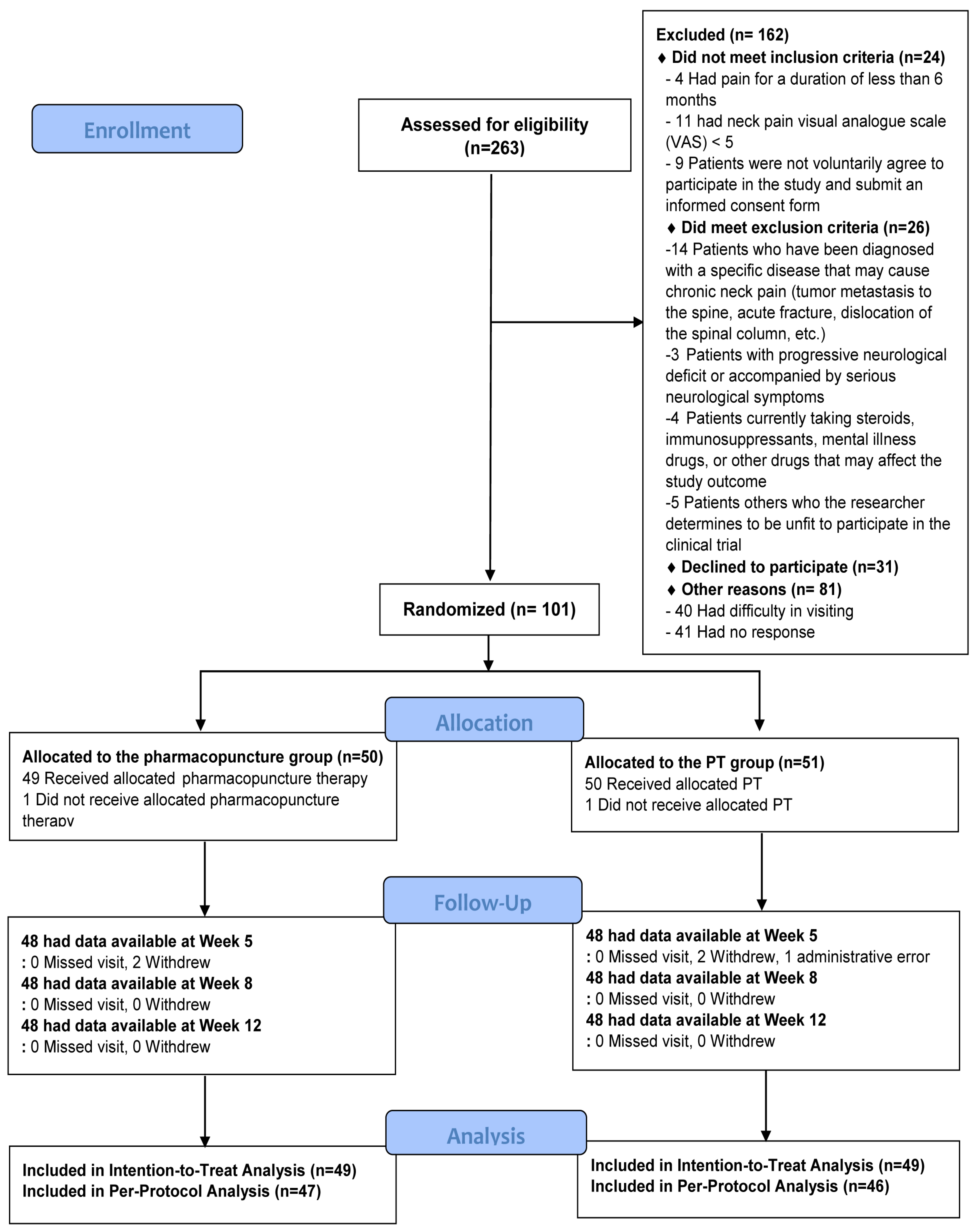
3.1. Flowchart of Participants
3.2. Baseline Characteristics
3.3. Treatment
3.4. Primary and Secondary Outcomes
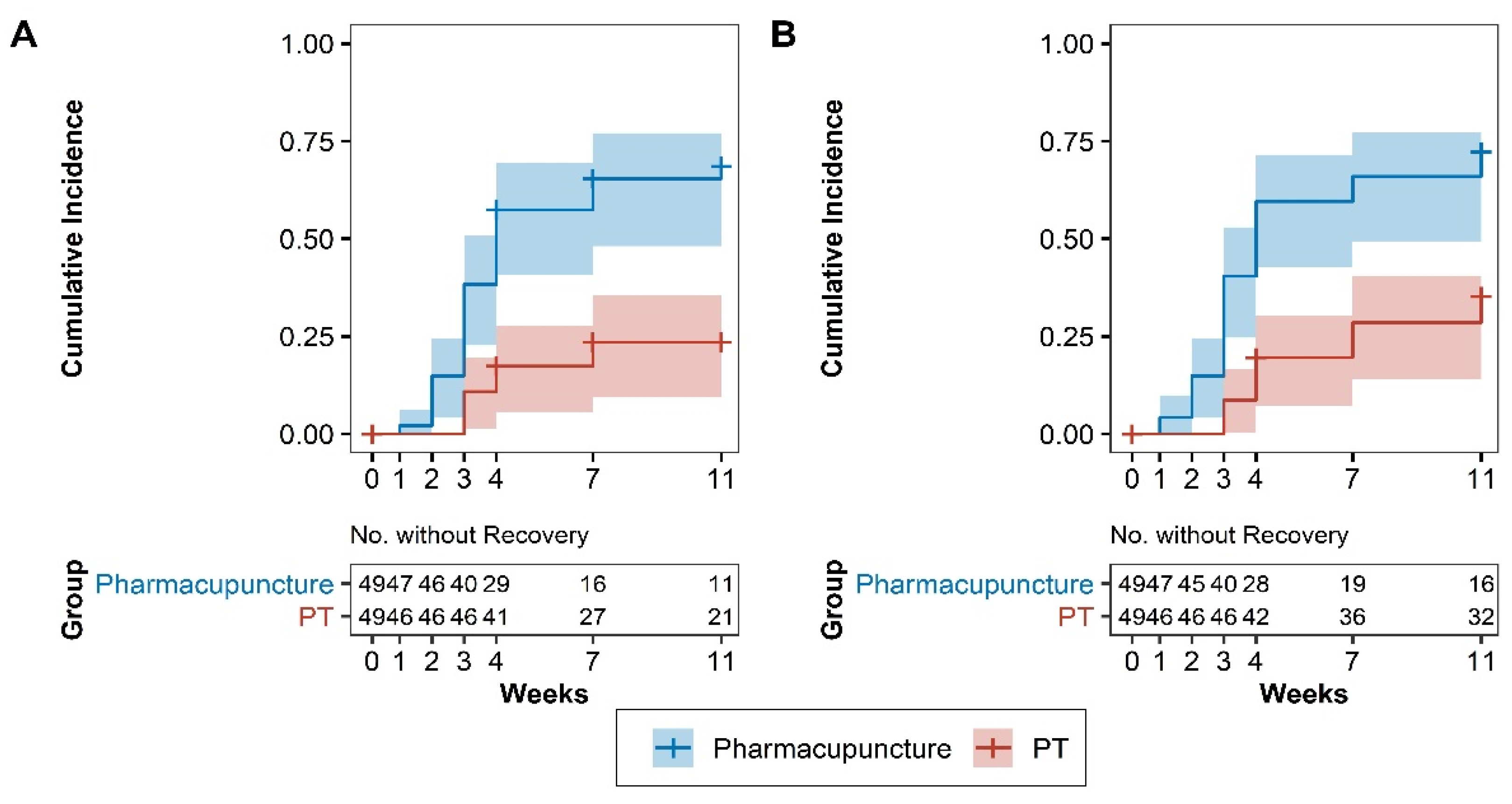
3.5. Survival Analysis
3.6. Adverse Events
4. Discussion
5. Conclusions
6. Protocol
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farì, G.; Fischetti, F.; Zonno, A.; Marra, F.; Maglie, A.; Bianchi, F.P.; Messina, G.; Ranieri, M.; Megna, M. Musculoskeletal Pain in Gymnasts: A Retrospective Analysis on a Cohort of Professional Athletes. Int. J. Environ. Res. Public Health 2021, 18, 5460. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safiri, S.; Kolahi, A.-A.; Hoy, D.; Buchbinder, R.; Mansournia, M.A.; Bettampadi, D.; Ashrafi-Asgarabad, A.; Almasi-Hashiani, A.; Smith, E.; Sepidarkish, M. Global, regional, and national burden of neck pain in the general population, 1990–2017: Systematic analysis of the global burden of disease study 2017. BMJ 2020, 368, m791. [Google Scholar] [CrossRef] [Green Version]
- Fejer, R.; Kyvik, K.O.; Hartvigsen, J. The prevalence of neck pain in the world population: A systematic critical review of the literature. Eur. Spine J. 2006, 15, 834–848. [Google Scholar] [CrossRef] [Green Version]
- Fernández-de-las-Peñas, C.; Hernández-Barrera, V.; Alonso-Blanco, C.; Palacios-Ceña, D.; Carrasco-Garrido, P.; Jiménez-Sánchez, S.; Jiménez-García, R. Prevalence of neck and low back pain in community-dwelling adults in Spain: A population-based national study. Spine 2011, 36, E213–E219. [Google Scholar] [CrossRef]
- Son, K.M.; Cho, N.H.; Lim, S.H.; Kim, H.A. Prevalence and risk factor of neck pain in elderly Korean community residents. J. Korean Med. Sci. 2013, 28, 680–686. [Google Scholar] [CrossRef] [Green Version]
- White, A.P.; Arnold, P.M.; Norvell, D.C.; Ecker, E.; Fehlings, M.G. Pharmacologic management of chronic low back pain: Synthesis of the evidence. Spine 2011, 36, S131–S143. [Google Scholar] [CrossRef] [PubMed]
- Fine, M. Quantifying the impact of NSAID-associated adverse events. Am. J. Manag. Care 2013, 19, s267–s272. [Google Scholar] [PubMed]
- Bier, J.D.; Scholten-Peeters, W.G.; Staal, J.B.; Pool, J.; van Tulder, M.W.; Beekman, E.; Knoop, J.; Meerhoff, G.; Verhagen, A.P. Clinical practice guideline for physical therapy assessment and treatment in patients with nonspecific neck pain. Phys. Ther. 2018, 98, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Strudwick, M.W.; Hinks, R.C.; Choy, S.B. Point injection as an alternative acupuncture technique–an exploratory study of responses in healthy subjects. Acupunct. Med. 2007, 25, 166–174. [Google Scholar] [CrossRef]
