Spectrum of DICER1 Germline Pathogenic Variants in Ovarian Sertoli–Leydig Cell Tumor
Abstract
1. Introduction
2. Genetics of DICER1
3. DICER1 Somatic Mutations in Ovarian Sertoli–Leydig Cell Tumor
4. DICER1 Pathogenic Germline Variants in Ovarian Sertoli–Leydig Cell Tumor
4.1. Literature Review
4.2. The Patients
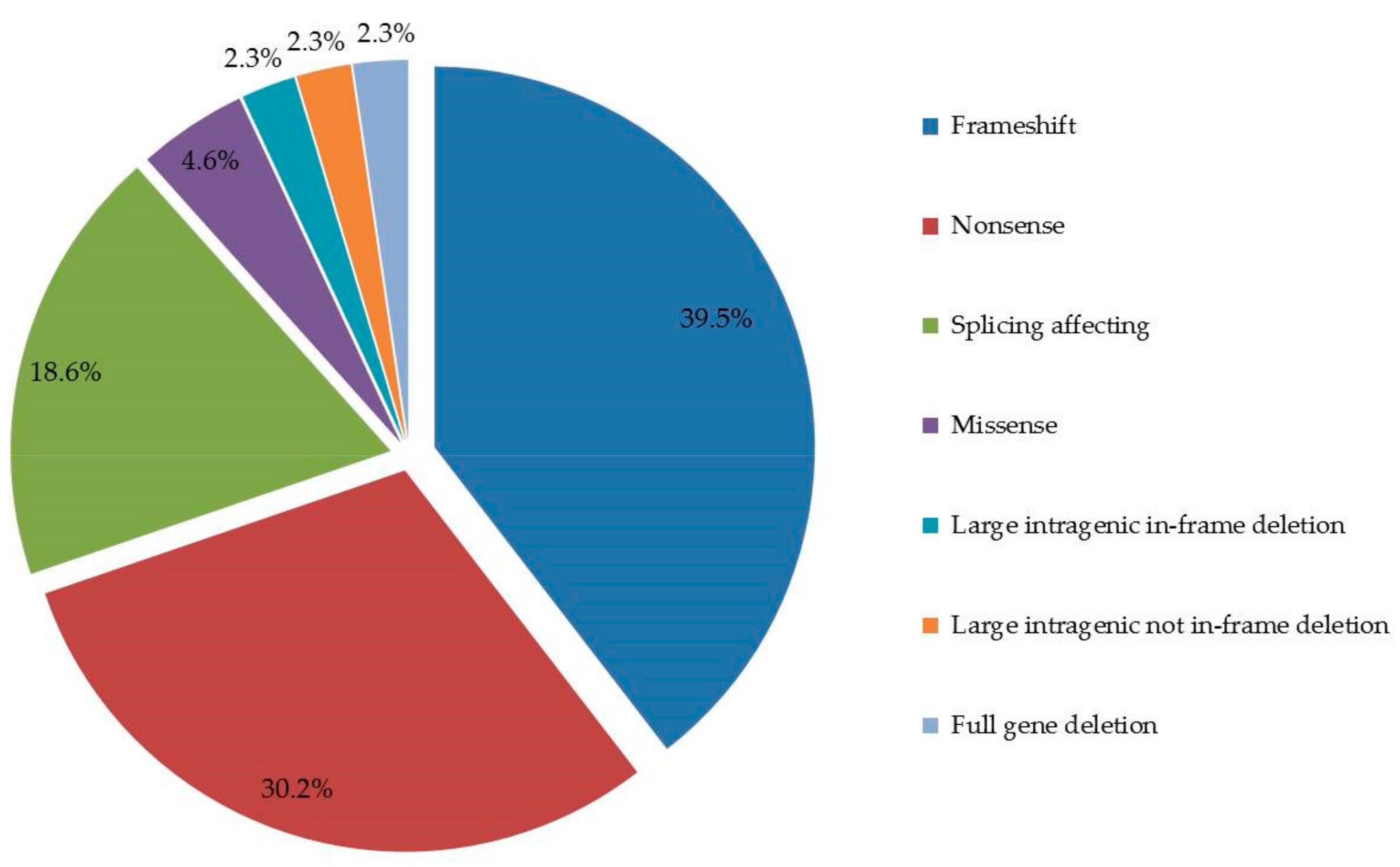
4.3. Germline DICER1 Alterations
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuller, P.J.; Leung, D.; Chu, S. Genetics and genomics of ovarian sex cord-stromal tumors. Clin. Genet. 2017, 91, 285–291. [Google Scholar] [CrossRef]
- Sigismondi, C.; Gadducci, A.; Lorusso, D.; Candiani, M.; Breda, E.; Raspagliesi, F.; Cormio, G.; Marinaccio, M.; Mangili, G. Ovarian Sertoli-Leydig cell tumors. A retrospective MITO study. Gynecol. Oncol. 2012, 125, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Macut, D.; Ilić, D.; Mitrović Jovanović, A.; Bjekić-Macut, J. Androgen-Secreting Ovarian Tumors. Front. Horm. Res. 2019, 53, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Cao, D.; Shen, K.; Yang, J.; Zhang, Y.; Yu, Q.; Wan, X.; Xiang, Y.; Xiao, Y.; Guo, L. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol. Oncol. 2012, 127, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Akman, L.; Ertas, I.E.; Gokcu, M.; Terek, M.C.; Sanci, M.; Sanli, U.A.; Zekioglu, O.; Ozsaran, A.A. Ovarian sertoli-leydig cell tumors: A multicenter long-term clinicopathological analysis of 27 patients. J. Cancer Res. Ther. 2016, 12, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.A.; Lim, Y.K.; Chia, Y.N.; Yam, K.L. Sertoli-Leydig cell tumor of the ovary: Analysis of a single institution database. J. Obstet. Gynaecol. Res. 2013, 39, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, B.; Zuo, J.; Feng, X.; Li, X.; Zhang, R.; Wu, L. Ovarian Sertoli-Leydig cell tumor: A report of seven cases and a review of the literature. Gynecol. Endocrinol. 2013, 29, 192–195. [Google Scholar] [CrossRef]
- Castro, B.G.R.; Souza, C.P.; Andrade, C.E.M.D.C.; Vieira, M.A.; Andrade, D.A.P.; Reis, R.D. Ovarian Sertoli-Leydig Cell Tumors: Epidemiological, Clinical and Prognostic Factors. Rev. Bras. Ginecol. Obstet. 2019, 41, 440–448. [Google Scholar] [CrossRef]
- WHO Classification of Tumours. Available online: https://tumourclassification.iarc.who.int/welcome/ (accessed on 23 April 2021).
