Nucleoside Analogue Reverse Transcriptase Inhibitors Improve Clinical Outcome in Transcriptional Active Human Parvovirus B19-Positive Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analysis of EMB
2.2.1. Genomic DNA Isolation from EMBs
2.2.2. Detection of Viral Genomes in EMBs by Nested-PCR and Sequencing
2.2.3. Measurement of Viral DNA Load by Quantitative Real-Time PCR (TaqMan qPCR)
2.2.4. RNA Isolation, Reverse Transcription (RT), and TaqMan qPCR for Measurement of Viral Transcripts
2.2.5. Histological and Immunohistochemical Staining for Assessment of Inflammation
2.3. Statistics
3. Results
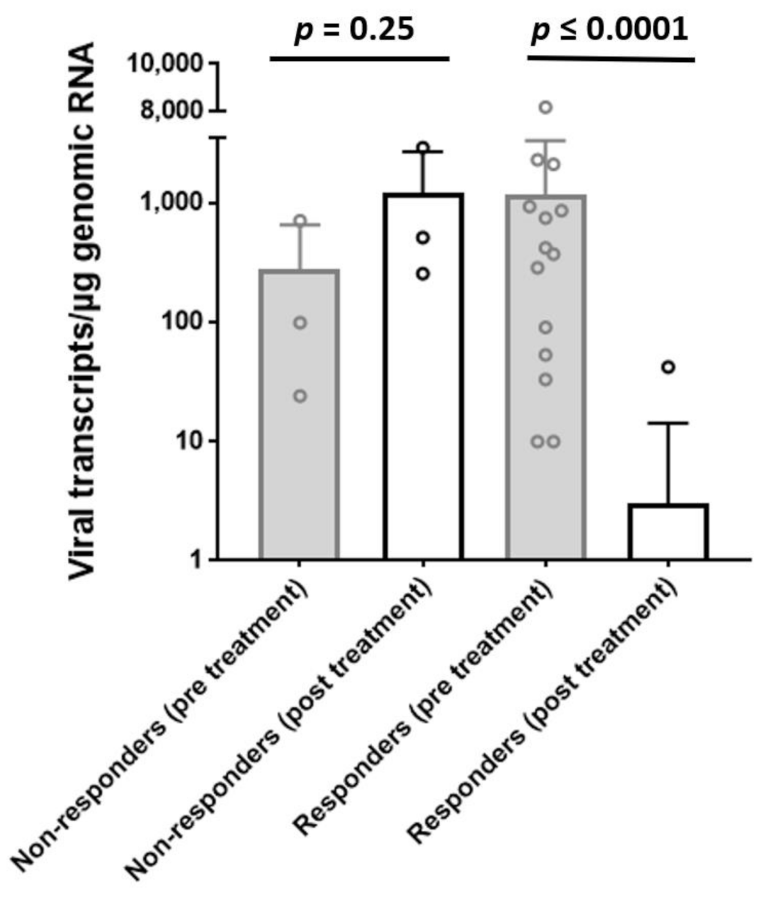
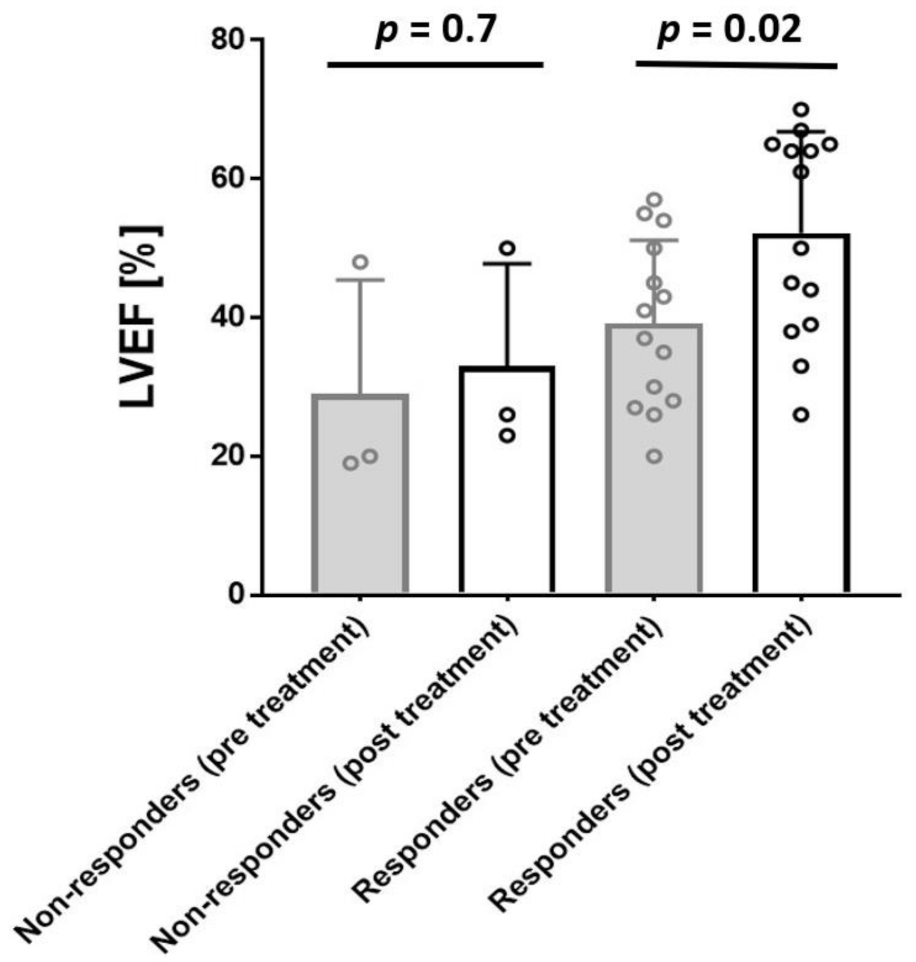
3.1. EMB Analyses at Baseline and Follow-Up
3.2. LTD Side Effects
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bock, C.-T.; Klingel, K.; Aberle, S.; Duechting, A.; Lupescu, A.; Lang, F.; Kandolf, R.; Bock, T. Human Parvovirus B19: A New Emerging Pathogen of Inflammatory Cardiomyopathy. J. Veter-Med. Ser. B 2005, 52, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.-P. High Prevalence of Viral Genomes and Multiple Viral Infections in the Myocardium of Adults with “Idiopathic” Left Ventricular Dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Adamson-Small, L.A.; Ignatovich, I.V.; Laemmerhirt, M.G.; Hobbs, J.A. Persistent parvovirus B19 infection in non-erythroid tissues: Possible role in the inflammatory and disease process. Virus Res. 2014, 190, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.; Hazebroek, M.; Merken, J.; Debing, Y.; Dennert, R.; Rocca, H.-P.B.-L.; Heymans, S. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: Review of the literature. Eur. J. Heart Fail. 2016, 18, 1430–1441. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Kühl, U.; Cooper, L.T. The management of myocarditis. Eur. Heart J. 2011, 32, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, C.; Liljeqvist, J.-Å.; Lindh, M.; Karason, K.; Bollano, E.; Oldfors, A.; Andersson, B. Parvovirus B19 in Endomyocardial Biopsy of Patients With Idiopathic Dilated Cardiomyopathy: Foe or Bystander? J. Card. Fail. 2019, 25, 60–63. [Google Scholar] [CrossRef]
- Verdonschot, J.A.; Cooper, L.T.; Heymans, S.R. Parvovirus B19 in Dilated Cardiomyopathy: There Is More Than Meets the Eye. J. Card. Fail. 2019, 25, 64–66. [Google Scholar] [CrossRef]
- Kuhl, U.; Lassner, D.; Dorner, A.; Rohde, M.; Escher, F.; Seeberg, B.; Hertel, E.; Tschope, C.; Skurk, C.; Gross, U.M.; et al. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res. Cardiol. 2013, 108, 1–10. [Google Scholar] [CrossRef]
