Clinical Associations between Serial Electrocardiography Measurements and Sudden Cardiac Death in Patients with End-Stage Renal Disease Undergoing Hemodialysis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Definitions
2.4. ECG Analysis
2.5. Patient Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. ECG Findings in the SCD Group
3.3. Changes in ECG Parameters before and after HD in the Two Groups
3.4. ECG Parameters as Predictors of SCD
3.5. Additional Analyses
4. Discussion
4.1. Clinical Implications of the QTpe Interval in This Study and Previous Studies
4.2. Comparison with Other Studies: An Explanatory Hypothesis
4.3. Clinical Implications of the QTc Interval and QT Dispersion in This Study and Previous Studies
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Albertus, P.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Cao, J.; Chen, J.L.; et al. Us renal data system 2016 annual data report: Epidemiology of kidney disease in the united states. Am. J. Kidney Dis. 2017, 69, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Makar, M.S.; Pun, P.H. Sudden cardiac death among hemodialysis patients. Am. J. Kidney Dis. 2017, 69, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.D.; Parekh, R.S. Calcium and sudden cardiac death in end-stage renal disease. Semin. Dial. 2015, 28, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin. J. Am. Soc. Nephrol. 2009, 4, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Guérin, A.P.; London, G.M.; Marchais, S.J.; Metivier, F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol. Dial. Transplant. 2015, 15, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Burton, J.O.; Selby, N.M.; Leccisotti, L.; Korsheed, S.; Baker, C.S.; Camici, P.G. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin. J. Am. Soc. Nephrol. 2008, 3, 19–26. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Hu, L.; O’Dea, R.; Murray, D.C.; Barre, P.E. Hypocalcemia, morbidity, and mortality in end-stage renal disease. Am. J. Nephrol. 1996, 16, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.H.; Middleton, J.P. Sudden cardiac death in hemodialysis patients: A comprehensive care approach to reduce risk. Blood Purif. 2012, 33, 183–189. [Google Scholar] [CrossRef]
