Screening and Confirmatory Testing for SARS-CoV-2 Antibodies: Comparison of Health and Non-Health Workers in a Nationwide Healthcare Organization in Central Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Outcomes
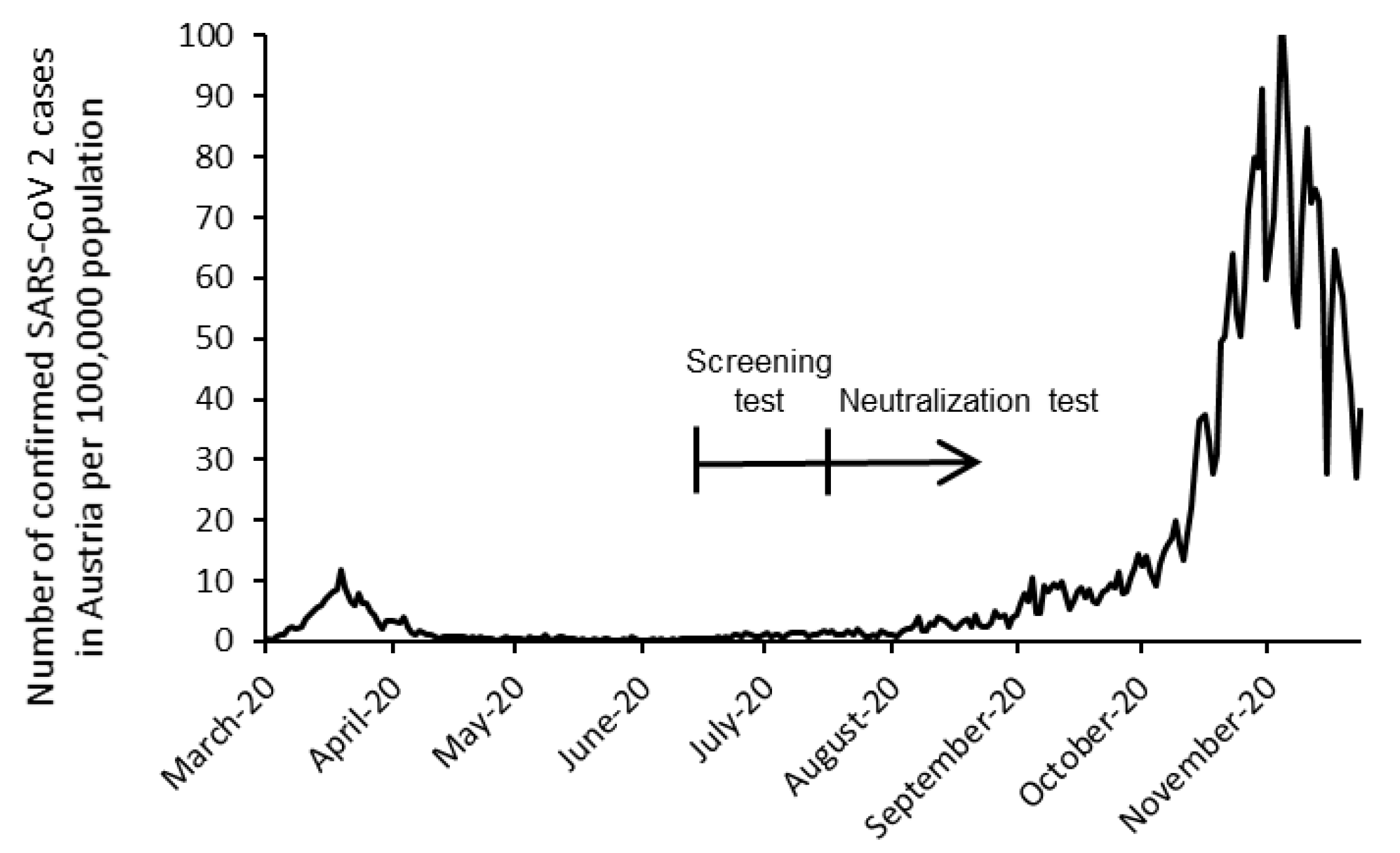
2.4. Screening and Confirmatory Testing
2.5. Statistics
3. Results
3.1. Screening Test (ST)
3.2. Confirmatory Testing (Neutralization Test, NT)
3.3. Healthcare Workers
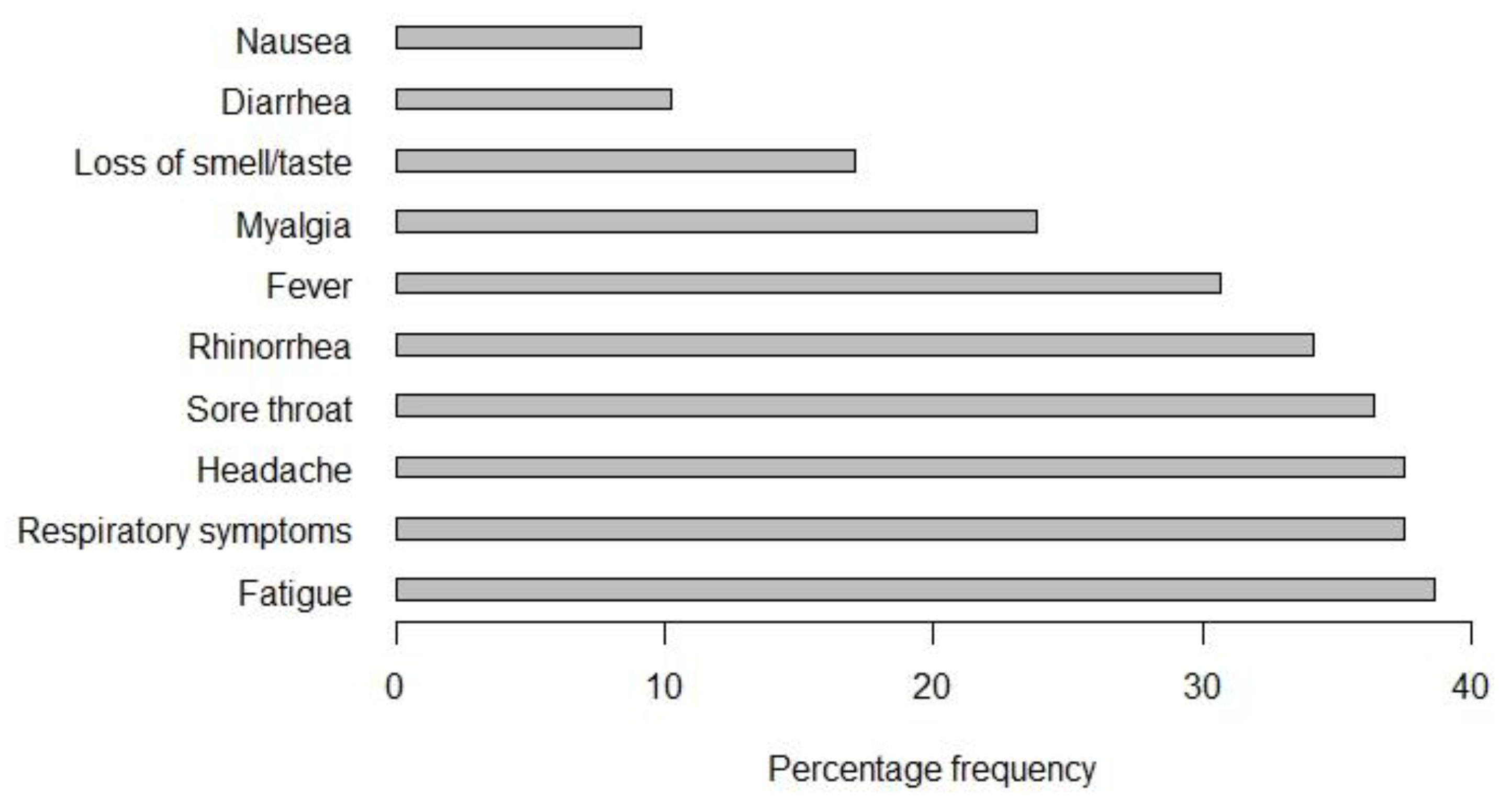
3.4. Symptoms
3.5. PCR
3.6. Performance of the Screening Test
3.7. Serologic Immunoassays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during Covid-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef]
- Pessoa-Amorim, G.; Camm, C.F.; Gajendragadkar, P.; De Maria, G.L.; Arsac, C.; Laroche, C.; Zamorano, J.L.; Weidinger, F.; Achenbach, S.; Maggioni, A.P.; et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: A survey by the European Society of Cardiology. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 210–216. [Google Scholar] [CrossRef]
- Lapolla, P.; Mingoli, A.; Lee, R. Deaths from COVID-19 in healthcare workers in Italy—What can we learn? Infect. Control Hosp. Epidemiol. 2021, 42, 364–365. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Zhou, C.; Liu, Q.; Li, S.; Sun, Q.; Wang, M.; Zhou, Q.; Wang, W. Analysis of the Infection Status of Healthcare Workers in Wuhan During the COVID-19 Outbreak: A Cross-sectional Study. Clin. Infect. Dis. 2020, 71, 2109–2113. