Prognostic Value of the Albumin-Bilirubin Grade for the Prediction of Post-Hepatectomy Liver Failure: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analysis
3. Results
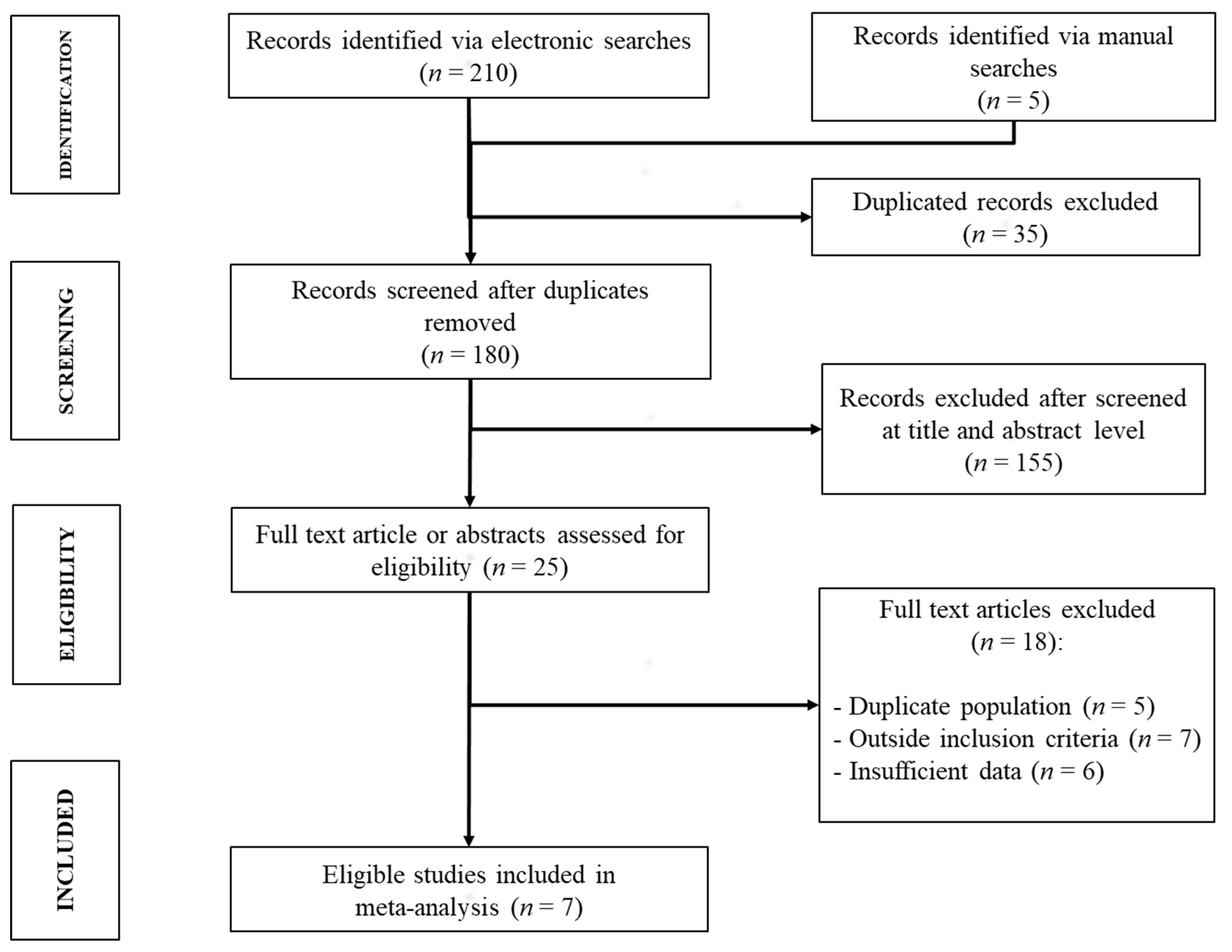
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
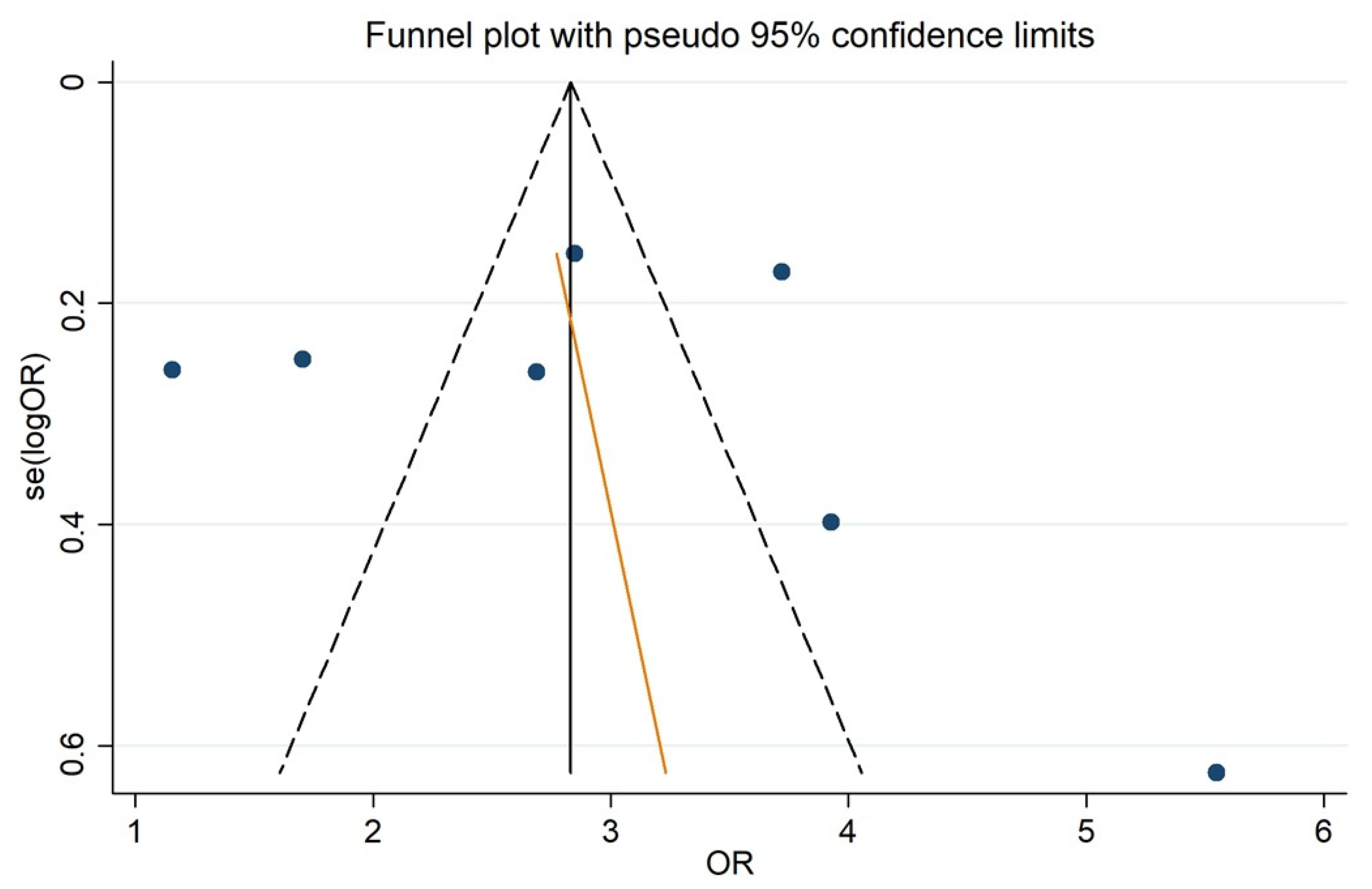
3.4. ALBI and PHLF Occurrence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, M.A.J.; Olde Damink, S.W.M.; Dejong, C.H.C.; Lang, H.; Malagó, M.; Jalan, R.; Saner, F.H. Liver failure after partial hepatic resection: Definition, pathophysiology, risk factors and treatment. Liver Int. 2008, 28, 767–780. [Google Scholar] [CrossRef]
- Søreide, J.A.; Deshpande, R. Post hepatectomy liver failure (PHLF)—Recent advances in prevention and clinical management. Eur. J. Surg. Oncol. 2020, 47, 216–224. [Google Scholar] [CrossRef]
- Sultana, A.; Brooke-Smith, M.; Ullah, S.; Figueras, J.; Rees, M.; Vauthey, J.N.; Conrad, C.; Hugh, T.J.; Garden, O.J.; Fan, S.T.; et al. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: An international multicentre study. HPB 2018, 20, 462–469. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Colecchia, A.; Milandri, M.; Rossini, B.; Alemanni, L.V.; Dajti, E.; Ravaioli, F.; Renzulli, M.; Golfieri, R.; Festi, D. Non-invasive tests for the prediction of post-hepatectomy liver failure in the elderly. Hepatoma Res. 2020, 2020. [Google Scholar] [CrossRef]
- Cucchetti, A.; Cescon, M.; Golfieri, R.; Piscaglia, F.; Renzulli, M.; Neri, F.; Cappelli, A.; Mazzotti, F.; Mosconi, C.; Colecchia, A.; et al. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J. Hepatol. 2016, 64, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Colecchia, A.; Dajti, E.; Ravaioli, F.; Cucchetti, A.; Cescon, M.; Festi, D. Prediction of posthepatectomy liver failure: Role of SSM and LSPS. J. Surg. Oncol. 2019, 119, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mehta, N.N.; Golhar, A.; Nundy, S. Post hepatectomy liver failure—A comprehensive review of current concepts and controversies. Ann. Med. Surg. 2018, 34, 4–10. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhao, X.-H.; Ma, L.; Ye, J.-Z.; Wu, F.-X.; Tang, J.; You, X.-M.; Xiang, B.-D.; Li, L.-Q. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J. Surg. Oncol. 2018, 118, 440–445. [Google Scholar] [CrossRef]
