Urinary Proteomics Reveals Promising Biomarkers in Menstrually Related and Post-Menopause Migraine
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Procedures
2.2. Sample Collection and Pre-Analytical Treatment
2.3. Mono-Dimensional Gel Electrophoresis (SDS-PAGE)
2.4. Two-Dimensional Gel Electrophoresis (2DE)
2.5. LC-MS/MS Analysis
2.6. Western Blot Analysis
2.7. Statistics
3. Results
3.1. SDS-PAGE and LC-MS/MS Analysis
3.2. DE and LC-MS/MS Identification
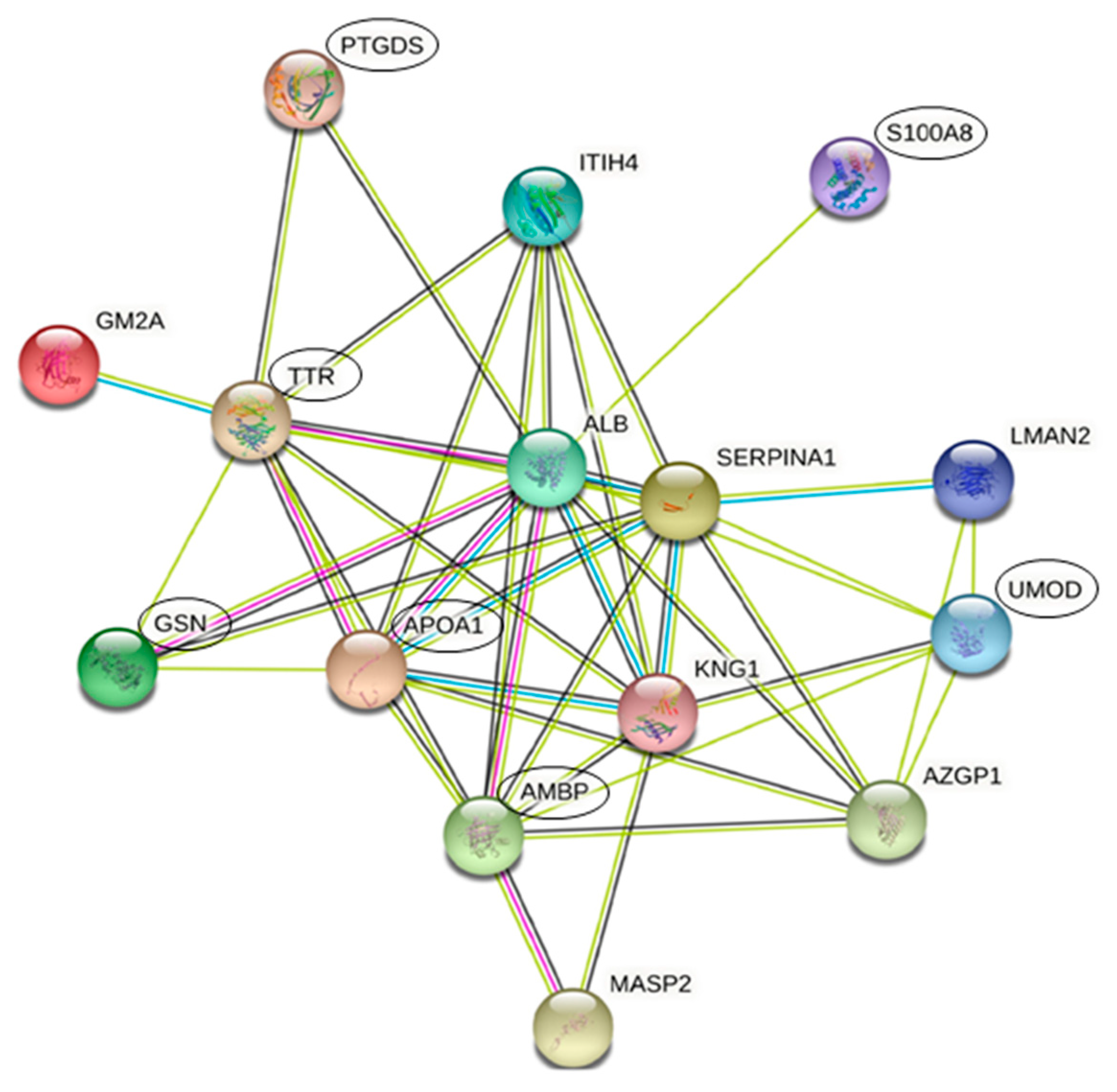
3.3. Protein Interactions Network by STRING
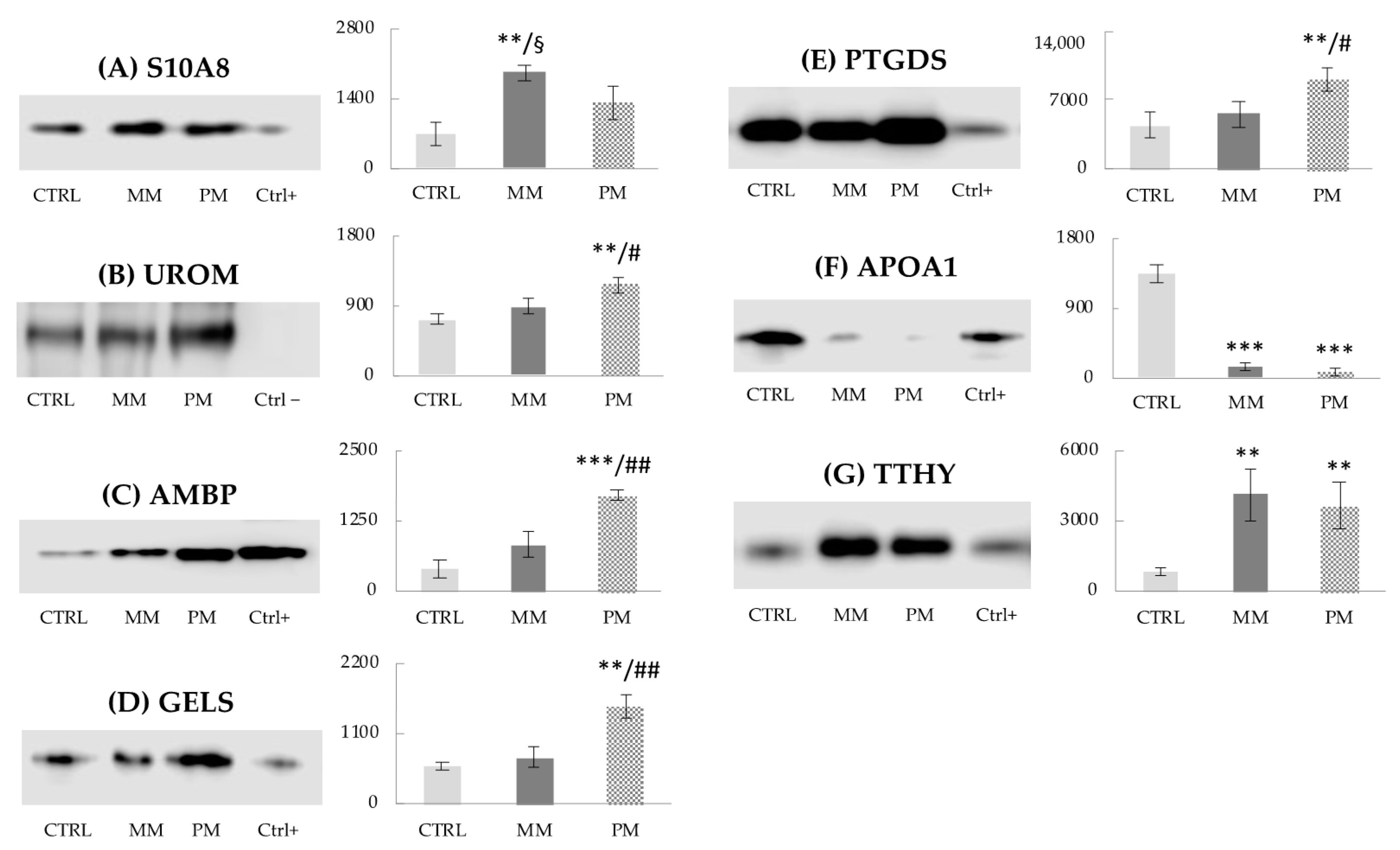
3.4. Western Blot Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A1AT | Alpha-1-antitrypsin |
| ALBU | Albumin |
| AMBP | Alpha-1-microglobulin |
| APOA1 | Apolipoprotein A-I |
| CVD | Cardiovascular disease |
| 2DE | Two-dimensional gel electrophoresis |
| GELS | Gelsolin |
| IGHG2 | Immunoglobulin heavy constant gamma 2 |
| IGKC | Immunoglobulin kappa constant |
| ITIH4 | Inter-alpha-trypsin inhibitor heavy chain H4 |
| KNG1 | Kininogen-1 |
| KVD20 | Immunoglobulin kappa variable 3D20 |
| LC-MS/MS | Liquid chromatography/mass spectrometry |
| LMAN2 | Vesicular integral-membrane protein VIP36 |
| MASP2 | Mannan-binding lectin serine protease 2 |
| MM | Menstrually related migraine group |
| MOH | Medication overuse headache |
| PEBP1 | Phosphatidylethanolamine-binding protein 1 |
| PEPA3 | Pepsin A-3 |
| PM | Post-menopause migraine group |
| PTGDS | Prostaglandin-H2 D-isomerase |
| RS27A | Ubiquitin-40S ribosomal protein S27a |
| S10A8 | Protein S100-A8 |
| SAP3 | Ganglioside GM2 activator |
| SDS-PAGE | Mono-dimensional gel electrophoresis |
| TTHY | Transthyretin |
| UROM | Uromodulin |
| ZA2G | Zinc-alpha-2-glycoprotein |
References
- Ansari, T.; Lagman-Bartolome, A.M.; Monsour, D.; Lay, C. Management of menstrual migraine. Curr. Neurol. Neurosci. Rep. 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A.; Victor, T.W.; Hu, X.; Xiang, Q.; Puenpatom, R.A.; Chen, W.; Campbell, J.C. Characteristics of menstrual vs nonmenstrual migraine: A post hoc, within-woman analysis of the usual-care phase of a nonrandomized menstrual migraine clinical trial. Headache 2010, 50, 528–538. [Google Scholar] [CrossRef]