- Alpert, M.A. Sudden cardiac arrest and sudden cardiac death on dialysis: Epidemiology, evaluation, treatment, and prevention. Hemodial. Int. 2011, 15, S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Waks, J.W.; Tereshchenko, L.G.; Parekh, R.S. Electrocardiographic predictors of mortality and sudden cardiac death in patients with end stage renal disease on hemodialysis. J. Electrocardiol. 2016, 49, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Lin, L.Y.; Wu, K.D. Qt interval dispersion in dialysis patients. Nephrology 2005, 10, 109–112. [Google Scholar] [CrossRef]
- Beaubien, E.R.; Pylypchuk, G.B.; Akhtar, J.; Biem, H.J. Value of corrected qt interval dispersion in identifying patients initiating dialysis at increased risk of total and cardiovascular mortality. Am. J. Kidney Dis. 2002, 39, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Lim, P.O.; Rana, B.S.; Struthers, A.D. Qt dispersion in medicine: Electrophysiological holy grail or fool’s gold? QJM Int. J. Med. 2000, 93, 425–431. [Google Scholar] [CrossRef][Green Version]
- Morris, S.T.; Galiatsou, E.; Stewart, G.A.; Rodger, R.S.; Jardine, A.G. Qt dispersion before and after hemodialysis. J. Am. Soc. Nephrol. 1999, 10, 160–163. [Google Scholar] [CrossRef]
- American College of Cardiology/American Heart Association Task Force on Clinical Data; Buxton, A.E.; Calkins, H.; Callans, D.J.; DiMarco, J.P.; Fisher, J.D.; Greene, H.L.; Haines, D.E.; Hayes, D.L.; Heidenreich, P.A.; et al. Acc/aha/hrs 2006 key data elements and definitions for electrophysiological studies and procedures: A report of the american college of cardiology/american heart association task force on clinical data standards (acc/aha/hrs writing committee to develop data standards on electrophysiology). Circulation 2006, 114, 2534–2570. [Google Scholar]