- Cescon, M.; Colecchia, A.; Cucchetti, A.; Peri, E.; Montrone, L.; Ercolani, G.; Festi, D.; Pinna, A.D. Value of Transient Elastography Measured With Fibroscan in Predicting the Outcome of Hepatic Resection for Hepatocellular Carcinoma. Ann. Surg. 2012, 256, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. A nssessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach—The albi grade. J. Clin. Oncol. 2015. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Yan, X.; Li, M.; Xia, J.; Liu, Y.; Chen, Y.; Jia, B.; Zhu, L.; Zhu, C.; et al. Albumin-Bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig. Liver Dis. 2019, 51, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Wang, Y.B.; Tan, Y.L.; Xi, C.; Xu, X.Z. Prognostic value of pretreatment albumin to bilirubin ratio in patients with hepatocellular cancer: A meta-analysis. Medicine 2019, 98, e14027. [Google Scholar] [CrossRef] [PubMed]
- Handbook for DTA Reviews | Cochrane Screening and Diagnostic Tests. Available online: http://methods.cochrane.org/sdt/handbook-dta-reviews (accessed on 29 May 2018).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Steichen, T. Nonparametric trim and fill analysis of publication bias in meta-analysis: Erratum. Stata Tech. Bull. 2001, 10. [Google Scholar]
- Thompson, S.G.; Sharp, S.J. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat. Med. 1999, 18, 2693–2708. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004, 23, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Manly, B.F.J. Randomization, Bootstrap and Monte Carlo Methods in Biology; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018; Chapter 4. [Google Scholar]
- Mai, R.Y.; Wang, Y.Y.; Bai, T.; Chen, J.; Xiang, B.D.; Wu, G.B.; Wu, F.X.; Li, L.Q.; Ye, J.Z. Combination of ALBI and APRI to predict posthepatectomy liver failure after liver resection for HBV-related HCC patients. Cancer Manag. Res. 2019, 11, 8799–8806. [Google Scholar] [CrossRef]
- Cai, W.; He, B.; Hu, M.; Zhang, W.; Xiao, D.; Yu, H.; Song, Q.; Xiang, N.; Yang, J.; He, S.; et al. A radiomics-based nomogram for the preoperative prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma. Surg. Oncol. 2019, 28, 78–85. [Google Scholar] [CrossRef]
- Ruzzenente, A.; De Angelis, M.; Conci, S.; Campagnaro, T.; Isa, G.; Bagante, F.; Ciangherotti, A.; Pedrazzani, C.; Capelli, P.; Iacono, C.; et al. The albumin-bilirubin score stratifies the outcomes of Child-Pugh class A patients after resection of hepatocellular carcinoma. Transl. Cancer Res. 2019, 8. [Google Scholar] [CrossRef]