- Luna, S.P.; Angeli, A.L.; Ferreira, C.L.; Lettry, V.; Scognamillo-Szabo, M. Comparison of pharmacopuncture, aquapuncture and acepromazine for sedation of horses. Evid.-Based Complementary Altern. Med. 2008, 5, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Shin, J.-S.; Lee, J.; Kim, M.-R.; Park, K.B.; Lee, H.D.; Lee, Y.; Hong, J.; Ha, I.-H. Usage report of pharmacopuncture in musculoskeletal patients visiting Korean medicine hospitals and clinics in Korea. BMC Complementary Altern. Med. 2016, 16, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.-S.; Shin, J.-S.; Lee, J.; Lee, Y.J.; Kim, M.-r.; Ahn, Y.-j.; Park, K.B.; Shin, B.-C.; Lee, M.S.; Kim, J.-H. A survey among Korea Medicine doctors (KMDs) in Korea on patterns of integrative Korean Medicine practice for lumbar intervertebral disc displacement: Preliminary research for clinical practice guidelines. BMC Complementary Altern. Med. 2015, 15, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Shin, J.-S.; Lee, J.; Ha, I.-H.; Kim, M.-R.; Koh, W.; Lee, S.-H.; Kim, S.; Cha, Y.-Y.; Lee, J.-H. Effectiveness of pharmacopuncture for cervical spondylosis: A systematic review and meta-analysis. Eur. J. Integr. Med. 2018, 20, 154–164. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Lee, Y.J.; Lee, J.; Ha, I.-H. A study on the effectiveness of pharmacopuncture for chronic neck pain: A protocol for a pragmatic randomized controlled trial. Medicine 2020, 99, e21406. [Google Scholar] [CrossRef]
- Choi, A.R.; Shin, J.-S.; Lee, J.; Lee, Y.J.; Kim, M.-R.; Oh, M.-S.; Lee, E.-J.; Kim, S.; Kim, M.; Ha, I.-H. Current practice and usual care of major cervical disorders in Korea: A cross-sectional study of Korean health insurance review and assessment service national patient sample data. Medicine 2017, 96, e8751. [Google Scholar] [CrossRef]
- Leak, A.; Cooper, J.; Dyer, S.; Williams, K.; Turner-Stokes, L.; Frank, A. The Northwick Park Neck Pain Questionnaire, devised to measure neck pain and disability. Rheumatology 1994, 33, 469–474. [Google Scholar] [CrossRef]