- Siscovick, D.S. Challenges in cardiac arrest research: Data collection to assess outcomes. Ann. Emerg. Med. 1993, 22, 92–98. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. esc/esh guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes-2016 abridged for primary care providers. Am. Diabetes Assoc. 2016, 34, 3–21. [Google Scholar]
- Page, R.L.; Joglar, J.A.; Caldwell, M.A.; Calkins, H.; Conti, J.B.; Deal, B.J.; Estes, N.A.M., 3rd; Field, M.E.; Goldberger, Z.D.; Hammill, S.C.; et al. 2015 acc/aha/hrs guideline for the management of adult patients with supraventricular tachycardia: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2016, 67, e27–e115. [Google Scholar] [CrossRef] [PubMed]
- Whitman, I.R.; Feldman, H.I.; Deo, R. Ckd and sudden cardiac death: Epidemiology, mechanisms, and therapeutic approaches. J. Am. Soc. Nephrol. 2012, 23, 1929–1939. [Google Scholar] [CrossRef]
- Panikkath, R.; Reinier, K.; Uy-Evanado, A.; Teodorescu, C.; Hattenhauer, J.; Mariani, R.; Gunson, K.; Jui, J.; Chugh, S.S. Prolonged tpeak-to-tend interval on the resting ecg is associated with increased risk of sudden cardiac death. Circ. Arrhythmia Electrophysiol. 2011, 4, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Patel, C.; Patel, H.; Narayanaswamy, S.; Malhotra, B.; Green, J.T.; Yan, G.X. T(p-e)/qt ratio as an index of arrhythmogenesis. J. Electrocardiol. 2008, 41, 567–574. [Google Scholar] [CrossRef]
- Watanabe, N.; Kobayashi, Y.; Tanno, K.; Miyoshi, F.; Asano, T.; Kawamura, M.; Mikami, Y.; Adachi, T.; Ryu, S.; Miyata, A.; et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J. Electrocardiol. 2004, 37, 191–200. [Google Scholar] [CrossRef]
- Lubinski, A.; Kornacewicz-Jach, Z.; Wnuk-Wojnar, A.M.; Adamus, J.; Kempa, M.; Królak, T.; Lewicka-Nowak, E.; Radomski, M.; Swiatecka, G. The terminal portion of the t wave: A new electrocardiographic marker of risk of ventricular arrhythmias. Pacing Clin. Electrophysiol. 2000, 23, 1957–1959. [Google Scholar] [CrossRef]