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Escribano, P.; Álvarez-Uría, A.; Alonso, R.; Catalán, P.; Alcalá, L.; Muñoz, P.; Guinea, J. Detection of SARS-CoV-2 antibodies is insufficient for the diagnosis of active or cured COVID-19. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Olivieri, L.G.; Rubin, D.; Vallari, A.; Pearce, S.; Olivo, A.; Prostko, J.; Nebbia, G.; Douthwaite, S.; Rodgers, M.; et al. A comparative evaluation between the Abbott Panbio™ COVID-19 IgG/IgM rapid test device and Abbott Architect™ SARS CoV-2 IgG assay. J. Clin. Virol. 2020, 132, 104645. [Google Scholar] [CrossRef] [PubMed]
- Haguet, H.; Douxfils, J.; Eucher, C.; Elsen, M.; Cadrobbi, J.; Tré-Hardy, M.; Dogné, J.; Favresse, J. Clinical performance of the Panbio assay for the detection of SARS-CoV-2 IgM and IgG in COVID-19 patients. J. Med. Virol. 2021, 93, 3277–3281. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Hoo, S.; Liang, Y.; Phua, S.; Aw, T. Performance of two rapid point of care SARS-COV-2 antibody assays against laboratory-based automated chemiluminescent immunoassays for SARS-COV-2 IG-G, IG-M and total antibodies. Pract. Lab. Med. 2021, 24, e00201. [Google Scholar] [CrossRef]
- Moshe, M.; Daunt, A.; Flower, B.; Simmons, B.; Brown, J.C.; Frise, R.; Penn, R.; Kugathasan, R.; Petersen, C.; Stockmann, H.; et al. SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (React 2): Diagnostic accuracy study. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Koblischke, M.; Traugott, M.T.; Medits, I.; Spitzer, F.S.; Zoufaly, A.; Weseslindtner, L.; Simonitsch, C.; Seitz, T.; Hoepler, W.; Puchhammer-Stöckl, E.; et al. Dynamics of CD4 T Cell and Antibody Responses in COVID-19 Patients with Different Disease Severity. Front. Med. 2020, 7, 592629. [Google Scholar] [CrossRef] [PubMed]