- Lee, K.-W.; Seo, H.-D.; Jung, K.-S.; Kim, S.-H.; Chung, Y.-J. Reliability and validity of Korean version northwick park neck pain questionnaire in neck pain patients. Phys. Ther. Korea 2010, 17, 68–76. [Google Scholar]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14(7), 409–415. [Google Scholar]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jo, M.-W.; Ahn, J.; Ock, M.; Shin, S.; Park, J. Assessment of psychometric properties of the Korean SF-12 v2 in the general population. BMC Public Health 2014, 14, 1086. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Ahn, J.; Ock, M.; Shin, S.; Park, J.; Luo, N.; Jo, M.-W. The EQ-5D-5L valuation study in Korea. Qual. Life Res. 2016, 25, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Ernst, E. A systematic review of randomized controlled trials of acupuncture for neck pain. Rheumatology 1999, 38, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Irnich, D.; Cummings, M.; Behrens, N.; Molzen, H.; König, A.; Gleditsch, J.; Krauss, M.; Natalis, M.; Senn, E.; Beyer, A. Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck painCommentary: Controls for acupuncture—can we finally see the light? BMJ 2001, 322, 1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreon, L.Y.; Glassman, S.D.; Campbell, M.J.; Anderson, P.A. Neck Disability Index, short form-36 physical component summary, and pain scales for neck and arm pain: The minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J. 2010, 10, 469–474. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, J.; Malo-Urriés, M.; Corral-de-Toro, J.; López-de-Celis, C.; Lucha-López, M.O.; Tricás-Moreno, J.M.; Lorente, A.I.; Hidalgo-García, C. Does the addition of manual therapy approach to a cervical exercise program improve clinical outcomes for patients with chronic neck pain in short-and mid-term? A randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 6601. [Google Scholar] [CrossRef]
- Arsh, A.; Darain, H.; Iqbal, M.; Rahman, M.U.; Ullah, I.; Khalid, S. Effectiveness of manual therapy to the cervical spine with and without manual therapy to the upper thoracic spine in the management of non-specific neck pain; a randomized controlled trial. JPMA 2020, 70, 399–403. [Google Scholar] [CrossRef]
- Dalewski, B.; Kamińska, A.; Szydłowski, M.; Kozak, M.; Sobolewska, E. Comparison of early effectiveness of three different intervention methods in patients with chronic orofacial pain: A randomized, controlled clinical trial. Pain Res. Manag. 2019, 2019, 7954291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calixtre, L.B.; Oliveira, A.B.; de Sena Rosa, L.R.; Armijo-Olivo, S.; Visscher, C.M.; Alburquerque-Sendín, F. Effectiveness of mobilisation of the upper cervical region and craniocervical flexor training on orofacial pain, mandibular function and headache in women with TMD. A randomised, controlled trial. J. Oral Rehabil. 2019, 46, 109–119. [Google Scholar] [CrossRef]