- Russolillo, N.; Forchino, F.; Conci, S.; Mele, C.; Langella, S.; Ruzzenente, A.; Scoleri, I.; Giuliante, F.; Guglielmi, A.; Ferrero, A. Validation of the albumin–indocyanine green evaluation model in patients with resected hepatocellular carcinoma and comparison with the albumin–bilirubin score. J. Hepatobiliary. Pancreat. Sci. 2019, 26, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.Y.; Ye, J.Z.; Long, Z.R.; Shi, X.M.; Bai, T.; Chen, J.; Li, L.Q.; Wu, G.B.; Wu, F.X. Preoperative aspartate aminotransferase-to-platelet-ratio index as a predictor of posthepatectomy liver failure for resectable hepatocellular carcinoma. Cancer Manag. Res. 2019, 11, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Koh, Y.X.; Syn, N.; Teo, J.Y.; Goh, B.K.P.; Cheow, P.C.; Chung, Y.F.A.; Ooi, L.L.; Chan, C.Y.; Lee, S.Y. Early Prediction of Post-hepatectomy Liver Failure in Patients Undergoing Major Hepatectomy Using a PHLF Prognostic Nomogram. World J. Surg. 2020, 44, 4197–4206. [Google Scholar] [CrossRef]
- Shi, J.Y.; Sun, L.Y.; Quan, B.; Xing, H.; Li, C.; Liang, L.; Pawlik, T.M.; Zhou, Y.H.; Wang, H.; Gu, W.M.; et al. A novel online calculator based on noninvasive markers (ALBI and APRI) for predicting post-hepatectomy liver failure in patients with hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Zhu, H.; Diao, Y.-K.; Xing, H.; Liang, L.; Li, J.; Zhou, Y.-H.; Gu, W.-M.; Chen, T.-H.; Zeng, Y.-Y.; et al. A novel online calculator based on albumin-bilirubin and aspartate transaminase-to-platelet ratio index for predicting postoperative morbidity following hepatectomy for hepatocellular carcinoma. Ann. Transl. Med. 2020, 8, 1591. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Li, J.; Dong, R.; Bai, X. An improved scoring system based on PALBI in predicting post-hepatectomy liver failure outcomes. Dig. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wen, Y.; Yuan, K.; Miao, X.Y.; Xiong, L.; Liu, K.J. Combining albumin-bilirubin score with future liver remnant predicts post-hepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018, 38, 494–502. [Google Scholar] [CrossRef]
- Zou, H.; Yang, X.; Li, Q.L.; Zhou, Q.X.; Xiong, L.; Wen, Y. A Comparative Study of Albumin-Bilirubin Score with Child-Pugh Score, Model for End-Stage Liver Disease Score and Indocyanine Green R15 in Predicting Posthepatectomy Liver Failure for Hepatocellular Carcinoma Patients. Dig. Dis. 2018, 36, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, L.; Zhang, N.; Zhu, W.; Zhou, J.; Pan, Q.; Mao, A.; Lin, Z.; Wang, L.; Zhao, Y. Predictive Value of Intraoperative Indocyanine Green Clearance Measurement on Postoperative Liver Function After Anatomic Major Liver Resection. J. Gastrointest. Surg. 2019. [Google Scholar] [CrossRef]