- Pavlovic, J.M. The impact of midlife on migraine in women: Summary of current views. Women Midlife Health 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.M.; Paemeleire, K.; Gobel, H.; Bonner, J.; Rapoport, A.; Kagan, R.; Zhang, F.; Picard, H.; Mikol, D.D. Efficacy and safety of erenumab in women with a history of menstrual migraine. J. Headache Pain 2020, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Roberts, L.; Elkins, G. Complementary and alternative medicine for menopause. J. Evid. Based Integr. Med. 2019, 24, 1–14. [Google Scholar] [CrossRef]
- Ripa, P.; Ornello, R.; Degan, D.; Tiseo, C.; Stewart, J.; Pistoia, F.; Carolei, A.; Sacco, S. Migraine in menopausal women: A systematic review. Int. J. Women Health 2015, 7, 773–782. [Google Scholar] [CrossRef]
- MacGregor, E.A. Perimenopausal migraine in women with vasomotor symptoms. Maturitas 2012, 71, 79–82. [Google Scholar] [CrossRef]
- Bernstein, C.; O’Neal, M.A. Migraine and menopause—A narrative review. Menopause 2021, 28, 96–101. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and their applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef]
- Alexovic, M.; Sabo, J.; Longuespée, R. Microproteomic sample preparation. Proteomics 2021, e2000318. [Google Scholar] [CrossRef]
- Lionetto, L.; Gentile, G.; Bellei, E.; Capi, M.; Sabato, D.; Marsibilio, F.; Simmaco, M.; Pini, L.A.; Martelletti, P. The omics in migraine. J. Headache Pain 2013, 14, 55. [Google Scholar] [CrossRef]
- Bellei, E.; Cuoghi, A.; Monari, E.; Bergamini, S.; Fantoni, L.I.; Zappaterra, M.; Guerzoni, S.; Bazzocchi, A.; Tomasi, A.; Pini, L.A. Proteomic analysis of urine in medication-overuse headache patients: Possible relation with renal damages. J. Headache Pain 2012, 13, 45–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellei, E.; Monari, E.; Cuoghi, A.; Bergamini, S.; Guerzoni, S.; Ciccarese, M.; Ozben, T.; Tomasi, A.; Pini, L.A. Discovery by a proteomic approach of possible early biomarkers of drug-induced nephrotoxicity in medication-overuse headache. J. Headache