- Sahoo, T.K.; Kar, T.; Kar, A.; Panda, S. Poorly differentiated Sertoli-Leydig cell tumour of ovary with heterologous elements. J. Clin. Diagn. Res. 2017, 11, XD01–XD02. [Google Scholar] [CrossRef]
- Young, R.H.; Scully, R.E. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am. J. Surg. Pathol. 1985, 9, 543–569. [Google Scholar] [CrossRef]
- Nam, S.M.; Kim, J.W.; Eoh, K.J.; Kim, H.M.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. A novel clinicopathological analysis of early stage ovarian Sertoli-Leydig cell tumors at a single institution. Obstet. Gynecol. Sci. 2017, 60, 39–45. [Google Scholar] [CrossRef]
- Appetecchia, M.; Cela, V.; Bernardi, F.; Burelli, A.; Cionini, R.; Pucci, E. Sertoli-Leydig cell androgens-estrogens secreting tumor of the ovary: Ultra-conservative surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 116, 113–116. [Google Scholar] [CrossRef]
- Brown, J.; Sood, A.K.; Deavers, M.T.; Milojevic, L.; Gershenson, D.M. Patterns of metastasis in sex cord-stromal tumors of the ovary: Can routine staging lymphadenectomy be omitted? Gynecol. Oncol. 2009, 113, 86–90. [Google Scholar] [CrossRef]
- Gouy, S.; Arfi, A.; Maulard, A.; Pautier, P.; Bentivegna, E.; Leary, A.; Chargari, C.; Genestie, C.; Morice, P. Results from a Monocentric Long-Term Analysis of 23 Patients with Ovarian Sertoli-Leydig Cell Tumors. Oncologist 2019, 24, 702–709. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Wang, Y.; Keul, J.; Tessier-Cloutier, B.; Magrill, J.; Kommoss, S.; Senz, J.; Yang, W.; Proctor, L.; Schmidt, D.; et al. DICER1 and FOXL2 Mutation Status Correlates with Clinicopathologic Features in Ovarian Sertoli-Leydig Cell Tumors. Am. J. Surg. Pathol. 2019, 43, 628–638. [Google Scholar] [CrossRef]
- Robertson, J.C.; Jorcyk, C.L.; Oxford, J.T. DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers 2018, 10, 143. [Google Scholar] [CrossRef]
- Schultz, K.A.P.; Stewart, D.R.; Kamihara, J.; Bauer, A.J.; Merideth, M.A.; Stratton, P.; Huryn, L.A.; Harris, A.K.; Doros, L.; Field, A.; et al. DICER1 Tumor Predisposition. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; 2014 Apr 24 [Updated 2020 Apr 30]; University of Washington: Seattle, WA, USA, 2014; pp. 1993–2021. [Google Scholar]
- DICER1 Syndrome Children’s Hospital of Philadelphia. Available online: http://www.chop.edu/conditions-diseases/dicer1-syndrome (accessed on 28 January 2021).