- Lee, B.; Seo, B.-K.; Kwon, O.-J.; Jo, D.-J.; Lee, J.-H.; Lee, S.J.T. Effect of Combined Bee Venom Acupuncture and NSAID Treatment for Non-Specific Chronic Neck Pain: A Randomized, Assessor-Blinded, Pilot Clinical Trial. Toxins 2021, 13, 436. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B. Treatment of cervical spondylopathy by acupoint injection: A report of 30 cases (in Chinese). Henan Tradit. Chin. Med. 2011, 31, 533–534. [Google Scholar]
- Kim, M.-R.; Shin, J.-S.; Lee, J.; Lee, Y.J.; Ahn, Y.-J.; Park, K.B.; Lee, H.D.; Lee, Y.; Kim, S.G.; Ha, I.-H. Safety of acupuncture and pharmacopuncture in 80,523 musculoskeletal disorder patients: A retrospective review of internal safety inspection and electronic medical records. Medicine 2016, 95, e3635. [Google Scholar] [CrossRef]
- Choi, H.S.; Lee, Y.J.; Kim, M.-R.; Cho, J.-H.; Kim, K.-W.; Kim, E.-J.; Ha, I.-H. Survey of Integrative Treatment Practices of Korean Medicine Doctors for Cervical Disc Herniation: Preliminary Data for Clinical Practice Guidelines. Evid.-Based Complementary Altern. Med. 2019, 2019, 2345640. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Shin, J.-S.; Lee, J.; Kim, M.-R.; Ahn, Y.-J.; Shin, Y.-S.; Park, K.B.; Shin, B.-C.; Lee, M.S.; Kim, J.-H.; et al. Survey of integrative lumbar spinal stenosis treatment in Korean medicine doctors: Preliminary data for clinical practice guidelines. BMC Complementary Altern. Med. 2017, 17, 425. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Hong, J.-Y.; Kim, W.K.; Shin, J.-S.; Lee, J.; Ha, I.-H.; Chung, H.-J.; Lee, S.K. Effects of SHINBARO2 on Rat Models of Lumbar Spinal Stenosis. Mediat. Inflamm. 2019, 2019, 7651470. [Google Scholar] [CrossRef]
- Brien, S.; Lewith, G.T.; McGregor, G.; Medicine, C. Devil’s Claw (Harpagophytum procumbens) as a treatment for osteoarthritis: A review of efficacy and safety. J. Altern. Complementary Med. 2006, 12, 981–993. [Google Scholar] [CrossRef]
- Chrubasik, S.; Zimpfer, C.; Schütt, U.; Ziegler, R.J.P. Effectiveness of Harpagophytum procumbens in treatment of acute low back pain. Phytomedicine 1996, 3, 1–10. [Google Scholar] [CrossRef]