Pain 2013, 14, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellei, E.; Monari, E.; Bergamini, S.; Cuoghi, A.; Tomasi, A.; Guerzoni, S.; Ciccarese, M.; Pini, L.A. Validation of potential candidate biomarkers of drug-induced nephrotoxicity and allodynia in medication-overuse headache. J. Headache Pain 2015, 16, 77. [Google Scholar] [CrossRef][Green Version]
- Pellesi, L.; Bellei, E.; Guerzoni, S.; Cainazzo, M.M.; Baraldi, C.; Monari, E.; Pini, L.A. Exploration of candidate serum biomarkers potentially related to the chronic pain conditions in medication-overuse headache. BMC Neurol. 2019, 19, 239. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Guerzoni, S.; Baraldi, C.; Cainazzo, M.M.; Pini, L.A.; Bellei, E. Identification of candidate proteomic markers in the serum of medication overuse headache patients: An exploratory study. Cephalalgia 2020, 40, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Bellei, E.; Vilella, A.; Monari, E.; Bergamini, S.; Tomasi, A.; Cuoghi, A.; Guerzoni, S.; Manca, L.; Zoli, M.; Pini, L.A. Serum protein changes in a rat model of chronic pain show a correlation between animal and humans. Sci. Rep. 2017, 7, 41723. [Google Scholar] [CrossRef] [PubMed]
- Bellei, E.; Rustichelli, C.; Bergamini, S.; Monari, E.; Baraldi, C.; Lo Castro, F.; Tomasi, A.; Ferrari, A. Proteomic serum profile in menstrual-related and post menopause migraine. J. Pharm. Biomed. Anal. 2020, 184, 113165. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Fazelinia, H.; Spruce, L.A.; Weiss, D.A.; Zderic, S.A.; Seeholzer, S.H. Urine proteomics: Evaluation of different sample preparation workflows for quantitative, reproducible, and improved depth of analysis. J. Proteome Res. 2020, 19, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Ferroni, P.; Barbanti, P.; Spila, A.; Fratangeli, F.; Aurilia, C.; Fofi, L.; Egeo, G.; Guadagni, F. Circulating biomarkers in migraine: New opportunities for precision medicine. Curr. Med. Chem. 2018, 26, 6191–6206. [Google Scholar] [CrossRef]