- Traugott, M.T.; Hoepler, W.; Seitz, T.; Baumgartner, S.; Karolyi, M.; Pawelka, E.; Friese, E.; Neuhold, S.; Kelani, H.; Thalhammer, F.; et al. Diagnosis of COVID-19 using multiple antibody assays in two cases with negative PCR results from nasopharyngeal swabs. Infection 2021, 49, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kreidl, P.; Schmid, D.; Maritschnik, S.; Richter, L.; Borena, W.; Genger, J.-W.; Popa, A.; Penz, T.; Bock, C.; Bergthaler, A.; et al. Emergence of coronavirus disease 2019 (COVID-19) in Austria. Wien. Klin. Wochenschr. 2020, 132, 645–652. [Google Scholar] [CrossRef]
- Lippi, G.; Simundic, A.-M.; Plebani, M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Mertens, A.N.; Crider, Y.S.; Nguyen, A.; Pokpongkiat, N.N.; Djajadi, S.; Seth, A.; Hsiang, M.S.; Colford, J.M.; Reingold, A.; et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Medical University of Vienna. Coronavirus SARS-Cov-2 Seroprävalenzstudie Bei 1655 Erwerbstätigen Erwachsenen in Einem Österreichischen Unternehmen: Immunitätslage von Berufstätigen Mit Verschiedenen Demographischen Faktoren und Arbeitsverhältnissen. Available online: https://www.meduniwien.ac.at/hp/fileadmin/tropenmedizin/Dokumente_Barbara/News/2020_07_05_Endbericht_Seropr%C3%A4valenz_MedUniWien.pdf (accessed on 8 January 2021).