- Castro Hevia, J.; Antzelevitch, C.; Tornés Bárzaga, F.; Dorantes Sánchez, M.; Dorticós Balea, F.; Zayas Molina, R.; Quiñones Pérez, M.A.; Fayad Rodríguez, Y. Tpeak-tend and tpeak-tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the brugada syndrome. J. Am. Coll. Cardiol. 2006, 47, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Letsas, K.P.; Weber, R.; Astheimer, K.; Kalusche, D.; Arentz, T. Tpeak-tend interval and tpeak-tend/qt ratio as markers of ventricular tachycardia inducibility in subjects with brugada ecg phenotype. EP Eur. 2010, 12, 271–274. [Google Scholar] [CrossRef]
- Rosenthal, T.M.; Stahls, P.F., 3rd; Abi Samra, F.M.; Bernard, M.L.; Khatib, S.; Polin, G.M.; Xue, J.Q.; Morin, D.P. T-peak to t-end interval for prediction of ventricular tachyarrhythmia and mortality in a primary prevention population with systolic cardiomyopathy. Heart Rhythm 2015, 12, 1789–1797. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Zhang, Z.-M.; Gregg, R.E.; Haisty, W.K.; Vitolins, M.Z.; Curtis, A.B.; Warren, J.; Horaĉek, M.B.; Zhou, S.H.; Soliman, E.Z. Normal standards for computer-ecg programs for prognostically and diagnostically important ecg variables derived from a large ethnically diverse female cohort: The women’s health initiative (whi). J. Electrocardiol. 2013, 46, 707–716. [Google Scholar] [CrossRef][Green Version]
- Kaesler, N.; Babler, A.; Floege, J.; Kramann, R. Cardiac remodeling in chronic kidney disease. Toxins 2020, 12, 161. [Google Scholar] [CrossRef]
- Morin, D.P.; Saad, M.N.; Shams, O.F.; Owen, J.S.; Xue, J.Q.; Abi-Samra, F.M.; Khatib, S.; Nelson-Twakor, O.S.; Milani, R.V. Relationships between the t-peak to t-end interval, ventricular tachyarrhythmia, and death in left ventricular systolic dysfunction. EP Eur. 2012, 14, 1172–1179. [Google Scholar] [CrossRef]
- Hassan, M.O.; Duarte, R.; Dix-Peek, T.; Vachiat, A.; Naidoo, S.; Dickens, C.; Grinter, S.; Manga, P.; Naicker, S. Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin. Nephrol. 2016, 86, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Saour, B.M.; Wang, J.H.; Lavelle, M.P.; Mathew, R.O.; Sidhu, M.S.; Boden, W.E.; Sacco, J.D.; Costanzo, E.J.; Hossain, M.A.; Vachharanji, T.; et al. Tpte and tpte/qt: Novel markers to predict sudden cardiac death in esrd? Braz. J. Nephrol. 