- Koike, A.; Arai, A.; Yamada, S.; Nagae, A.; Saita, N.; Itoh, H.; Uemoto, S.; Totani, M.; Ikemoto, M. Dynamic mobility of immunological cells expressing S100A8 and S100A9 in vivo: A variety of functional roles of the two proteins as regulators in acute inflammatory reaction. Inflammation 2012, 35, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Wu, Y. Contact pathway of coagulation and inflammation. Thromb. J. 2015, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Mukhtar, A.; Yan, S.; Holland, A. Kallikreins and kinins in inflammatory-like events in the reproductive tract. Pharmacol. Res. 1997, 35, 537–540. [Google Scholar] [CrossRef]
- Grande, G.; Milardi, D.; Vincenzoni, F.; Ricciardi, D.; Mancini, F.; Di Nicuolo, F.; Castagnola, M.; Pontecorvi, A.; Marana, R. Identification of novel putative urinary markers of endometriosis by high-resolution quantitative proteomics. Biomed. J. Sci. Tech. Res. 2020, 28, 21475–21481. [Google Scholar] [CrossRef]
- Abdullah-Soheimi, S.S.; Lim, B.K.; Hashim, O.H.; Shuib, A.S. Patients with ovarian carcinoma excrete altered levels of urine CD59, kininogen-1 and fragments of inter-alpha-trypsin inhibitor heavy chain H4 and albumin. Proteome Sci. 2010, 8, 58. [Google Scholar] [CrossRef]
- Devuyst, O.; Bochud, M. Uromodulin, kidney function, cardiovascular disease, and mortality. Kidney Int. 2015, 88, 944–946. [Google Scholar] [CrossRef]
- Vyssoulis, G.P.; Tousoulis, D.; Antoniades, C.; Dimitrakopoulos, S.; Zervoudaki, A.; Stefanadis, C. α-1-microglobulin as a new inflammatory marker in newly diagnosed hypertensive patients. Am. J. Hypertens. 2007, 20, 1016–1021. [Google Scholar] [CrossRef][Green Version]
- Bettencourt, C.; Foti, S.C.; Miki, Y.; Botia, J.; Chatterjee, A.; Warner, T.T.; Revesz, T.; Lashley, T.; Balazs, R.; Viré, E.; et al. White matter DNA methylation profiling reveals deregulation of HIP1, LMAN2, MOBP, and other loci in multiple system atrophy. Acta Neuropathol. 2020, 139, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Zinc α2-glycoprotein: A multidisciplinary protein. Mol. Cancer Res. 2008, 6, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Ryan, A.S. Zinc-α2-glycoprotein expression in adipose tissue of obese postmenopausal women before and after weight loss and exercise + weight loss. Metab. Clin. Exp. 2014, 63, 995–999. [Google Scholar] [CrossRef]