- De Kock, L.; Wu, M.K.; Foulkes, W.D. Ten years of DICER1 mutations: Provenance, distribution, and associated phenotypes. Hum. Mutat. 2019, 40, 1939–1953. [Google Scholar] [CrossRef]
- Thunders, M.; Delahunt, B. Gene of the month: DICER1: Ruler and controller. J. Clin. Pathol. 2021, 74, 69–72. [Google Scholar] [CrossRef]
- Song, M.S.; Rossi, J.J. Molecular mechanisms of Dicer: Endonuclease and enzymatic activity. Biochem. J. 2017, 474, 1603–1618. [Google Scholar] [CrossRef]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Yang, N.; Greshock, J.; Megraw, M.S.; Giannakakis, A.; Liang, S.; Naylor, T.L.; Barchetti, A.; Ward, M.R.; et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 9136–9141. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, M.; Romano, G.; Wernicke, D.; Croce, C.M. MicroRNA and cancer-a brief overview. Adv. Biol. Regul. 2015, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kian, R.; Moradi, S.; Ghorbian, S. Role of components of microRNA machinery in carcinogenesis. Exp. Oncol. 2018, 40, 2–9. [Google Scholar] [CrossRef]
- Guillerman, R.P.; Foulkes, W.D.; Priest, J.R. Imaging of DICER1 syndrome. Pediatr. Radiol. 2019, 49, 1488–1505. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Priest, J.R.; Duchaine, T.F. DICER1: Mutations, microRNAs and mechanisms. Nat. Rev. Cancer 2014, 14, 662–672. [Google Scholar] [CrossRef]
- Brenneman, M.; Field, A.; Yang, J.; Williams, G.; Doros, L.; Rossi, C.; Hill, D.A. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in pleuropulmonary blastoma/DICER1 syndrome: A unique variant of the two-hit tumor suppression model. F1000 Res. 2015, 4, 214. [Google Scholar] [CrossRef]
- Stewart, D.R.; Best, A.F.; Williams, G.M.; Harney, L.A.; Carr, A.G.; Harris, A.K.; Kratz, C.P.; Dehner, L.P.; Messinger, Y.H.; Rosenberg, P.S.; et al. Neoplasm Risk Among Individuals with a Pathogenic Germline Variant in DICER1. J. Clin. Oncol. 2019, 37, 668–676. [Google Scholar] [CrossRef]
- de Kock, L.; Wang, Y.C.; Revil, T.; Badescu, D.; Rivera, B.; Sabbaghian, N.; Wu, M.; Weber, E.; Sandoval, C.; Hopman, S.M.; et al. High-sensitivity sequencing reveals multi-organ somatic mosaicism causing DICER1 syndrome. J. Med. Genet. 2016, 53, 43–52. [Google Scholar] [CrossRef]
- Klein, S.; Lee, H.; Ghahremani, S.; Kempert, P.; Ischander, M.; Teitell, M.A.; Nelson, S.F.; Martinez-Agosto, J.A. Expanding the phenotype of mutations in DICER1: Mosaic missense mutations in the RNase IIIb domain of DICER1 cause GLOW syndrome. J. Med. Genet. 2014, 51, 294–302. [Google Scholar] [CrossRef]
- Rakheja, D.; Chen, K.S.; Liu, Y.; Shukla, A.A.; Schmid, V.; Chang, T.C.; Khokhar, S.; Wickiser, J.E.; Karandikar, N.J.; Malter, J.S.; et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat. Commun. 2014, 2, 4802. [Google Scholar] [CrossRef]
- Schultz, K.A.; Pacheco, M.C.; Yang, J.; Williams, G.M.; Messinger, Y.; Hill, D.A.; Dehner, L.P.; Priest, J.R. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: A report from the International Pleuropulmonary Blastoma Registry. Gynecol. Oncol. 2011, 122, 246–250. [Google Scholar] [CrossRef]
- Heravi-Moussavi, A.; Anglesio, M.S.; Cheng, S.W.; Senz, J.; Yang, W.; Prentice, L.; Fejes, A.P.; Chow, C.; Tone, A.; Kalloger, S.E.; et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N. Engl. J. Med. 2012, 366, 234–242. [Google Scholar] [CrossRef]