- Andersen, M.L.; Santos, E.H.; Maria de Lourdes, V.S.; da Silva, A.A.; Tufik, S. Evaluation of acute and chronic treatments with Harpagophytum procumbens on Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2004, 91, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, I.M.; Ojewole, J.A. Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytother. Res. 2004, 18, 982–989. [Google Scholar] [CrossRef] [PubMed]
- McGregor, G.; Fiebich, B.; Wartenberg, A.; Brien, S.; Lewith, G.; Wegener, T. Devil’s claw (Harpagophytum procumbens): An anti-inflammatory herb with therapeutic potential. Phytochem. Rev. 2005, 4, 47–53. [Google Scholar] [CrossRef]
- Kim, W.K.; Chung, H.-J.; Pyee, Y.; Choi, T.J.; Park, H.J.; Hong, J.-Y.; Shin, J.-S.; Lee, J.H.; Ha, I.-H.; Lee, S.K. Effects of intra-articular SHINBARO treatment on monosodium iodoacetate-induced osteoarthritis in rats. Chin. Med. 2016, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venancio, R.C.; Pelegrini, S.; Gomes, D.Q.; Nakano, E.Y.; Liebano, R.E. Effects of carrier frequency of interferential current on pressure pain threshold and sensory comfort in humans. Arch. Phys. Med. Rehabil. 2013, 94, 95–102. [Google Scholar] [CrossRef]
- Fuentes, J.; Armijo-Olivo, S.; Magee, D.J.; Gross, D.P. A preliminary investigation into the effects of active interferential current therapy and placebo on pressure pain sensitivity: A random crossover placebo controlled study. Physiotherapy 2011, 97, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, C.A.; Draper, D.O.; Feland, J.B.; Jutte, L.S.; Eggett, D.L. Paraspinal musculature and skin temperature changes: Comparing the Thermacare HeatWrap, the Johnson & Johnson Back Plaster, and the ABC Warme-Pflaster. J. Orthop. Sports Phys. Ther. 2004, 34, 549–558. [Google Scholar]
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Alayat, M.S.M.; Atya, A.M.; Ali, M.M.E.; Shosha, T.M. Long-term effect of high-intensity laser therapy in the treatment of patients with chronic low back pain: A randomized blinded placebo-controlled trial. Lasers Med Sci. 2014, 29, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Santamato, A.; Solfrizzi, V.; Panza, F.; Tondi, G.; Frisardi, V.; Leggin, B.G.; Ranieri, M.; Fiore, P. Short-term effects of high-intensity laser therapy versus ultrasound therapy in the treatment of people with subacromial impingement syndrome: A randomized clinical trial. Phys. Ther. 2009, 89, 643–652. [Google Scholar] [CrossRef]