- Nishiura, H.; Kobayashi, T.; Miyama, T.; Suzuki, A.; Jung, S.-M.; Hayashi, K.; Kinoshita, R.; Yang, Y.; Yuan, B.; Akhmetzhanov, A.R.; et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int. J. Infect. Dis. 2020, 94, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; Felix, S.E.B.; Tie, Y.; Fullerton, K.E. Coronavirus Disease2019 Case Surveillance—United States, 22 January–30 May 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef]
- Fennelly, K.P. Particle sizes of infectious aerosols: Implications for infection control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, A.D.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Ran, L.; Chen, X.; Wang, Y.; Wu, W.; Zhang, L.; Tan, X. Risk Factors of Healthcare Workers with Coronavirus Disease 2019: A Retrospective Cohort Study in a Designated Hospital of Wuhan in China. Clin. Infect. Dis. 2020, 71, 2218–2221. [Google Scholar] [CrossRef]
- Wilson, N.M.; Norton, A.; Young, F.P.; Collins, D.W. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: A narrative review. Anaesthesia 2020, 75, 1086–1095. [Google Scholar] [CrossRef]
- Ye, G.; Lin, H.; Chen, S.; Wang, S.; Zeng, Z.; Wang, W.; Zhang, S.; Rebmann, T.; Li, Y.; Pan, Z.; et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J. Infect. 2020, 81, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-D.; Wang, Z.-Y.; Zhang, S.-F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.-B.; Dong, Y.-Z.; Chi, X.-Y.; et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26, 1583–1591. [Google Scholar] [CrossRef]
- Grant, J.J.; Wilmore, S.M.; McCann, N.S.; Donnelly, O.; Lai, R.W.; Kinsella, M.J.; Rochford, H.L.; Patel, T.; Kelsey, M.C.; Andrews, J.A. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect. Control Hosp. Epidemiol. 2021, 42, 212–214. [Google Scholar] [CrossRef]
- Rudberg, A.-S.; Havervall, S.; Månberg, A.; Falk, A.J.; Aguilera, K.; Ng, H.; Gabrielsson, L.; Salomonsson, A.-C.; Hanke, L.; Murrell, B.; et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Nie, S.; Li, H.; Kong, Y.; Liang, M.; Hou, J.; Huang, X.; Li, D.; Ma, T.; et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat. Med. 2020, 26, 1193–1195. [Google Scholar] [CrossRef]
- Brehm, T.T.; Schwinge, D.; Lampalzer, S.; Schlicker, V.; Küchen, J.; Thompson, M.; Ullrich, F.; Huber, S.; Schmiedel, S.; Addo, M.M.; et al. Seroprevalence of SARS-CoV-2 antibodies among hospital workers in a German tertiary care center: A sequential follow-up study. Int. J. Hyg. Environ. Health 2021, 232, 113671. [Google Scholar] [CrossRef]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M.; et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020, 128, 104437. [Google Scholar] [CrossRef]
- Moscola, J.; Sembajwe, G.; Jarrett, M.; Farber, B.; Chang, T.; McGinn, T.; Davidson, K.W. Prevalence of SARS-CoV-2 Antibodies in Health Care Personnel in the New York City Area. JAMA 2020, 324, 893–895. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Tesoriero, J.M.; Rosenthal, E.M.; Chung, R.; Barranco, M.A.; Styer, L.M.; Parker, M.M.; Leung, S.-Y.J.; Morne, J.E.; Greene, D.; et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann. Epidemiol. 2020, 48, 23–29.e4. [Google Scholar] [CrossRef]