- Wan, Z.; Zhi, N.; Wong, S.; Keyvanfar, K.; Liu, D.; Raghavachari, N.; Munson, P.J.; Su, S.; Malide, D.; Kajigaya, S.; et al. Human parvovirus B19 causes cell cycle arrest of human erythroid progenitors via deregulation of the E2F family of transcription factors. J. Clin. Investig. 2010, 120, 3530–3544. [Google Scholar] [CrossRef]
- Heegaard, E.D.; Brown, K.E.; Ablashi, D.V.; Chatlynne, L.G.; Whitman, J.J.E.; Cesarman, E. Human Parvovirus B19. Clin. Microbiol. Rev. 2002, 15, 439–464. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Z.; Xiong, M.; Zou, W.; Deng, X.; Ganaie, S.S.; Kleiboeker, S.; Peng, J.; Liu, K.; Wang, S.; et al. Parvovirus B19 NS1 protein induces cell cycle arrest at G2-phase by activating the ATR-CDC25C-CDK1 pathway. PLoS Pathog. 2017, 13, e1006266. [Google Scholar] [CrossRef]
- Rogo, L.D.; Mokhtari-Azad, T.; Kabir, M.H.; Rezaei, F. Human parvovirus B19: A review. Acta Virol. 2014, 58, 199–213. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Zobel, T.; Escher, F.; Tschöpe, C.; Lassner, D.; Kühl, U.; Gubbe, K.; Volk, H.-D.; Schultheiss, H.-P. Human Parvovirus B19 (B19V) Up-regulates CXCR4 Surface Expression of Circulating Angiogenic Cells: Implications for Cardiac Ischemia in B19V Cardiomyopathy. J. Infect. Dis. 2017, 217, 456–465. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Zobel, T.; Schrepfer, S.; Kuhl, U.; Wang, D.; Klingel, K.; Becher, P.M.; Fechner, H.; Pozzuto, T.; Van Linthout, S.; et al. Impaired Endothelial Regeneration Through Human Parvovirus B19–Infected Circulating Angiogenic Cells in Patients With Cardiomyopathy. J. Infect. Dis. 2015, 212, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Anderson, S.M.; Young, N.S. Erythrocyte P antigen: Cellular receptor for B19 parvovirus. Science 1993, 262, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Bültmann, B.D.; Sotlar, K.; Klingel, K. Parvovirus B19. N. Engl. J. Med. 2004, 350, 2006–2007. [Google Scholar] [CrossRef]
- Kühl, U.; Pauschinger, M.; Bock, T.; Klingel, K.; Schwimmbeck, C.P.L.; Seeberg, B.; Krautwurm, L.; Poller, W.; Schultheiss, H.-P.; Kandolf, R. Parvovirus B19 Infection Mimicking Acute Myocardial Infarction. Circulation 2003, 108, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Bültmann, B.D.; Klingel, K.; Sotlar, K.; Bock, C.; Baba, H.A.; Sauter, M.; Kandolf, R. Fatal parvovirus B19–associated myocarditis clinically mimicking ischemic heart disease: An endothelial cell–mediated disease. Hum. Pathol. 