- Witkowski, L.; Mattina, J.; Schönberger, S.; Murray, M.J.; Choong, C.S.; Huntsman, D.G.; Reis-Filho, J.S.; McCluggage, W.G.; Nicholson, J.C.; Coleman, N.; et al. DICER1 hotspot mutations in non-epithelial gonadal tumours. Br. J. Cancer. 2013, 109, 2744–2750. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.H.; Yoo NJLee, S.H. DICER1 exons 25 and 26 mutations are rare in common human tumours besides Sertoli-Leydig cell tumour. Histopathology 2013, 63, 436–438. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, M.Z.; Liu, F.Y.; Yang, B.C.; Wang, L.Q.; Wang, F.; Yu, X.H.; Wan, L.; Wan, X.D.; Xu, X.Y.; et al. Absence of DICER1, CTCF, RPL22, DNMT3A, TRRAP, IDH1 and IDH2 hotspot mutations in patients with various subtypes of ovarian carcinomas. Biomed. Rep. 2015, 3, 33–37. [Google Scholar] [CrossRef]
- Goulvent, T.; Ray-Coquard, I.; Borel, S.; Haddad, V.; Devouassoux-Shisheboran, M.; Vacher-Lavenu, M.C.; Pujade-Laurraine, E.; Savina, A.; Maillet, D.; Gillet, G.; et al. DICER1 and FOXL2 mutations in ovarian sex cord-stromal tumours: A GINECO Group study. Histopathology 2016, 68, 279–285. [Google Scholar] [CrossRef]
- Conlon, N.; Schultheis, A.M.; Piscuoglio, S.; Silva, A.; Guerra, E.; Tornos, C.; Reuter, V.E.; Soslow, R.A.; Young, R.H.; Oliva, E.; et al. A survey of DICER1 hotspot mutations in ovarian and testicular sex cord-stromal tumors. Mod. Pathol 2015, 28, 1603–1612. [Google Scholar] [CrossRef]
- Kato, N.; Kusumi, T.; Kamataki, A.; Tsunoda, R.; Fukase, M.; Kurose, A. DICER1 hotspot mutations in ovarian Sertoli-Leydig cell tumors: A potential association with androgenic effects. Hum. Pathol. 2017, 59, 41–47. [Google Scholar] [CrossRef]
- de Kock, L.; Terzic, T.; McCluggage, W.G.; Stewart, C.J.R.; Shaw, P.; Foulkes, W.D.; Clarke, B.A. DICER1 Mutations Are Consistently Present in Moderately and Poorly Differentiated Sertoli-Leydig Cell Tumors. Am. J. Surg. Pathol. 2017, 41, 1178–1187. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Yang, W.; Mo, F.; Senz, J.; Yap, D.; Anglesio, M.S.; Gilks, B.; Morin, G.B.; Huntsman, D.G. The oncogenic roles of DICER1 RNase IIIb domain mutations in ovarian Sertoli-Leydig cell tumors. Neoplasia 2015, 17, 650–660. [Google Scholar] [CrossRef]
- Canfarotta, M.; Riba-Wolman, R.; Orsey, A.D.; Balarezo, F.; Finck, C. DICER1 syndrome and thyroid disease. J. Pediatr. Surg. Case Rep. 2016, 11, 31–34. [Google Scholar] [CrossRef][Green Version]
- Haley, M.; Bindal, P.; McAuliffe, A.; Vredenburgh, J. A family with Sertoli-Leydig cell tumour, multinodular goiter, and DICER1 mutation. Curr. Oncol. 2019, 26, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Huo, X.; Jiang, D.; Yu, M.; Cao, D.; Wu, H.; Shen, K.; Yang, J.; Zhang, Y.; Zhou, H.; et al. Clinical Characteristics and Mutation Analyses of Ovarian Sertoli-Leydig Cell Tumors. Oncologist 2020, 25, e1396–e1405. [Google Scholar] [CrossRef]
- Rio Frio, T.; Bahubeshi, A.; Kanellopoulou, C.; Hamel, N.; Niedziela, M.; Sabbaghian, N.; Pouchet, C.; Gilbert, L.; O’Brien, P.K.; Serfas, K.; et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA 2011, 305, 68–77. [Google Scholar] [CrossRef]
- Cowan, M.; Suntum, T.; Olivas, A.D.; Perpich, M.; Applebaum, M.A.; Lastra, M.M.; Yamada, S.D. Second primary rhabdomyosarcoma of the uterine cervix presenting with synchronous ovarian Sertoli-Leydig cell tumor: An illustrative case of DICER1 syndrome. Gynecol. Oncol. Rep. 2018, 25, 94–97. [Google Scholar] [CrossRef]