- Malfliet, A.; Lluch Girbés, E.; Pecos-Martin, D.; Gallego-Izquierdo, T.; Valera-Calero, A. The influence of treatment expectations on clinical outcomes and cortisol levels in patients with chronic neck pain: An experimental study. Pain Pract. 2019, 19, 370–381. [Google Scholar] [CrossRef] [PubMed]

| Pharmacopuncture | PT | p-Value | |
|---|---|---|---|
| (n = 49) | (n = 49) | ||
| Sex | |||
| Female | 34 (69.4) | 35 (71.4) | 0.8249 |
| Male | 15 (30.6) | 14 (28.6) | |
| Age | 49.59 ± 12.23 | 47.65 ± 9.88 | 0.3901 |
| Height (cm) | 163.20 ± 7.93 | 163.50 ± 8.31 | 0.8166 |
| Body weight (kg) | 65.08 ± 12.38 | 64.47 ± 11.18 | 0.7978 |
| BMI (kg/m2) | 24.39 ± 4.24 | 24.03 ± 3.26 | 0.6331 |
| Credibility and expectancy of improvement | 6.92 ± 1.32 | 5.61 ± 1.59 | <0.0001 |
| Duration of neck pain (months) | 28.49 ± 33.30 | 28.37 ± 23.35 | 0.9832 |
| Severity of neck pain | |||
| Mild | 1 (2.0) | 0 (0.0) | 0.2714 |
| Moderate | 20 (40.8) | 17 (34.7) | |
| Severe (not requiring treatment) | 11 (22.5) | 19 (38.8) | |
| Severe (requiring treatment) | 17 (34.7) | 13 (26.5) | |
| VAS | |||
| Neck pain | 63.94 ± 11.05 | 65.33 ± 10.71 | 0.5292 |
| Arm pain | 41.96 ± 27.78 | 37.16 ± 29.71 | 0.4112 |
| NRS | |||
| Neck pain | 6.41 ± 1.12 | 6.57 ± 1.12 | 0.4713 |
| Arm pain | 4.12 ± 2.80 | 3.92 ± 2.96 | 0.7267 |
| NDI | 36.48 ± 12.71 | 32.79 ± 9.09 | 0.1015 |
| NPQ | 43.49 ± 13.12 | 40.97 ± 10.13 | 0.2907 |
| EQ-5D-5L | 0.69 ± 0.13 | 0.76 ± 0.10 | 0.0036 |
| SF-12 | |||
| MCS | 45.48 ± 9.30 | 48.04 ± 9.71 | 0.1853 |
| PCS | 38.66 ± 8.44 | 40.47 ± 7.94 | 0.2749 |
| Week 5 | Week 8 | Week 12 | ||
|---|---|---|---|---|
| VAS for neck pain | Pharmacopuncture (n = 49) | 33.15 (27.83, 38.48) | 34.07 (19.68, 48.46) | 34.72 (14.17, 55.28) |
| PT (n = 49) | 17.35 (12.16, 22.55) | 22.16 (−1.17, 45.49) | 27.48 (2.1, 52.86) | |
| Difference in decrease (95% CI) | 16.66 (9.9, 23.42) | 12.85 (−0.57, 26.27) | 8.07 (−2.4, 18.53) | |
| p-value | <0.0001 | 0.060 | 0.131 | |
| VAS for arm pain | Pharmacopuncture (n = 49) | 18.91 (13.04, 24.79) | 19.00 (10.6, 27.39) | 21.38 (13.62, 29.15) |
| PT (n = 49) | 9.97 (4.1, 15.85) | 10.82 (1.5, 20.13) | 11.64 (2.31, 20.98) | |
| Difference in decrease (95% CI) | 6.79 (0.22, 13.35) | 5.87 (−2.07, 13.82) | 7.11 (−1.5, 15.72) | |
| p-value | 0.043 | 0.147 | 0.106 | |
| NRS for neck pain | Pharmacopuncture (n = 49) | 3.23 (2.7, 3.77) | 3.28 (2.68, 3.88) | 3.41 (2.83, 3.99) |
| PT (n = 49) | 1.68 (1.17, 2.2) | 1.88 (1.33, 2.43) | 2.24 (1.63, 2.86) | |