2019, 41, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.M.; Kors, J.A.; De Bruin, M.L.; van der Hooft, C.S.; Hofman, A.; Heeringa, J.; Deckers, J.W.; Kingma, J.H.; Sturkenboom, M.C.; Stricker, B.H.; et al. Prolonged qtc interval and risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 2006, 47, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Drug and Therapeutics Bulletin. QT interval and drug therapy. BMJ 2016, 353, i2732. [Google Scholar]
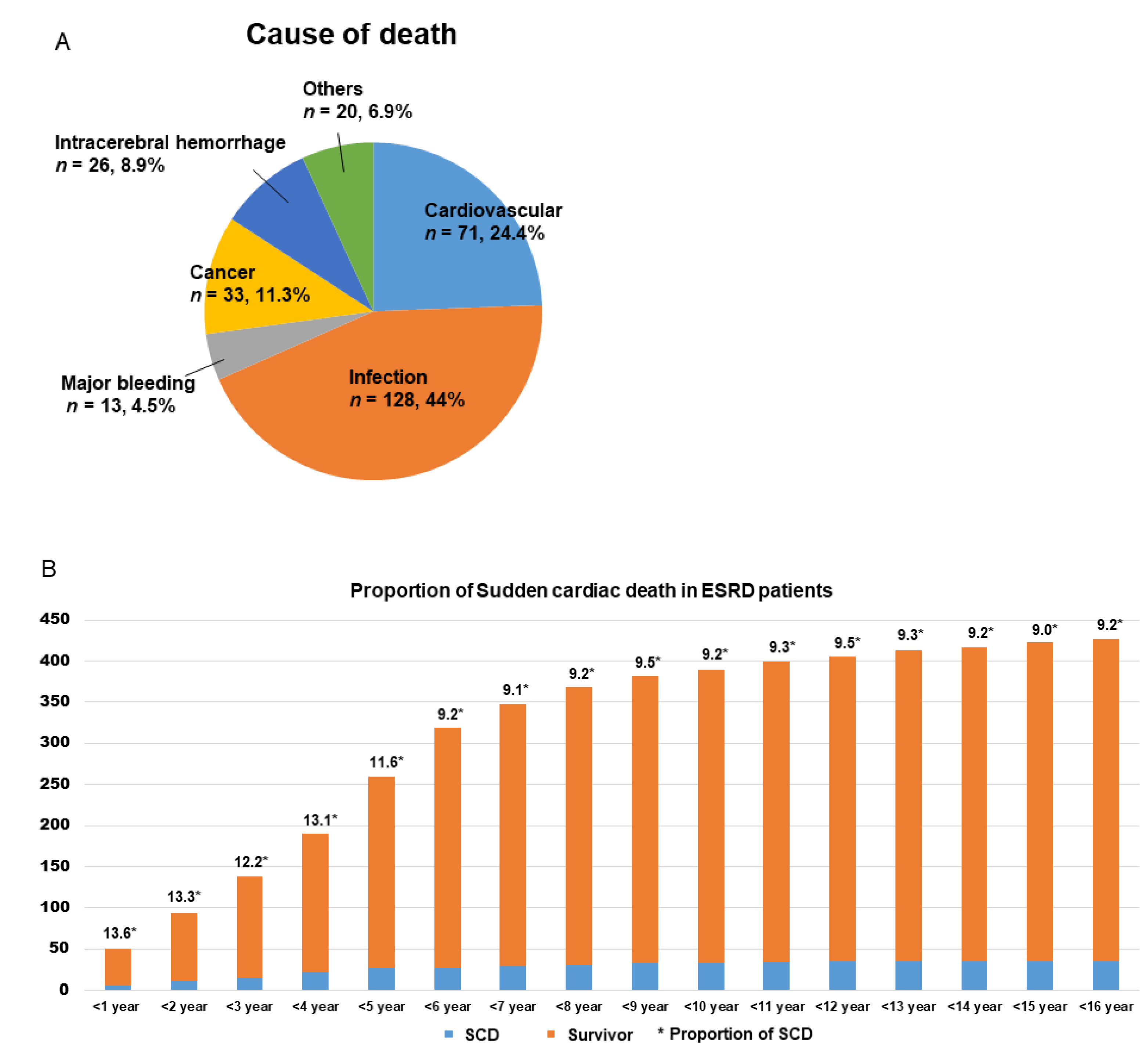
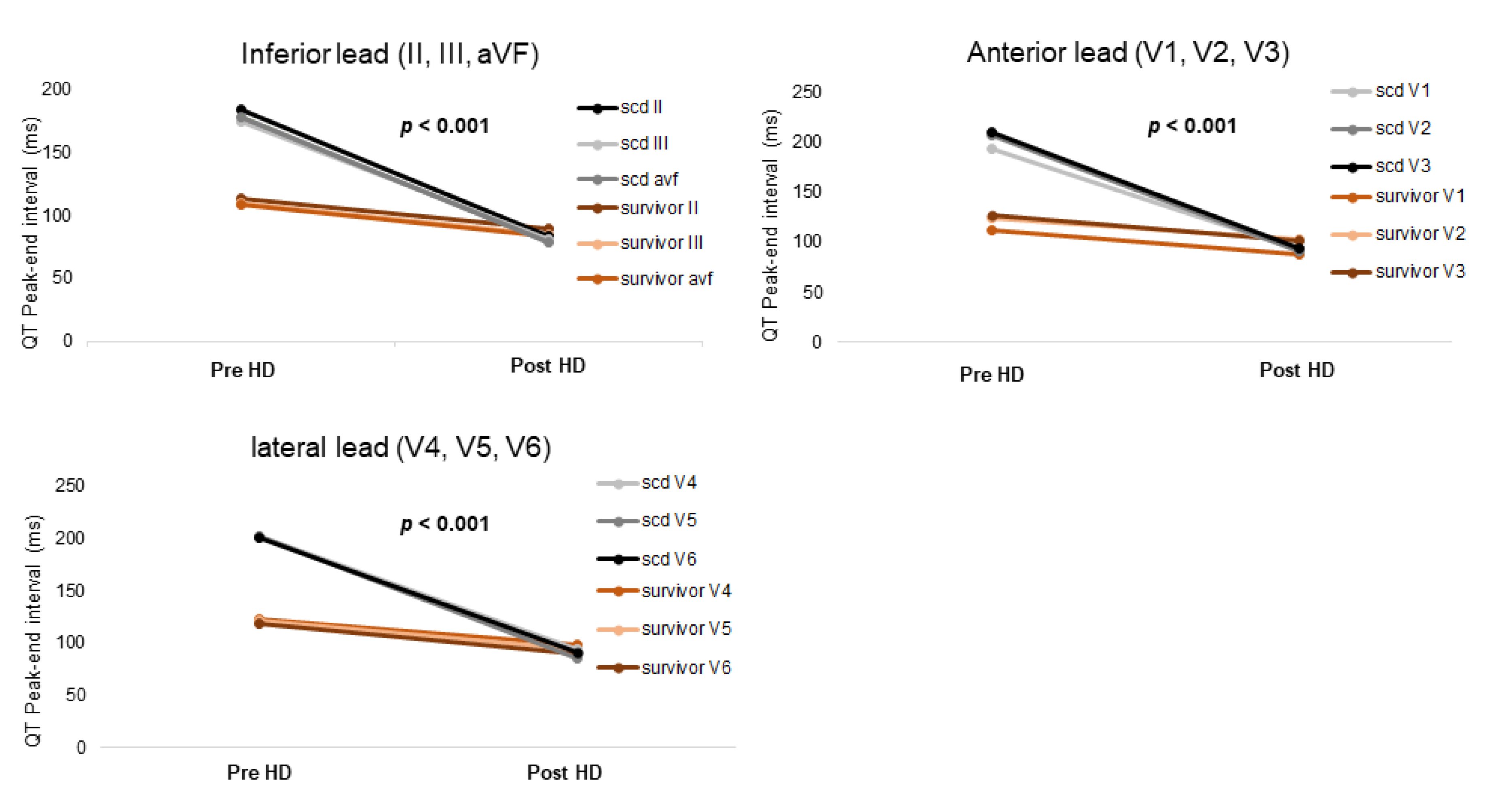
| Variable | Survivor (n = 387) | SCD (n = 39) | p-Value |
|---|---|---|---|
| Age (years) | 59.8 ± 13.7 | 69.2 ± 12.5 | <0.001 |
| Male (n, %) | 189 (48.8) | 25 (64.1) | 0.069 |
| Body surface area (m2) | 1.64 ± 0.19 | 1.66 ± 0.22 | 0.571 |
| Body mass index (kg/m2) | 23.0 ± 3.7 | 22.8 ± 3.9 | 0.78 |
| Hypertension (n, %) | 349 (90.2) | 33 (84.6) | 0.387 |
| Diabetes mellitus (n, %) | 206 (53.2) | 30 (76.9) | 0.003 |
| Duration of HD (months) | 64.5 ± 53.0 | 54.9 ± 52.8 | 0.294 |
| Echocardiography before HD | |||
| Pre-HD LVEF (%) | 59.4 ± 11.3 | 54.8 ± 12.4 | 0.037 |
| Pre-HD LVEDD (mm) | 51.9 ± 6.6 | 53.9 ± 10.1 | 0.367 |
| Pre-HD LVESD (mm) | 35.5 ± 7.3 | 38.1 ± 11.2 | 0.329 |
| Pre-HD E/E′ | 15.6 ± 6.6 | 19.4 ± 7.7 | 0.011 |
| Echocardiography after HD | |||
| Post-HD- HD LVEF (%) | 58.4 ± 11.6 | 46 ± 11.8 | <0.001 |
| Post-HD LVEDD (mm) | 51.2 ± 6.0 | 52.7 ± 10.5 | 0.493 |
| Post-HD LVESD (mm) | 35.3 ± 7.1 | 39.9 ± 10.7 | 0.051 |
| Post-HD E/E′ | 17.2 ± 7.9 | 22.9 ± 9.4 | 0.018 |
| Laboratory findings after HD | |||
| Hb (g/dL) | 10.5 ± 1.4 | 9.3 ± 1.5 | <0.001 |
| Hct (%) | 32.6 ± 7.8 | 28.2 ± 4.8 | 0.017 |
| BUN (mg/dL) | 54.8 ± 21.9 | 58.5 ± 30.7 | 0.611 |
| Cr (mg/dL) | 8.4 ± 3.2 | 6.8 ± 3.7 | 0.039 |
| eGFR (EPI) | 6.5 ± 5.6 | 10.0 ± 8.4 | 0.091 |
| Sodium (mEq/L) | 137 ± 9.8 | 137.6 ± 5.4 | 0.764 |
| Potassium (mEq/L) | 4.9 ± 0.8 | 4.8 ± 1.3 | 0.642 |
| T.Calcium (mg/dL) | 8.9 ± 1.0 | 8.6 ± 0.7 | 0.352 |
| Variable | Survivor (n = 387) | SCD (n = 39) | p-Value |
|---|---|---|---|
| Atrial fibrillation (n, %) | 5 (1.3) | 3 (7.7) | 0.028 |
| Heart rate (/min) | 76.2 ± 14.6 | 80.6 ± 16.7 | 0.141 |
| PR interval (ms) | 168.9 ± 27.6 | 175.2 ± 37.9 | 0.377 |