- Pugh, T.J.; Yu, W.; Yang, J.; Field, A.L.; Ambrogio, L.; Carter, S.L.; Cibulskis, K.; Giannikopoulos, P.; Kiezun, A.; Kim, J.; et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene 2014, 33, 5295–5302. [Google Scholar] [CrossRef]
- Stewart, D.R.; Messinger, Y.; Williams, G.M.; Yang, J.; Field, A.; Schultz, K.A.; Harney, L.A.; Doros, L.A.; Dehner, L.P.; Hill, D.A. Nasal chondromesenchymal hamartomas arise secondary to germline and somatic mutations of DICER1 in the pleuropulmonary blastoma tumor predisposition disorder. Hum. Genet. 2014, 133, 1443–1450. [Google Scholar] [CrossRef]
- Schultz, K.A.; Yang, J.; Doros, L.; Williams, G.M.; Harris, A.; Stewart, D.R.; Messinger, Y.; Field, A.; Dehner, L.P.; Hill, D.A. DICER1-pleuropulmonary blastoma familial tumor predisposition syndrome: A unique constellation of neoplastic conditions. Pathol. Case Rev. 2014, 19, 90–100. [Google Scholar] [CrossRef]
- Darrat, I.; Bedoyan, J.K.; Chen, M.; Schuette, J.L.; Lesperance, M.M. Novel DICER1 mutation as cause of multinodular goiter in children. Head Neck 2013, 35, E369–E371. [Google Scholar] [CrossRef]
- Oost, E.E.; Charles, A.; Choong, C.S.; Leung, Y.C.; Salfinger, S.; Sonnendecker, H.; Tan, J.; Townshend, S.; Witkowski, L.; Foulkes, W.D.; et al. Ovarian sex cord-stromal tumors in patients with probable or confirmed germline DICER1 mutations. Int. J. Gynecol. Pathol. 2015, 34, 266–274. [Google Scholar] [CrossRef]
- Schrader, K.A.; Cheng, D.T.; Joseph, V.; Prasad, M.; Walsh, M.; Zehir, A.; Ni, A.; Thomas, T.; Benayed, R.; Ashraf, A.; et al. Germline Variants in Targeted Tumor Sequencing Using Matched Normal DNA. JAMA Oncol. 2016, 2, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Luke, A.M.; Moroney, J.W.; Snitchler, A.; Whiteway, S.L. Ovarian Sertoli-Leydig Cell Tumor with Elevated Inhibin B as a Cause of Secondary Amenorrhea in an Adolescent with Germ Line DICER1 Mutation. J. Pediatr. Adolesc. Gynecol. 2017, 30, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Slade, I.; Bacchelli, C.; Davies, H.; Murray, A.; Abbaszadeh, F.; Hanks, S.; Barfoot, R.; Burke, A.; Chisholm, J.; Hewitt, M.; et al. DICER1 syndrome: Clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J. Med. Genet. 2011, 48, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, M.; Hong, Y.; Zhong, Y.; Cong, X.; Chen, C.; Liu, Z.; Man, Y.; Yang, L. Sertoli-Leydig cell tumor in two siblings with DICER1 syndrome: A case report and literature review. Medicine 2020, 99, e20806. [Google Scholar] [CrossRef]
- Sabbaghian, N.; Srivastava, A.; Hamel, N.; Plourde, F.; Gajtko-Metera, M.; Niedziela, M.; Foulkes, W.D. Germ-line deletion in DICER1 revealed by a novel MLPA assay using synthetic oligonucleotides. Eur. J. Hum. Genet. 2014, 22, 564–567. [Google Scholar] [CrossRef]
- Chen, K.S.; Stuart, S.H.; Stroup, E.K.; Shukla, A.S.; Wang, J.; Rajaram, V.; Vujanic, G.M.; Slone, T.; Rakheja, D.; Amatruda, J.F. Distinct DICER1 Hotspot Mutations Identify Bilateral Tumors as Separate Events. JCO Precis. Oncol. 2018, 2, PO.17.00113. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, D.; Li, Y.; Bian, L.; Ma, T.; Xie, M. DICER1 mutations in a patient with an ovarian Sertoli-Leydig tumor, well-differentiated fetal adenocarcinoma of the lung, and familial multinodular goiter. Eur. J. Med. Genet. 2014, 57, 621–625. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Bahubeshi, A.; Hamel, N.; Pasini, B.; Asioli, S.; Baynam, G.; Choong, C.S.; Charles, A.; Frieder, R.P.; Dishop, M.K.; et al. Extending the phenotypes associated with DICER1 mutations. Hum. Mutat. 2011, 32, 1381–1384. [Google Scholar] [CrossRef]