- Rashid-Abdi, M.; Krifors, A.; Sälléber, A.; Eriksson, J.; Månsson, E. Low rate of COVID-19 seroconversion in health-care workers at a Department of Infectious Diseases in Sweden during the later phase of the first wave; a prospective longitudinal seroepidemiological study. Infect. Dis. 2021, 53, 169–175. [Google Scholar] [CrossRef]
- Augustine, R.; Das, S.; Hasan, A.; Abdul Salam, S.; Augustine, P.; Dalvi, Y.B.; Varghese, R.; Primavera, R.; Yassine, H.M.; Thakor, A.S.; et al. Rapid Antibody-Based COVID-19 Mass Surveillance: Relevance, Challenges, and Prospects in a Pandemic andPost-Pandemic World. J. Clin. Med. 2020, 9, 3372. [Google Scholar] [CrossRef]
- Stubblefield, W.B.; Talbot, H.K.; Feldstein, L.R.; Tenforde, M.W.; Rasheed, M.A.U.; Mills, L.; Lester, S.N.; Freeman, B.; Thornburg, N.J.; Jones, I.D.; et al. Seroprevalence of SARS-CoV-2 Among Frontline Healthcare Personnel During the First Month of Caring for Patients With COVID-19—Nashville, Tennessee. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Weidner, L.; Gänsdorfer, S.; Unterweger, S.; Weseslindtner, L.; Drexler, C.; Farcet, M.; Witt, V.; Schistal, E.; Schlenke, P.; Kreil, T.R.; et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J. Clin. Virol. 2020, 129, 104540. [Google Scholar] [CrossRef]
- Tenny, S.; Hoffman, M.R. Prevalence; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ainsworth, M.; Andersson, M.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Beveridge, A.; Bibi, S.; Blackwell, L.; Borak, M.; et al. Performance characteristics of five immunoassays for SARS-CoV-2: A head-to-head benchmark comparison. Lancet Infect. Dis. 2020, 20, 1390–1400. [Google Scholar] [CrossRef]
- Oved, K.; Olmer, L.; Shemer-Avni, Y.; Wolf, T.; Supino-Rosin, L.; Prajgrod, G.; Shenhar, Y.; Payorsky, I.; Cohen, Y.; Kohn, Y.; et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine 2020, 29-30, 100651. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef]
- Hanson, E.K.; Caliendo, A.M.; Arias, A.C.; Englund, A.J.; Hayden, M.K.; Lee, M.J.; Loeb, M.; Patel, R.; Altayar, O.; El Alayli, A.; et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19:Serologic Testing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Ko, J.-H.; Joo, E.-J.; Park, S.-J.; Baek, J.Y.; Kim, W.D.; Jee, J.; Kim, C.J.; Jeong, C.; Kim, Y.-J.; Shon, H.J.; et al. Neutralizing Antibody Production in Asymptomatic and Mild COVID-19 Patients, in Comparison with Pneumonic COVID-19 Patients. J. Clin. Med. 2020, 9, 2268. [Google Scholar] [CrossRef] [PubMed]

| HCW (n = 2242) | Non-HCW (n = 5301) | |
|---|---|---|
| Age Group, n (%) | ||
| 18–39 years | 1071 (48) | 1399 (26) |
| 40–49 years | 589 (26) | 1502 (28) |
| 50–59 years | 521 (23) | 2143 (41) |
| 60–69 years | 61 (3) | 257 (5) |
| Gender, n (%) | ||
| Female | 1118 (50) | 4194 (79) |
| Male | 1124 (50) | 1107 (21) |