| Difference in decrease (95% CI) | 1.66 (1, 2.32) | 1.51 (0.75, 2.27) | 1.27 (0.49, 2.04) | |
| p-value | <0.0001 | 0.000 | 0.001 | |
| NRS of arm pain | Pharmacopuncture (n = 49) | 1.70 (1.12, 2.27) | 1.76 (1.1, 2.42) | 2.07 (1.41, 2.72) |
| PT (n = 49) | 1.12 (0.57, 1.67) | 1.22 (0.65, 1.79) | 1.14 (0.33, 1.95) | |
| Difference in decrease (95% CI) | 0.49 (−0.12, 1.1) | 0.45 (−0.27, 1.17) | 0.82 (−0.01, 1.65) | |
| p-value | 0.117 | 0.217 | 0.053 | |
| NDI | Pharmacopuncture (n = 49) | 14.40 (10.87, 17.93) | 16.73 (12.73, 20.74) | 17.38 (13.21, 21.56) |
| PT (n = 49) | 8.03 (5.28, 10.78) | 10.25 (7.12, 13.39) | 10.83 (7.46, 14.20) | |
| Difference in decrease (95% CI) | 4.83 (0.82, 8.85) | 4.73 (0.14, 9.31) | 4.75 (−0.08, 9.58) | |
| p-value | 0.018 | 0.043 | 0.054 | |
| NPQ | Pharmacopuncture (n = 49) | 17.14 (13.42, 20.86) | 17.86 (13.5, 22.22) | 20.00 (15.75, 24.25) |
| PT (n = 49) | 10.32 (7.09, 13.55) | 12.33 (8.75, 15.91) | 12.64 (8.74, 16.54) | |
| Difference in decrease (95% CI) | 5.70 (1.36, 10.03) | 4.25 (−0.78, 9.29) | 6.16 (0.99, 11.32) | |
| p-value | 0.010 | 0.098 | 0.020 | |
| EQ-5D-5L | Pharmacopuncture (n = 49) | −0.10 (−0.13, −0.06) | −0.11 (−0.15, −0.07) | −0.13 (−0.17, −0.09) |
| PT (n = 49) | −0.03 (−0.06, −0.01) | −0.04 (−0.07, −0.01) | −0.06 (−0.10, −0.03) | |
| Difference in decrease (95% CI) | −0.01 (−0.05, 0.02) | −0.01 (−0.04, 0.02) | −0.01 (−0.05, 0.03) | |
| p-value | 0.371 | 0.640 | 0.500 | |
| SF-12 (MCS) | Pharmacopuncture (n = 49) | −4.44 (−6.98, −1.91) | −4.09 (−7.28, −0.9) | −5.80 (−9.00, −2.61) |
| PT (n = 49) | −4.48 (−7.23, −1.73) | −4.35 (−7.19, −1.51) | −5.03 (−7.78, −2.28) | |
| Difference in decrease (95% CI) | 1.53 (−1.53, 4.58) | 1.82 (−1.82, 5.45) | 0.76 (−2.81, 4.34) | |
| p-value | 0.327 | 0.327 | 0.676 | |
| SF-12 (PCS) | Pharmacopuncture (n = 49) | −5.29 (−7.77, −2.81) | −6.68 (−9.00, −4.35) | −6.98 (−9.44, −4.52) |
| PT (n = 49) | −1.68 (−3.84, 0.49) | −2.61 (−4.89, −0.33) | −2.82 (−5.16, −0.49) | |
| Difference in decrease (95% CI) | −2.63 (−5.4, 0.14) | −3.05 (−5.73, −0.37) | −3.11 (−5.88, −0.34) | |
| p-value | 0.063 | 0.026 | 0.028 | |
| PGIC | Pharmacopuncture (n = 49) | 2.39 (−2.3, 7.08) | 2.52 (−2.42, 7.46) | 2.35 (−2.26, 6.96) |
| PT (n = 49) | 3.10 (−2.98, 9.19) | 3.13 (−3.01, 9.28) | 3.15 (−3.03, 9.33) | |
| Difference in decrease (95% CI) | −0.71 (−0.99, −0.44) | −0.62 (−0.99, −0.24) | −0.8 (−1.12, −0.48) | |