- Moller-Kristensen, M.; Jensenius, J.C.; Jensen, L.; Thielens, N.; Rossi, V.; Arlaud, G.; Thiel, S. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J. Immunol. Methods 2003, 282, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, C.L.; Petri Nahas, E.A.; Nahas-Neto, J.; Orsatti, F.L.; Linhares, I.M.; Witkin, S.S. Mannose-binding lectin gene polymorphism and risk factors for cardiovascular disease in postmenopausal women. Mol. Immunol. 2014, 61, 23–27. [Google Scholar] [CrossRef]
- Orsini, F.; Chrysanthou, E.; Dudler, T.; Cummings, W.J.; Takahashi, M.; Fujita, T.; Demopulos, G.; De Simoni, M.; Schwaeble, W. Mannan binding lectin-associated serine protease-2 (MASP-2) critically contributes to post-ischemic brain injury independent of MASP-1. J. Neuroinflamm. 2016, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Al-Kraity, W.R.H.; Al-Dujaili, A.N.G. Assessment of gelsolin level in women with heart disease after menopause. Res. J. Pharm. Technol. 2017, 10, 1657–1660. [Google Scholar] [CrossRef]
- Pikel, E.; Levental, I.; Durnas, B.; Janmey, P.A.; Bucki, R. Plasma gelsolin: Indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int. J. Mol. Sci. 2018, 19, 2516. [Google Scholar] [CrossRef]
- Cupini, L.M.; Corbelli, I.; Sarchelli, P. Menstrual migraine: What it is and does it matter? J. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, I.A.L.; da Cruz, E.J.S.N.; Fontenele, A.M.M.; Figueiredo Neto, J.A.d. Alterations in postmenopausal plasmatic lipidome. PLoS ONE 2018, 13, e0203027. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Summ, O.; Gregor, N.; Marziniak, M.; Gralow, I.; Husstedt, I.W.; Evers, S. Cluster headache and alpha 1-antitrypsin deficiency. Cephalalgia 2010, 30, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.; Alves, C.H.; Quintela, T.; Baltazar, G.; Socorro, S.; Saraiva, M.J.; Abreu, R.; Santos, C.R.A. Transthyretin is up-regulated by sex hormones in mice liver. Mol. Cell. Biochem. 