2003, 34, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Elsanhoury, A.; Klein, O.; Sosnowski, M.; Miteva, K.; Lassner, D.; Abou-El-Enein, M.; Pieske, B.; Kühl, U.; Tschöpe, C. Telbivudine in chronic lymphocytic myocarditis and human parvovirus B19 transcriptional activity. ESC Heart Fail. 2018, 5, 818–829. [Google Scholar] [CrossRef]
- Lai, C.-L.; Gane, E.; Liaw, Y.-F.; Hsu, C.-W.; Thongsawat, S.; Wang, Y.; Chen, Y.; Heathcote, E.J.; Rasenack, J.; Bzowej, N.; et al. Telbivudine versus Lamivudine in Patients with Chronic Hepatitis B. N. Engl. J. Med. 2007, 357, 2576–2588. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Telbivudine. Drugs 2007, 67, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.B. Telbivudine: A new nucleoside analogue for the treatment of chronic hepatitis B. Expert Opin. Investig. Drugs 2005, 14, 511–519. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Ye, B.; Yang, X.; Wu, W.; Chen, B.; Pan, X.; Cao, H.; Li, L. Effect of telbivudine therapy on the cellular immune response in chronic hepatitis B. Antivir. Res. 2011, 91, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, M.; Liu, Y.; She, W.; Li, L.; Wang, J.; Jiang, W. Telbivudine therapy may shape CD4+ T-cell response to prevent liver fibrosis in patients with chronic hepatitis B. Liver Int. 2015, 35, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Huang, H.; Sun, X.; Pan, M.; He, Y.; Tan, S.; Zeng, Y.; Li, L.; Deng, G.; Yan, Z.; et al. Telbivudine Prevents Vertical Transmission of Hepatitis B Virus From Women With High Viral Loads: A Prospective Long-Term Study. Clin. Gastroenterol. Hepatol. 2015, 13, 1170–1176. [Google Scholar] [CrossRef]
- Zobel, T.; Bock, C.-T.; Kühl, U.; Rohde, M.; Lassner, D.; Schultheiss, H.-P.; Schmidt-Lucke, C. Telbivudine Reduces Parvovirus B19-Induced Apoptosis in Circulating Angiogenic Cells. Viruses 2019, 11, 227. [Google Scholar] [CrossRef]
- Aretz, H.T. Myocarditis: The Dallas criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef]
- Kühl, U.; Lassner, D.; Pauschinger, M.; Gross, U.; Seeberg, B.; Noutsias, M.; Poller, W.; Schultheiss, H.-P. Prevalence of erythrovirus genotypes in the myocardium of patients with dilated cardiomyopathy. J. Med. Virol. 2008, 80, 1243–1251. [Google Scholar] [CrossRef]