- Lu, L.H.; Zhang, Y.F.; Mu-Yan, C.; Kan, A.; Zhong, X.P.; Mei, J.; Ling, Y.H.; Li, S.H.; Shi, M.; Wei, W.; et al. Platelet-albumin-bilirubin grade: Risk stratification of liver failure, prognosis after resection for hepatocellular carcinoma. Dig. Liver Dis. 2019, 51, 1430–1437. [Google Scholar] [CrossRef]
- Andreatos, N.; Amini, N.; Gani, F.; Margonis, G.A.; Sasaki, K.; Thompson, V.M.; Bentrem, D.J.; Hall, B.L.; Pitt, H.A.; Wilson, A.; et al. Albumin-Bilirubin Score: Predicting Short-Term Outcomes Including Bile Leak and Post-hepatectomy Liver Failure Following Hepatic Resection. J. Gastrointest. Surg. 2017, 21, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Xiong, L.; Zhou, J.J.; Miao, X.Y.; Li, Q.L.; Wen, Y.; Zou, H. Ability of the ALBI grade to predict posthepatectomy liver failure and long-term survival after liver resection for different BCLC stages of HCC 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. World J. Surg. Oncol. 2018, 16. [Google Scholar] [CrossRef]
- Fagenson, A.M.; Gleeson, E.M.; Pitt, H.A.; Lau, K.N. Albumin-Bilirubin Score vs Model for End-Stage Liver Disease in Predicting Post-Hepatectomy Outcomes. J. Am. Coll. Surg. 2020, 230, 637–645. [Google Scholar] [CrossRef]
- Taylor, G.A.; Fagenson, A.M.; Kuo, L.E.; Pitt, H.A.; Lau, K.N. Predicting Outcomes of Surgery in Patients with Liver Disease: Albumin-Bilirubin Score vs Model for End-stage Liver Disease-Sodium Score. J. Am. Coll. Surg. 2020. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhong, J.H.; Su, Z.Y.; Huang, J.F.; Lu, S.D.; Xiang, B.D.; Ma, L.; Qi, L.N.; Ou, B.N.; Li, L.Q. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br. J. Surg. 2016, 103, 725–734. [Google Scholar] [CrossRef]
- Sposito, C.; Monteleone, M.; Aldrighetti, L.; Cillo, U.; Dalla Valle, R.; Guglielmi, A.; Ettorre, G.M.; Ferrero, A.; Di Benedetto, F.; Rossi, G.E.; et al. Preoperative predictors of liver decompensation after mini-invasive liver resection. Surg. Endosc. 2020, 35. [Google Scholar] [CrossRef]
- Chong, C.; Wong, G.; Fung, A.; Lok, H.-T.; Fong, A.; Cheung, S.; Wong, J.; Lee, K.-F.; Lai, P. Albumin-bilirubin versus liver stiffness measurement versus child’s pugh grade as a predictor of postoperative outcome following hepatectomy for hepatocellular carcinoma. HPB 2018, 20, S316. [Google Scholar] [CrossRef][Green Version]