- Rossing, M.; Gerdes, A.M.; Juul, A.; Rechnitzer, C.; Rudnicki, M.; Nielsen, F.C.; Vo Hansen, T. A novel DICER1 mutation identified in a female with ovarian Sertoli-Leydig cell tumor and multinodular goiter: A case report. J. Med. Case Rep. 2014, 8, 112. [Google Scholar] [CrossRef]
- de Kock, L.; Sabbaghian, N.; Druker, H.; Weber, E.; Hamel, N.; Miller, S.; Choong, C.S.; Gottardo, N.G.; Kees, U.R.; Rednam, S.P.; et al. Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol. 2014, 128, 583–595. [Google Scholar] [CrossRef]
- van Engelen, K.; Villani, A.; Wasserman, J.D.; Aronoff, L.; Greer, M.C.; Tijerin Bueno, M.; Gallinger, B.; Kim, R.H.; Grant, R.; Meyn, M.S.; et al. DICER1 syndrome: Approach to testing and management at a large pediatric tertiary care center. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef]
- Apellaniz-Ruiz, M.; de Kock, L.; Sabbaghian, N.; Guaraldi, F.; Ghizzoni, L.; Beccuti, G.; Foulkes, W.D. Familial multinodular goiter and Sertoli-Leydig cell tumors associated with a large intragenic in-frame DICER1 deletion. Eur. J. Endocrinol. 2018, 178, K11–K19. [Google Scholar] [CrossRef]
- Verrier, F.; Dubois d’Enghien, C.; Gauthier-Villars, M.; Bonadona, V.; Faure-Conter, C.; Dijoud, F.; Stoppa-Lyonnet, D.; Houdayer, C.; Golmard, L. Mutiple DICER1-related lesions associated with a germline deep intronic mutation. Pediatr. Blood Cancer 2018, 65, e27005. [Google Scholar] [CrossRef]
- Rutter, M.M.; Jha, P.; Schultz, K.A.; Sheil, A.; Harris, A.K.; Bauer, A.J.; Field, A.L.; Geller, J.; Hill, D.A. DICER1 Mutations and Differentiated Thyroid Carcinoma: Evidence of a Direct Association. J. Clin. Endocrinol. Metab. 2016, 101, 1–5. [Google Scholar] [CrossRef]
- Wu, M.K.; de Kock, L.; Conwell, L.S.; Stewart, C.J.; King, B.R.; Choong, C.S.; Hussain, K.; Sabbaghian, N.; MacRae, I.J.; Fabian, M.R.; et al. Functional characterization of multiple DICER1 mutations in an adolescent. Endocr. Relat. Cancer 2016, 23, L1–5. [Google Scholar] [CrossRef][Green Version]
- Moke, D.J.; Thomas, S.M.; Hiemenz, M.C.; Nael, A.; Wang, K.; Shillingford, N.; Biegel, J.A.; Mascarenhas, L. Three synchronous malignancies in a patient with DICER1 syndrome. Eur. J. Cancer 2018, 93, 140–143. [Google Scholar] [CrossRef]
- Herriges, J.C.; Brown, S.; Longhurst, M.; Ozmore, J.; Moeschler, J.B.; Janze, A.; Meck, J.; South, S.T.; Andersen, E.F. Identification of two 14q32 deletions involving DICER1 associated with the development of DICER1-related tumors. Eur. J. Med. Genet. 2019, 62, 9–14. [Google Scholar] [CrossRef]
- de Kock, L.; Geoffrion, D.; Rivera, B.; Wagener, R.; Sabbaghian, N.; Bens, S.; Ellezam, B.; Bouron-Dal Soglio, D.; Ordóñez, J.; Sacharow, S.; et al. Multiple DICER1-related tumors in a child with a large interstitial 14q32 deletion. Genes Chromosomes Cancer 2018, 57, 223–230. [Google Scholar] [CrossRef]
- Schultz, K.A.; Harris, A.; Messinger, Y.; Sencer, S.; Baldinger, S.; Dehner, L.P.; Hill, D.A. Ovarian tumors related to intronic mutations in DICER1: A report from the international ovarian and testicular stromal tumor registry. Fam. Cancer 2016, 15, 105–110. [Google Scholar] [CrossRef]
- Schultz, K.A.P.; Williams, G.M.; Kamihara, J.; Stewart, D.R.; Harris, A.K.; Bauer, A.J.; Turner, J.; Shah, R.; Schneider, K.; Schneider, K.W.; et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018, 24, 2251–2261. [Google Scholar] [CrossRef]