2008, 317, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Prendecki, M.; Kozubski, W.; Lianeri, M.; Dorszewska, J. Molecular factors in migraine. Oncotarget 2016, 7, 50708–50718. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, Y.; Wang, C.; Liao, H.; Zhou, S.; Li, D.; Huang, S.; Shi, Y.; Wang, Z.; Chen, J.; et al. Brain structural and functional differences between pure menstrual migraine and menstrually-related migraine. Sci. Rep. 2020, 10, 16454. [Google Scholar] [CrossRef]
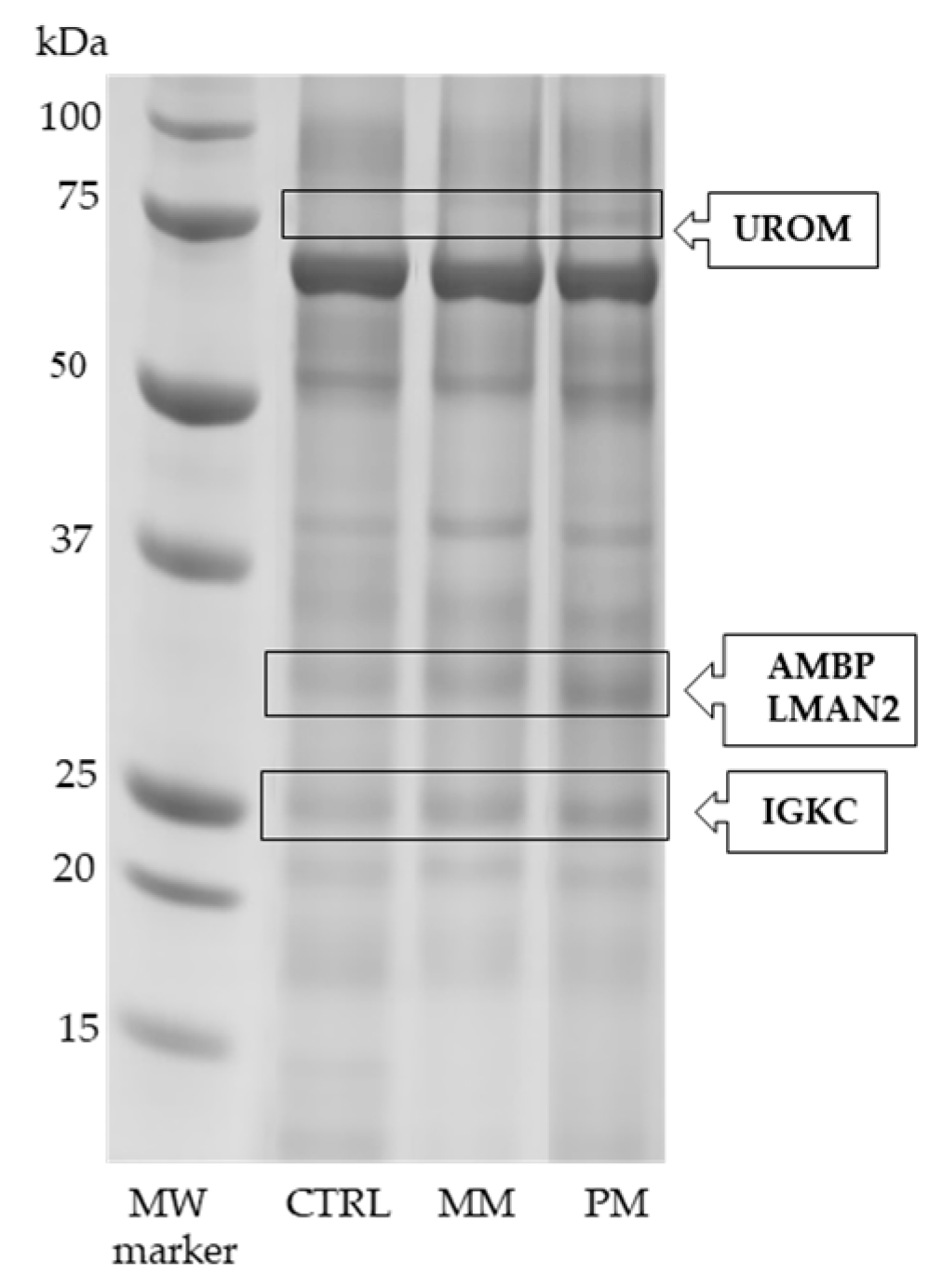

| Protein Full Name | Entry Name (a) | Fold Change (b) | p-Value (c) |
|---|---|---|---|
| Uromodulin | UROM | +2.00 * +1.95 # | 0.013 0.011 |
| Alpha-1-microglobulin Vesicular integral-membrane protein VIP36 | +2.51 * +1.67 # | 0.001 0.005 | |
| Immunoglobulin kappa constant | IGKC | +1.58 * | 0.013 |
| Entry (a) | Protein Full Name | Acc. (b) | Gene (c) | Score (d) | Pep./Seq. (e) | emPAI (f) | Change (g) | Function (h) |
|---|---|---|---|---|---|---|---|---|
| S10A8 | Protein S100-A8 | P05109 | S100A8 | 174 | 9/5 | 4.31 | Up (1) | Inflammation |
| KNG1 | Kininogen-1 | P01042 | KNG1 | 314 | 12/10 | 0.68 | Up (1) | Inflammation |
| ALBU | Albumin | P02768 | ALB | 122 | 8/8 | 0.54 | Up (1) | Multifunction |
| IGHG2 | Immunoglobulin heavy constant gamma 2 | P01859 | IGHG2 | 98 | 4/4 | 0.51 | Up (1) | Immune response |
| PEBP1 | Phosphatidylethanolamine-binding protein1 | P30086 | PEBP1 | 50 | 5/5 | 1.45 | Up (1) | Neurotransmitter synthesis |
| ITIH4 | Inter-alpha-trypsin inhibitor heavy chain H4 (fragment) | Q14624 | ITIH4 | 125 | 6/4 | 0.16 | Up (2) | Inflammation |
| UROM | Uromodulin | P07911 | UMOD | 190 | 3/3 | 1.10 | Up (2) | Regulation |
| AMBP | Alpha-1-microglobulin | P02760 | AMBP | 818 | 41/10 | 2.10 | Up (2) | Inhibition |
| LMAN2 | Vesicular integral-membrane protein VIP36 | Q12907 | LMAN2 | 260 | 16/8 | 1.10 | Up (2) | Transport |
| ZA2G | Zinc-alpha-2-glycoprotein | P25311 | AZGP1 | 65 | 3/2 | 0.12 | Up (2) | Immune response |
| MASP2 | Mannan-binding lectin serine protease 2 | O00187 | MASP2 | 192 | 11/5 | 0.28 | Up (2) | Inflammation modulation |
| GELS | Gelsolin | P06396 | GSN | 30 | 3/3 | 0.14 | Up (2) | Inflammation |