- Fagenson, A.M.; Gleeson, E.M.; Karhadkar, S.; Di Carlo, A.; Pitt, H.A.; Lau, K.N. Comparison of Albumin-Bilirubin and Model for End-Stage Liver Disease in Predicting Post-Hepatectomy Liver Failure. J. Am. Coll. Surg. 2019, 229, S170–S171. [Google Scholar] [CrossRef]
- Fukutomi, S.; Nomura, Y.; Muroya, D.; Goto, Y.; Sakai, H.; Akagi, Y.; Okuda, K. The validity of albumin-bilirubin model (ALBI) in predicting surgical outcome after hepatectomy for hepatocellular carcinoma-comparison to child pugh score. J. Hepatobiliary. Pancreat. Sci. 2017, 24, A289. [Google Scholar]
- Imai, D.; Maeda, T.; Kayashima, H. Usefulness of ALBI grade in post-hepatectomy liver failure of early stage HCC. J. Hepatobiliary. Pancreat. Sci. 2017, 24, A147. [Google Scholar]
- Shum, J.K.; Ng, S.W.Y.; Wong, B.; Chan, W.L.; Lok, H.T.; Fung, A.K.Y.; Cheung, Y.S.; Wong, J.; Lee, K.F.; Lai, P.B.S.; et al. Higher albumin-bilirubin grade and liver stiffness measurement were associated with more morbidities and mortality after hepatectomy for hepatocellular carcinoma. Surg. Pract. 2018, 22, 20. [Google Scholar]
- Mai, R.Y.; Zeng, J.; Ye, J.Z.; Su, Q.B.; Long, Z.R.; Shi, X.M.; Huang, S.; Wu, F.X.; Li, L.Q.; Lian, F.; et al. Clinical value of preoperative aspartate aminotransferase-to-platelet ratio index in predicting liver failure after hepatectomy for primary liver cancer. Acad. J. Second Mil. Med. Univ. 2019, 40, 61–67. [Google Scholar] [CrossRef]
- Geng, L.; Zong, R.; Shi, Y.; Xu, K. Prognostic role of preoperative albumin-bilirubin grade on patients with hepatocellular carcinoma after surgical resection: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 769–778. [Google Scholar] [CrossRef]
- Mohammadi, H.; Abuodeh, Y.; Jin, W.; Frakes, J.; Friedman, M.; Biebel, B.; Choi, J.; El-Haddad, G.; Kis, B.; Sweeney, J.; et al. Using the Albumin-Bilirubin (ALBI) grade as a prognostic marker for radioembolization of hepatocellular carcinoma. J. Gastrointest. Oncol. 2018, 9, 840–846. [Google Scholar] [CrossRef]
- Na, S.K.; Yim, S.Y.; Suh, S.J.; Jung, Y.K.; Kim, J.H.; Seo, Y.S.; Yim, H.J.; Yeon, J.E.; Byun, K.S.; Um, S.H. ALBI versus Child-Pugh grading systems for liver function in patients with hepatocellular carcinoma. J. Surg. Oncol. 2018, 117, 912–921. [Google Scholar] [CrossRef]