| p-value | <0.0001 | 0.001 | <0.0001 |
| Pharmacopuncture | PT | Mean Difference | p-Value | |
|---|---|---|---|---|
| VAS score for neck pain | 396.90 (332.65, 461.15) | 524.27 (443.17, 605.37) | −127.37 (−196.85, −57.88) | 0.0003 |
| VAS score for arm pain | 288.12 (226.88, 349.35) | 313.54 (236.59, 390.48) | −25.42 (−119.11, 68.27) | 0.5949 |
| NRS score for neck pain | 40.90 (36.26, 45.54) | 55.04 (50.95, 59.13) | −14.14 (−20.25, −8.02) | <0.0001 |
| NRS score for arm pain | 29.39 (23.25, 35.54) | 32.52 (25.14, 39.9) | −3.13 (−12.71, 6.46) | 0.5225 |
| NDI | 257.10 (220.38, 293.82) | 275.04 (246.8, 303.28) | −17.94 (−63.75, 27.88) | 0.4429 |
| NPQ | 315.89 (278.82, 352.95) | 346.16 (315.57, 376.74) | −30.27 (−77.83, 17.29) | 0.2123 |
| EQ-5D-5L score | 8.60 (8.38, 8.82) | 8.78 (8.58, 8.98) | −0.18 (−0.47, 0.12) | 0.2385 |
| SF-12 (MCS) score | 541.75 (518.54, 564.96) | 569.44 (546.41, 592.48) | −27.69 (−60.32, 4.94) | 0.0963 |
| SF-12 (PCS) score | 481.06 (462.22, 499.91) | 465.86 (445, 486.71) | 15.20 (−12.74, 43.15) | 0.2863 |
| PGIC score | 17.11 (15.71, 18.51) | 21.93 (20.42, 23.44) | −4.82 (−6.82, −2.82) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-S.; Kim, S.; Kim, C.; Seo, J.-Y.; Cho, H.; Kim, S.-D.; Lee, Y.-J.; Lee, J.; Ha, I.-H. A Comparative Study of the Effectiveness of Pharmacopuncture Therapy for Chronic Neck Pain: A Pragmatic, Randomized, Controlled Trial. J. Clin. Med. 2022, 11, 12. https://doi.org/10.3390/jcm11010012
Park K-S, Kim S, Kim C, Seo J-Y, Cho H, Kim S-D, Lee Y-J, Lee J, Ha I-H. A Comparative Study of the Effectiveness of Pharmacopuncture Therapy for Chronic Neck Pain: A Pragmatic, Randomized, Controlled Trial. Journal of Clinical Medicine. 2022; 11(1):12. https://doi.org/10.3390/jcm11010012
Chicago/Turabian StylePark, Kyoung-Sun, Suna Kim, Changnyun Kim, Ji-Yeon Seo, Hyunwoo Cho, Sang-Don Kim, Yoon-Jae Lee, Jinho Lee, and In-Hyuk Ha. 2022. "A Comparative Study of the Effectiveness of Pharmacopuncture Therapy for Chronic Neck Pain: A Pragmatic, Randomized, Controlled Trial" Journal of Clinical Medicine 11, no. 1: 12. https://doi.org/10.3390/jcm11010012
APA StylePark, K.-S., Kim, S., Kim, C., Seo, J.-Y., Cho, H., Kim, S.-D., Lee, Y.-J., Lee, J., & Ha, I.-H. (2022). A Comparative Study of the Effectiveness of Pharmacopuncture Therapy for Chronic Neck Pain: A Pragmatic, Randomized, Controlled Trial. Journal of Clinical Medicine, 11(1), 12. https://doi.org/10.3390/jcm11010012