- Kim, J.; Field, A.; Schultz, K.A.P.; Hill, D.A.; Stewart, D.R. The prevalence of DICER1 pathogenic variation in population databases. Int. J. Cancer 2017, 141, 2030–2036. [Google Scholar] [CrossRef]
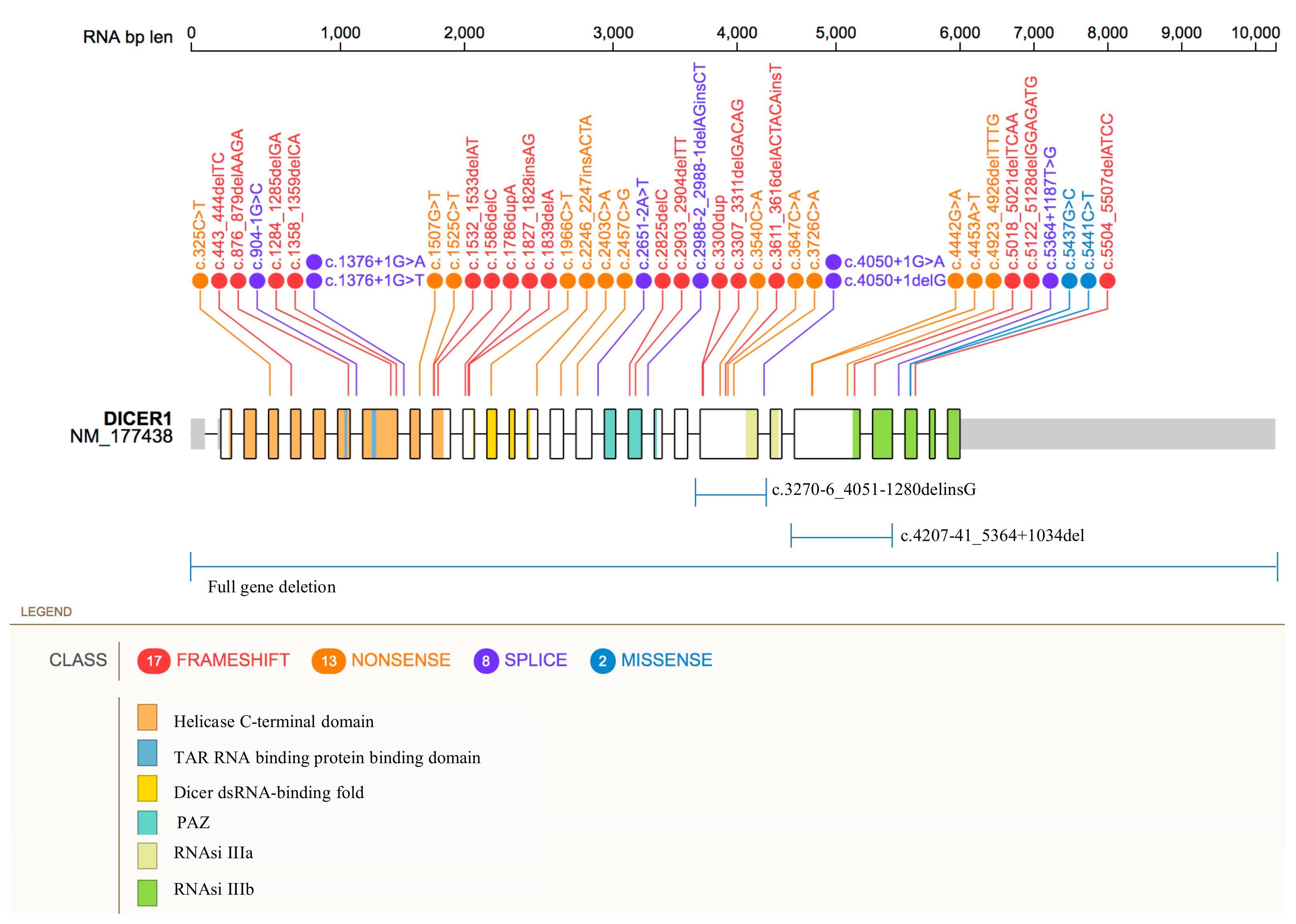
| Coding DNA Reference Sequence & | Exon/Intron | Codon | Protein Change | Protein Domain Δ | Variant Classification ◊ | Patient Phenotype † | Ref. |
|---|---|---|---|---|---|---|---|
| c.325C>T | 4 | 109 | p.Gln109Ter | Helicase ATP-binding | Pathogenic | SLCT (7y), MNG (11y) | [44] |
| c.325C>T | 4 | 109 | p.Gln109Ter | Helicase ATP-binding | Pathogenic | MNG, SLCT (38y) | [45] |
| c.443_444delTC* (c.5425G>A) | 5 (25) | 148 (1809) | p.Leu148HisfsTer22 (p.Gly1809Arg) | Helicase ATP-binding (RNase IIIb) | Pathogenic | SCLT (14y) | [46] |
| c.876_879delAAGA | 7 | 293 | p.Arg293IlefsTer4 | TAR RNA binding protein binding domain | Pathogenic | MNG (9y), SLCT (14y), Polyps-GI | [47] |
| c.904-1G>C (c.5439G>A) | Intron 7 (25) | - (1813) | - (p.Glu1813Glu) | - | Likely Pathogenic | MNG (12y), cERMS (17y), SLCT (18y) | [48] |
| c.1284_1285delGA (c.5437G>C) | 8 (25) | 429 (1813) | p.Lys429AlafsTer47 (p.Glu1813Gln) | TAR RNA binding protein binding domain (RNase IIIb) | Pathogenic | SLCT (54y), Pulmonary Bullae | [42] |
| c.1358_1359delCA (c.5125G>A) | 8 (24) | 453 (1709) | p.Thr453SerfsTer23 (p.Asp1709Asn) | Helicase C-terminal (RNase IIIb) | Pathogenic | SLCT (12y) | [46] |
| c.1376+1G>T | Intron 8 | - | - | - | Pathogenic | SLCT | [34] |
| c.1376+1G>T | Intron 8 | - | - | - | Pathogenic | PPB type II, MNG, Thyroid-PTC, SLCT | [49] |
| c.1376+1G>T | Intron 8 | - | - | - | Pathogenic | PPB Type II, NCMH, SLCT, Thyroid-DTC, Peritoneal Cyst | [29] |
| c.1376+1G>T | Intron 8 | - | - | - | Pathogenic | PPB (70y), NCMH (13y), Thyroid-PTC, SLCT | [50] |
| c.1376+1G>A (c.5439G>T) | Intron 8 (25) | - (1813) | - (p.Glu1813Asp) | - (RNase IIIb) | Pathogenic | PPB-Type-II (5.5y),Thyroid-DTC (8y), SLCT (13.5y), NCMH (13.5y) | [51] |
| c.1376+1G>A (c.5439G>T) | Intron 8 (25) | - (1813) | - (p.Glu1813Asp) | - (RNase IIIb) | Pathogenic | SLCT (13y), PPB, Thyroid-Nodule, Nasal Polyp/s | [42] |
| c.1507G>T | 9 | 503 | p.Glu503Ter | Helicase C-terminal | Pathogenic | SLCT | [34] |
| c.1525C>T | 10 | 509 | p.Arg509Ter | Helicase C-terminal | Pathogenic | SLCT-bilateral (8y, 14y), MNG (14y) | [52] |
| c.1525C>T (c.5125G>A) | 10 (24) | 509 (1709) | p.Arg509Ter (p.Asp1709Asn) | Helicase C-terminal (RNase IIIb) | Pathogenic | SLCT (13y), Thyroid-Nodule | [42] |
| c.1532_1533delAT (c.5429A>T) | 10 (25) | 511 (1810) | p.His511ArgfsTer16 (p.Asp1810Val) | Helicase C-terminal (RNase IIIb) | Pathogenic | SLCT (16y) | [53] |
| c.1532_1533delAT (c.5438A>C) | 10 (25) | 511 (1813) | p.His511ArgfsTer16 (p.Glu1813Ala) | Helicase C-terminal (RNase IIIb) | Pathogenic | SLCT (28y), Thyroid-Cyst | [42] |
| c.1532_1533delAT (c.5113G>A) | 10 (24) | 511 (1705) | p.His511ArgfsTer16 (p.Glu1705Lys) | Helicase C-terminal (RNase IIIb) | Pathogenic | SLCT (15y) | [42] |
| c.1532_1533delAT (c.5429A>T) | 10 (25) | 511 (1810) | p.His511ArgfsTer16 (p.Asp1810Val) | Helicase C-terminal (RNase IIIb) | Pathogenic | SLCT (16y)/JGCT | [42] |
| c.1586delC (c.5437G>C) | 10 (25) | 529 (1813) | p.Pro529GlnfsTer33 (p.Glu1813Gln) | Helicase C-terminal (RNase IIIb) | Pathogenic | SLCT-bilateral (20y, 29y) | [54] |
| c.1786dupA (No somatic variant detected) | 11 | 596 | p.Thr596AsnfsTer3 | Helicase C-terminal | Pathogenic | SLCT (59y) | [46] |