- Xu, L.; Wu, J.; Lu, W.; Yang, C.; Liu, H. Application of the Albumin-Bilirubin Grade in Predicting the Prognosis of Patients With Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Transplant. Proc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maluccio, M.; Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012, 62, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Fukumoto, T.; Kuramitsu, K.; Kido, M.; Takebe, A.; Tanaka, M.; Itoh, T.; Ku, Y. Assessment of ISGLS Definition of Posthepatectomy Liver Failure and Its Effect on Outcome in Patients with Hepatocellular Carcinoma. J. Gastrointest. Surg. 2014, 18, 729–736. [Google Scholar] [CrossRef]



| Author, Year | Country | Design of the Study | Total Pts | Age | Sex (Male), n. (%) | Etiology, % | Child Pugh, n. (%) | Extent of Hepatectomy n. (%) | Outcome | ALBI Grade n., (%) | Total PHLF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang, 2016 [43] | China | Retrospective | 1242 | >60: 223 (18%) | 1072 (86.3) | HBV 85.3% | A 1189 (95.7), B 53 (4.3), C 0 (0) | Minor 975 (78.5), Major 267 (21.5) | PHLF A + B + C | ALBI 1, 850 (68.4), ALBI 2, 390 (31.4), ALBI 3, 2(0⋅2) | 166, Grade A 58, Grade B 91, Grade C 17 |
| Chong, 2018 [45] | China | Retrospective | 396 | 59.7 c | 334 (84.3) | HBV 80.3% | A 397 (97.7), B 9 (2.3), C 0 (0) | Minor 243 (61.4), Major 153 (38.6) | PHLF A + B + C | ALBI 1, 302 (76.25), ALBI 2, 93 (23.5), ALBI 3, 1 (0.25) | 109, Grade A 52, Grade B/C 57 |
| Zhang, 2018 [40] | China | Retrospective | 338 | 52 [44–66] b | 299 (88.5) | HBV 82.2% | A 308 (91.1), B 30 (8.9), C 0 (0) | N/A | PHLF A + B + C | ALBI 1, 134 (39.6), ALBI 2, 198 (58.6), ALBI 3, 6 (1.8) | 26, Grade A 8, Grade B 13, Grade C 5 |
| Zou, 2018 [36] | China | Retrospective | 473 | 52 (18–77) a | 411 (86.9) | HBV 85.4% | A 427 (90.3), B 46 (9.7), C 0 (0) | Minor 356 (75,3), Major 117 (24.7) | PHLF A + B + C | ALBI 1, 189 (40), ALBI 2, 282 (59.6), ALBI 3, 2 (0.4) | 50 |
| Lu, 2019 [38] | China | Retrospective | 2038 | > 50: 948 (46.5%) | 1810 (88.8) | HBV 88.9% | A 2038 (100), B 0 (0), C 0 (0) | Minor 1501 (73.7), Major 537 (26.3) | PHLF B + C | ALBI 1, 1570 (77), ALBI 2, 468 (23), ALBI 3, 0 (0) | 196 |
| Russolillo, 2019 [29] | Italy | Retrospective | 400 | 70 (24–86) a | 339 (84.8) | Mixed (HCV 40%) | A 385 (96.25), B 15 (3.75), C 0 (0) | Minor 299 (74.7), Major 101 (25.3) | PHLF A + B + C | ALBI 1, 208 (52), ALBI 2, 188 (47), ALBI 3, 4 (1) | 82, Grade A 48, Grade B/C 34 |
| Sposito, 2020 [44] | Italy | Prospective | 490 | 68.6 [61.4–74.7] b | 360 (73.5) | Mixed (HCV 59%) | A 463 (94.5), B 27 (5.5), C 0 (0) | Minor 457 (93.3), Major 33 (6.7) | PHLF B + C | ALBI 1, 217 (44.3), ALBI 2, 268 (54.7), ALBI 3, 5 (1.0) | 89 |
| Study | Patient Selection | Index Test | Reference Standard | Flow and Timing | |||