- Pietsch, H.; Escher, F.; Aleshcheva, G.; Lassner, D.; Bock, C.-T.; Schultheiss, H.-P. Detection of parvovirus mRNAs as markers for viral activity in endomyocardial biopsy-based diagnosis of patients with unexplained heart failure. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Lassner, D.; Poller, W.; Westermann, D.; Pieske, B.; Tschöpe, C.; Schultheiss, H.-P. Long-term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive therapy. Clin. Res. Cardiol. 2016, 105, 1011–1020. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Bock, C.-T. Parvovirus B19: A New Emerging Pathogenic Agent of Inflammatory Cardiomyopathy. Chronic Viral Inflamm. Cardiomyopathy 2006, 83–97. [Google Scholar] [CrossRef]
- Bock, C.-T.; Klingel, K.; Kandolf, R. Human Parvovirus B19–Associated Myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.-T.; et al. Presentation, Patterns of Myocardial Damage, and Clinical Course of Viral Myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Rohde, M.; Lassner, D.; Gross, U.; Escher, F.; Schultheiss, H.-P. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012, 37, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Piper, C.; Sowade, O.; Waagstein, F.; Kapp, J.-F.; Wegscheider, K.; Groetzbach, G.; Pauschinger, M.; Escher, F.; Arbustini, E.; et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-β treatment in patients with chronic viral cardiomyopathy. Clin. Res. Cardiol. 2016, 105, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Spillmann, F.; Bock, T.; Kühl, U.; Van Linthout, S.; Schultheiss, H.; Tschöpe, C. Interferon Beta Modulates Endothelial Damage in Patients with Cardiac Persistence of Human Parvovirus B19 Infection. J. Infect. Dis. 2010, 201, 936–945. [Google Scholar] [CrossRef]
- Bonvicini, F.; Bua, G.; Manaresi, E.; Gallinella, G. Enhanced inhibition of parvovirus B19 replication by cidofovir in extendedly exposed erythroid progenitor cells. Virus Res. 2016, 220, 47–51. [Google Scholar] [CrossRef]
- Ganaie, S.S.; Qiu, J. Recent Advances in Replication and Infection of Human Parvovirus B19. Front. Cell. Infect. Microbiol. 2018, 8, 166. [Google Scholar] [CrossRef]
- Luo, Y.; Qiu, J. Human parvovirus B19: A mechanistic overview of infection and DNA replication. Futur. Virol. 2015, 10, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Gish, R.G. Improving outcomes for patients with chronic hepatitis B. Hepatol. Res. 2007, 37, S67–S78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thongsawat, S.; Gane, E.J.; Liaw, Y.; Jia, J.; Hou, J.; Chan, H.L.Y.; Papatheodoridis, G.; Wan, M.; Niu, J.; et al. Efficacy and safety of continuous 4-year telbivudine treatment in patients with chronic hepatitis B. J. Viral Hepat. 2012, 20, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q. Role of natural killer cells (NK) and toll-like receptor 9 (TLR9) in early responder to telbuvidine for chronic hepatitis B patients. Hepatol. Int. 2012, 6, 61. [Google Scholar]