| QRS duration (ms) | 92.6 ± 14.0 | 100.6 ± 14.9 | 0.015 |
| QT interval (ms) | 405.5 ± 47.9 | 395.6 ± 49.0 | 0.307 |
| QTc interval (ms) | 451.8 ± 28.8 | 456.2 ± 29.9 | 0.449 |
| Dispersion of QT (ms) | 55.5 ± 32.4 | 67.9 ± 22.7 | 0.052 |
| QT Peak-end interval (ms) | |||
| II | 113.4 ± 50.0 | 183.9 ± 67.4 | <0.001 |
| III | 110.0 ± 69.2 | 174.8 ± 72.3 | <0.001 |
| aVF | 108.4 ± 48.4 | 178.4 ± 70.8 | <0.001 |
| V1 | 112.0 ± 49.6 | 192.5 ± 70.5 | <0.001 |
| V2 | 123.9 ± 51.5 | 206.8 ± 67.7 | <0.001 |
| V3 | 126.3 ± 53.3 | 209.7 ± 75.9 | <0.001 |
| V4 | 122.2 ± 54.5 | 201.8 ± 71.8 | <0.001 |
| V5 | 121.1 ± 53.6 | 202.1 ± 76.7 | <0.001 |
| V6 | 118.5 ± 54.4 | 200.3 ± 80.8 | <0.001 |
| Variable | Survivor (n = 387) | SCD (n = 39) | p-Value |
|---|---|---|---|
| Atrial fibrillation (n, %) | 12 (3.1) | 3 (7.7) | 0.138 |
| Heart rate (/min) | 81.9 ± 15.6 | 89.8 ± 22.3 | 0.037 |
| PR interval (ms) | 171.2 ± 31.8 | 151.0 ± 44.6 | 0.106 |
| QRS duration (ms) | 95.2 ± 26.1 | 105.6 ± 33.0 | 0.099 |
| QT interval (ms) | 409.0 ± 51.4 | 396.9 ± 62.7 | 0.279 |
| QTc interval (ms) | 459.6 ± 46.8 | 464.1 ± 47.3 | 0.601 |
| Dispersion of QT (ms) | 52.0 ± 21 | 66.6 ± 43.8 | 0.075 |
| QT Peak-end interval (ms) | |||
| II | 89.1 ± 37.0 | 83.3 ± 37.1 | 0.419 |
| III | 84.2 ± 52.5 | 81.5 ± 35.3 | 0.781 |
| aVF | 83.3 ± 20.3 | 78.4 ± 31.9 | 0.413 |
| V1 | 88.3 ± 22.9 | 92.0 ± 22.0 | 0.407 |
| V2 | 102.1 ± 50.5 | 90.4 ± 21.4 | 0.232 |
| V3 | 102.0 ± 20.0 | 94.2 ± 24.8 | 0.096 |
| V4 | 98.4 ± 26.8 | 93.7 ± 28.3 | 0.382 |
| V5 | 93.5 ± 21.9 | 86.0 ± 22.2 | 0.079 |
| V6 | 90.0 ± 20.5 | 91.3 ± 25.8 | 0.802 |
| Variable | Survivor (n = 387) | SCD (n = 39) | p-Value |
|---|---|---|---|
| QT interval (ms) | −6 (−36, 26) | 38 (−25, 84) | 0.963 |
| QTc interval (ms) | −8.5 (−32, 12) | 1 (−13.5, 58) | 0.937 |
| Dispersion of QT (ms) | 4.3 (−13.9, 27.8) | 32 (1, 48) | 0.02 |
| QT Peak-end interval (ms) | |||
| II | 8 (−9.4, −71.2) | 112 (82, 128) | <0.001 |
| III | 12 (−8, 69.4) | 96 (66, 140) | <0.001 |
| aVF | 12 (−8, 61.8) | 132 (96, 142) | <0.001 |
| V1 | 8 (−12, 64.4) | 140 (98.0−150) | <0.001 |