| PTGDS | Prostaglandin-H2 D-isomerase | P41222 | PTGDS | 466 | 20/6 | 3.06 | Up (2) | Inflammatory mediator |
| SAP3 | Ganglioside GM2 activator | P17900 | GM2A | 162 | 10/6 | 1.84 | Up (2) | Lipid transport |
| IGKC | Immunoglobulin kappa constant | P01834 | IGKC | 228 | 10/4 | 2.41 | Up (2) | Immunity |
| KVD20 | Immunoglobulin kappa variable 3D20 | A0C4DH25 | IGKV3D-20 | 122 | 4/3 | 1.39 | Up (2) | Immunity |
| RS27A | Ubiquitin-40S ribosomal protein S27a | P62979 | RPS27A | 104 | 8/4 | 1.24 | Up (2) | Metabolic process |
| APOA1 | Apolipoprotein A-I | P02647 | APOA1 | 50 | 3/3 | 0.45 | Down (3) | Lipid metabolism |
| A1AT | Alpha-1-antitrypsin | P01009 | SERPINA1 | 400 | 19/13 | 2.16 | Down (3) | Anti-inflammatory |
| TTHY | Transthyretin | P02766 | TTR | 50 | 3/2 | 0.59 | Up (3) | Hormone binding |
| PEPA3 | Pepsin A-3 | PP0DJD8 | PGA3 | 98 | 6/2 | 0.35 | Up (3) | Regulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellei, E.; Bergamini, S.; Rustichelli, C.; Monari, E.; Dal Porto, M.; Fiorini, A.; Tomasi, A.; Ferrari, A. Urinary Proteomics Reveals Promising Biomarkers in Menstrually Related and Post-Menopause Migraine. J. Clin. Med. 2021, 10, 1854. https://doi.org/10.3390/jcm10091854
Bellei E, Bergamini S, Rustichelli C, Monari E, Dal Porto M, Fiorini A, Tomasi A, Ferrari A. Urinary Proteomics Reveals Promising Biomarkers in Menstrually Related and Post-Menopause Migraine. Journal of Clinical Medicine. 2021; 10(9):1854. https://doi.org/10.3390/jcm10091854
Chicago/Turabian StyleBellei, Elisa, Stefania Bergamini, Cecilia Rustichelli, Emanuela Monari, Michele Dal Porto, Alessandro Fiorini, Aldo Tomasi, and Anna Ferrari. 2021. "Urinary Proteomics Reveals Promising Biomarkers in Menstrually Related and Post-Menopause Migraine" Journal of Clinical Medicine 10, no. 9: 1854. https://doi.org/10.3390/jcm10091854
APA StyleBellei, E., Bergamini, S., Rustichelli, C., Monari, E., Dal Porto, M., Fiorini, A., Tomasi, A., & Ferrari, A. (2021). Urinary Proteomics Reveals Promising Biomarkers in Menstrually Related and Post-Menopause Migraine. Journal of Clinical Medicine, 10(9), 1854. https://doi.org/10.3390/jcm10091854