- Ma, L.; Cai, Y.-J.; Yu, L.; Feng, J.-Y.; Wang, J.; Li, C.; Niu, J.-Q.; Jiang, Y.-F. Treatment with Telbivudine Positively Regulates Antiviral Immune Profiles in Chinese Patients with Chronic Hepatitis B. Antimicrob. Agents Chemother. 2013, 57, 1304–1311. [Google Scholar] [CrossRef]
- Pan, X.; Yao, W.; Fu, J.; Liu, M.; Li, L.; Gao, X. Telbivudine improves the function of myeloid dendritic cells in patients with chronic hepatitis B. Acta Virol. 2012, 56, 31–38. [Google Scholar] [CrossRef][Green Version]
- Tzang, B.-S.; Chiu, C.-C.; Tsai, C.-C.; Lee, Y.-J.; Lu, I.-J.; Shi, J.-Y.; Hsu, T.-C. Effects of human parvovirus B19 VP1 unique region protein on macrophage responses. J. Biomed. Sci. 2009, 16, 13. [Google Scholar] [CrossRef]
- Meng, N.; Gao, X.; Yan, W.; Wang, M.; Liu, P.; Lu, X.-D.; Zhang, S.-J.; Lu, Y.-Q.; Tang, W.-X. Efficacy of telbivudine in the treatment of chronic hepatitis b and liver cirrhosis and its effect on immunological responses. Acta Acad. Med. 2015, 35, 230–234. [Google Scholar] [CrossRef]
- Zou, W.; Wang, Z.; Xiong, M.; Chen, A.Y.; Xu, P.; Ganaie, S.S.; Badawi, Y.; Kleiboeker, S.; Nishimune, H.; Ye, S.Q.; et al. Human Parvovirus B19 Utilizes Cellular DNA Replication Machinery for Viral DNA Replication. J. Virol. 2017, 92. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Tattersall, P. Parvovirus DNA Replication. Cold Spring Harb. Monogr. Arch. 1996, 799–813. [Google Scholar]
- Hazebroek, M.R.; Henkens, M.T.; Raafs, A.G.; Verdonschot, J.A.; Merken, J.J.; Dennert, R.M.; Eurlings, C.; Hamid, M.A.A.; Wolffs, P.F.; Winkens, B.; et al. Intravenous immunoglobulin therapy in adult patients with idiopathic chronic cardiomyopathy and cardiac parvovirus B19 persistence: A prospective, double-blind, randomized, placebo-controlled clinical trial. Eur. J. Heart Fail. 2021, 23, 302–309. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | |
| Men, n (%) | 13 (65) |
| Age, years, mean ± SD | 45.7 ± 13.9 |
| History, weeks ± SD | 18.9 ± 11.4 |
| LVEF, % ± SD; [range 25–75] | 37.7 ± 13.5 [26.5–51.0] |
| Systolic blood pressure, mmHg ± SD | 102 ± 27 |
| Diastolic blood pressure, mmHg ± SD | 69 ± 8 |
| Infection preceding onset of symptoms < 12 weeks, % | 70.7 |
| Complaints at Baseline Biopsy | |