| V2 | 12 (−8, 63) | 140 (94, 168) | <0.001 |
| V3 | 8.7 (−8, 70.2) | 152 (114, 186) | <0.001 |
| V4 | 8 (−10, 64.7) | 140 (80, 164) | <0.001 |
| V5 | 12 (−9.2, 96.6) | 160 (142, 176) | <0.001 |
| V6 | 12 (−4.2, 66.2) | 152 (90, 174) | <0.001 |
| Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Age | 1.051 | 1.005–1.100 | 0.030 |
| Sex, male | 1.846 | 0.623–5.470 | 0.269 |
| Diabetes mellitus | 3.276 | 0.719–14.936 | 0.125 |
| Atrial fibrillation before hemodialysis | 8.061 | 1.886–34.449 | 0.005 |
| Ejection fraction in echocardiography before hemodialysis | 0.975 | 0.934–1.019 | 0.259 |
| QTpe interval at the V2 lead > 148.1 ms before hemodialysis | 33.793 | 4.446–256.845 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Choe, A.R.; Lee, H.; Ryu, D.R.; Kang, E.W.; Park, J.T.; Lee, S.H.; Park, J. Clinical Associations between Serial Electrocardiography Measurements and Sudden Cardiac Death in Patients with End-Stage Renal Disease Undergoing Hemodialysis. J. Clin. Med. 2021, 10, 1933. https://doi.org/10.3390/jcm10091933
Lee HJ, Choe AR, Lee H, Ryu DR, Kang EW, Park JT, Lee SH, Park J. Clinical Associations between Serial Electrocardiography Measurements and Sudden Cardiac Death in Patients with End-Stage Renal Disease Undergoing Hemodialysis. Journal of Clinical Medicine. 2021; 10(9):1933. https://doi.org/10.3390/jcm10091933
Chicago/Turabian StyleLee, Hyun Jin, A Reum Choe, HaeJu Lee, Dong Ryeol Ryu, Ea Wha Kang, Jung Tak Park, Su Hwan Lee, and Junbeom Park. 2021. "Clinical Associations between Serial Electrocardiography Measurements and Sudden Cardiac Death in Patients with End-Stage Renal Disease Undergoing Hemodialysis" Journal of Clinical Medicine 10, no. 9: 1933. https://doi.org/10.3390/jcm10091933
APA StyleLee, H. J., Choe, A. R., Lee, H., Ryu, D. R., Kang, E. W., Park, J. T., Lee, S. H., & Park, J. (2021). Clinical Associations between Serial Electrocardiography Measurements and Sudden Cardiac Death in Patients with End-Stage Renal Disease Undergoing Hemodialysis. Journal of Clinical Medicine, 10(9), 1933. https://doi.org/10.3390/jcm10091933