|---|---|---|---|---|---|---|---|
| Risk of Bias | Concerns about Applicability | Risk of Bias | Concerns about Applicability | Risk of Bias | Concerns about Applicability | Risk of Bias | |
| Wang, 2016 [43] | L | L | L | L | U | L | U |
| Chong, 2018 [45] | L | L | L | L | U | L | U |
| Zhang, 2018 [40] | U | L | L | L | U | L | U |
| Zou, 2018 [36] | U | L | L | L | U | L | U |
| Lu, 2019 [38] | U | L | L | L | U | H | U |
| Russolillo, 2019 [29] | L | L | L | L | U | L | U |
| Sposito, 2020 [44] | L | L | L | L | U | H | U |
| Covariates | Number of Studies | Beta Coefficient ± SE | Adjusted R2 (%) | p Value | p Value ± SE after Montecarlo Permutation |
|---|---|---|---|---|---|
| Country | 7 | 0.308 ± 0.369 | −2.03 | 0.371 | 0.432 ± 0.007 |
| Age | 5 | 0.871 ± 0.074 | 29.67 | 0.202 | 0.283 ± 0.006 |
| Sex (Male) | 7 | 1.097 ± 0.126 | −9.15 | 0.460 | 0.485 ± 0.007 |
| Child–Pugh A | 7 | 1.014 ± 0.017 | −6.57 | 0.442 | 0.437 ± 0.007 |
| Major hepatectomy | 5 | 0.956 ± 0.046 | −3.46 | 0.424 | 0.316 ± 0.007 |
| PHLF definition | 7 | 1.462 ± 1.900 | −21.97 | 0.782 | 0.852 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasco, G.; Alemanni, L.V.; Colecchia, A.; Festi, D.; Bazzoli, F.; Mazzella, G.; Montagnani, M.; Azzaroli, F. Prognostic Value of the Albumin-Bilirubin Grade for the Prediction of Post-Hepatectomy Liver Failure: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2011. https://doi.org/10.3390/jcm10092011
Marasco G, Alemanni LV, Colecchia A, Festi D, Bazzoli F, Mazzella G, Montagnani M, Azzaroli F. Prognostic Value of the Albumin-Bilirubin Grade for the Prediction of Post-Hepatectomy Liver Failure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(9):2011. https://doi.org/10.3390/jcm10092011
Chicago/Turabian StyleMarasco, Giovanni, Luigina Vanessa Alemanni, Antonio Colecchia, Davide Festi, Franco Bazzoli, Giuseppe Mazzella, Marco Montagnani, and Francesco Azzaroli. 2021. "Prognostic Value of the Albumin-Bilirubin Grade for the Prediction of Post-Hepatectomy Liver Failure: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 9: 2011. https://doi.org/10.3390/jcm10092011
APA StyleMarasco, G., Alemanni, L. V., Colecchia, A., Festi, D., Bazzoli, F., Mazzella, G., Montagnani, M., & Azzaroli, F. (2021). Prognostic Value of the Albumin-Bilirubin Grade for the Prediction of Post-Hepatectomy Liver Failure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(9), 2011. https://doi.org/10.3390/jcm10092011