| MLHFQ total score, mean ± SD | 68.1 ± 17.7 |
| 6MWD, m, mean ± SD | 468.8 ± 73.3 |
| Dyspnea on exertion, n (%) | 15 (88.2) |
| NYHA class I/II/III/IV, % | 0/47.0/47.0/5.9 |
| Heart Failure Medication | |
| ACE inhibitors/Angiotensin receptor blockers, n (%) | 17 (100) |
| Beta-blockers, n (%) | 15 (88.2) |
| Aldosterone-antagonists, n (%) | 13 (76.4) |
| Diuretics, n (%) | 16 (94.1) |
| Baseline | Follow-Up | p-Value | |
|---|---|---|---|
| LVEF, % ± SD, [range 25–75] | 39.6 ± 12.4 [27.7–52.5] | 52.9 ± 15.7 [38.7–65.0] | p = 0.02 |
| MLHFQ total score, mean ± SD | 66.6 ± 16.4 | 43.2 ± 23.9 | p = 0.03 |
| 6MWD, m, mean ± SD | 465.9 ± 82.8 | 559.8 ± 71.7 | p = 0.04 |
| Dyspnea on exertion, n (%) | 12 (85.7) | 4 (28.5) | p = 0.0006 |
| NYHA class I/II/III/IV, % | 0/57.1/35.7/7.1 | 35.7/64.2/0/0 | p = 0.001 |
| Baseline | Follow-Up | p-Value | |
|---|---|---|---|
| CD3+ cells/mm2 | 8.7 ± 7.7 | 4.7 ± 4.0 | 0.1 |
| LFA-1+ cells/mm2 | 15.9 ± 11.6 | 10.9 ± 8.3 | 0.09 |
| CD45R0+ cells/mm2 | 23.5 ± 15.0 | 20.5 ± 17.5 | 0.4 |
| MAC-1+ cells/mm2 | 42.9 ± 41.5 | 28.7 ± 17.9 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultheiss, H.-P.; Bock, T.; Pietsch, H.; Aleshcheva, G.; Baumeier, C.; Fruhwald, F.; Escher, F. Nucleoside Analogue Reverse Transcriptase Inhibitors Improve Clinical Outcome in Transcriptional Active Human Parvovirus B19-Positive Patients. J. Clin. Med. 2021, 10, 1928. https://doi.org/10.3390/jcm10091928
Schultheiss H-P, Bock T, Pietsch H, Aleshcheva G, Baumeier C, Fruhwald F, Escher F. Nucleoside Analogue Reverse Transcriptase Inhibitors Improve Clinical Outcome in Transcriptional Active Human Parvovirus B19-Positive Patients. Journal of Clinical Medicine. 2021; 10(9):1928. https://doi.org/10.3390/jcm10091928
Chicago/Turabian StyleSchultheiss, Heinz-Peter, Thomas Bock, Heiko Pietsch, Ganna Aleshcheva, Christian Baumeier, Friedrich Fruhwald, and Felicitas Escher. 2021. "Nucleoside Analogue Reverse Transcriptase Inhibitors Improve Clinical Outcome in Transcriptional Active Human Parvovirus B19-Positive Patients" Journal of Clinical Medicine 10, no. 9: 1928. https://doi.org/10.3390/jcm10091928
APA StyleSchultheiss, H.-P., Bock, T., Pietsch, H., Aleshcheva, G., Baumeier, C., Fruhwald, F., & Escher, F. (2021). Nucleoside Analogue Reverse Transcriptase Inhibitors Improve Clinical Outcome in Transcriptional Active Human Parvovirus B19-Positive Patients. Journal of Clinical Medicine, 10(9), 1928. https://doi.org/10.3390/jcm10091928