| Healthcare Workers (n = 23) | Non-Healthcare Workers (n = 65) | |
|---|---|---|
| Age group, n (%) | ||
| 18–39 years | 8 (35) | 14 (22) |
| 40–49 years | 6 (26) | 23 (35) |
| 50–59 years | 9 (39) | 26 (40) |
| 60–69 years | 0 | 2 (3) |
| Gender, n (%) | ||
| Female | 15 (65) | 42 (65) |
| Male | 8 (35) | 23 (35) |
| Job function, n (%) | ||
| Nurse | 9 (39) | – |
| Nursing assistance | 4 (17) | – |
| Physician | 4 (17) | – |
| Physiotherapist | 2 (9) | – |
| Dental assistance | 2 (9) | – |
| Other healthcare personnel | 2 (9) | |
| Administrative and clerical | – | 59 (91) |
| Cleaning service | – | 4 (6) |
| Dietary service | – | 1 (1.5) |
| Maintenance service | – | 1 (1.5) |
| Chronic medical conditions, n (%) | ||
| Hypertension | 6 (26) | 6 (9) |
| Cancer | 0 | 0 |
| Diabetes mellitus | 0 | 3 (5) |
| Chronic lung disease | 2 (9) | 3 (5) |
| Autoimmune disease | 1 (4) | 2 (3) |
| Cardiac disease | 1 (4) | 1 (1.5) |
| Previous PCR testing, n (%) | ||
| Positive | 5 (22) | 8 (12) |
| Negative | 4 (17) | 6 (9) |
| Not performed | 14 (61) | 51 (79) |
| Neutralization Test | Level | Negative | Positive |
|---|---|---|---|
| n | 39 | 88 | |
| Diasorin (%) | neg | 38 (97.4) | 52 (59.1) |
| pos | 1 (2.6) | 36 (40.9) | |
| Euroimmun (%) | neg | 39 (100) | 51 (58) |
| pos | 0 (0) | 37 (42) | |
| Abbott (%) | neg | 37 (94.9) | 50 (56.8) |
| pos | 2 (5.1) | 38 (43.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartko, J.; Zehetmayer, S.; Weseslindtner, L.; Stiasny, K.; Schloegl, A.; Forjan, E.; Zwettler, E.; Krauter, A.; Keil, F.; Sédille-Mostafaie, N. Screening and Confirmatory Testing for SARS-CoV-2 Antibodies: Comparison of Health and Non-Health Workers in a Nationwide Healthcare Organization in Central Europe. J. Clin. Med. 2021, 10, 1909. https://doi.org/10.3390/jcm10091909
Bartko J, Zehetmayer S, Weseslindtner L, Stiasny K, Schloegl A, Forjan E, Zwettler E, Krauter A, Keil F, Sédille-Mostafaie N. Screening and Confirmatory Testing for SARS-CoV-2 Antibodies: Comparison of Health and Non-Health Workers in a Nationwide Healthcare Organization in Central Europe. Journal of Clinical Medicine. 2021; 10(9):1909. https://doi.org/10.3390/jcm10091909
Chicago/Turabian StyleBartko, Johann, Sonja Zehetmayer, Lukas Weseslindtner, Karin Stiasny, Andrea Schloegl, Ernst Forjan, Elisabeth Zwettler, Andreas Krauter, Felix Keil, and Nazanin Sédille-Mostafaie. 2021. "Screening and Confirmatory Testing for SARS-CoV-2 Antibodies: Comparison of Health and Non-Health Workers in a Nationwide Healthcare Organization in Central Europe" Journal of Clinical Medicine 10, no. 9: 1909. https://doi.org/10.3390/jcm10091909
APA StyleBartko, J., Zehetmayer, S., Weseslindtner, L., Stiasny, K., Schloegl, A., Forjan, E., Zwettler, E., Krauter, A., Keil, F., & Sédille-Mostafaie, N. (2021). Screening and Confirmatory Testing for SARS-CoV-2 Antibodies: Comparison of Health and Non-Health Workers in a Nationwide Healthcare Organization in Central Europe. Journal of Clinical Medicine, 10(9), 1909. https://doi.org/10.3390/jcm10091909