| c.1827_1828insAG | 11 | 610 | p.Val610ArgfsTer8 | Between Helicase C-terminal and Dicer dsRNA-binding fold | Pathogenic | PPB Type Ir, CN, SLCT | [29] |
| c.1839delA | 11 | 614 | p.Tyr614MetfsTer3 | Between Helicase C-terminal and Dicer dsRNA-binding fold | Pathogenic | SLCT-bilateral (8y, 14y) | [55] |
| c.1966C>T | 12 | 656 | p.Arg656Ter | Dicer dsRNA-binding fold | Pathogenic | SLCT-bilateral (12y,14y) | [56] |
| c.2246_2247insACTA | 14 | 749 | p.Tyr749Ter | Between Dicer dsRNA-binding fold and PAZ | Pathogenic | SLCT | [34] |
| c.2403C>A (c.5113G>A) | 15 (24) | 801 (1705) | p. Cys801Ter (p.Glu1705Lys) | Between Dicer dsRNA-binding fold and PAZ (RNase IIIb) | Pathogenic | MNG (20y), SCLT (21y) | [57] |
| c.2457C>G | 16 | 819 | p. Tyr819Ter | Between Dicer dsRNA-binding fold and PAZ | Pathogenic/Likely Pathogenic | MNG (9y), SLCT (14y) | [47] |
| c.2457C>G (c.5437G>A) | 16 (25) | 819 (1813) | p.Tyr819Ter (p.Glu1813Lys) | Between Dicer dsRNA-binding fold and PAZ (RNase IIIb) | Pathogenic/Likely Pathogenic | MNG (9y), SLCT (14y) | [35] |
| c.2651-2A>T* (c.5425G > A) | Intron 16 (25) | - (1809) | - (p.Gly1809Arg) | - (RNase IIIb) | Pathogenic | SLCT (14y) | [46] |
| c.2825delC (c.5125G>A) | 18 (24) | 942 (1709) | p.Pro942LysfsTer6 (p.Asp1709Asn) | PAZ (RNase IIIb) | Pathogenic | SLCT | [35] |
| c.2903_2904delTT (c.5437G>C) | 18 (25) | 968 (1813) | p.Phe968CysfsTer5 (p.Glu1813Gln) | PAZ (RNase IIIb) | Pathogenic | SLCT (20y) | [42] |
| c.2988-2_2988-1delAGinsCT | Intron 18 | - | - | - | Pathogenic | WT (6y), MNG (9y), SLCT (12y) | [56] |
| c.3270-6_4051-1280delinsG | 21 | 1091 | p.Tyr1091SerfsTer28 | Between PAZ and RNase IIIa | Pathogenic | SLCT (6y), MNG (14y) | [58] |
| c.3300dup (c.5437G>A) | 21 (25) | 1101 (1813) | p.Ser1101IlefsTer3 (p.Glu1813Lys) | Between PAZ and RNase IIIa (RNase IIIb) | Pathogenic | SLCT (53y) | [42] |
| c.3300dup (c.5113G>A) | 21 (24) | 1101 (1705) | p. Ser1101IlefsTer3 (p.Glu1705Lys) | Between PAZ and RNase IIIa (RNase IIIb) | Pathogenic | PPB-Type-I (1y), LC (1.8y), SLCT (18y) | [42] |
| c.3307_3311delGACAG (c.5439G>T; c.5439G>C) | 21 (25) | 1103 (1813) | p.Asp1103GlnfsTer8 (p.Glu1813Asp) | Between PAZ and RNase IIIa (RNase IIIb) | Pathogenic | WT-DA (5y), ASK (10y), Thyroid-FA (12y), ERMS-bladder (13y), SLCT-bilateral (15y) | [59] |
| c.3540C>A ** | 21 | 1180 | p.Tyr1180Ter | Between PAZ and RNase IIIa | Pathogenic | SLCT (16y), WDFA (16y) | [60] |
| c.3611_3616delACTACAinsT | 21 | 1204 | p.Tyr1204LeufsTer29 | Between PAZ and RNase IIIa | Pathogenic | MNG (26y), SLCT (29y) | [61] |
| c.3611_3616delACTACAinsT (c.5113G>A) | 21 (24) | 1204 (1705) | p.Tyr1204LeufsTer29 (p.Glu1705Lys) | Between PAZ and RNase IIIa (RNase IIIb) | Pathogenic | MNG (26y), SLCT (29y) | [35] |
| c.3647C>A *** | 21 | 1216 | p. Ser1216Ter | Between PAZ and RNase IIIa (RNase IIIb) | Pathogenic | MNG (13y), SLCT (13y) | [62] |
| c.3726C>A (c.5439G>T) | 21 (25) | 1242 (1813) | p. Tyr1242Ter (p.Glu1813Asp) | Between PAZ and RNase IIIa (RNase IIIb) | Pathogenic | SLCT (13y), NCMH (21y), PPB (27y), MNG | [50] |
| c.4050+1delG | Intron 21 | - | - | - | Pathogenic | SLCT (9y), MNG (20y), cPNET (20y) | [61] |
| c.4050+1G>A | Intron 21 | - | - | - | Pathogenic | PinB (10y), ERMS-cervix, SLCT, FEP-cervix, FEP-vagina, ERMS-brainstem | [63] |
| c.4442G>A ($) | 23 | 1481 | p. Trp1481Ter | Between RNase IIIa and IIIb | Pathogenic | SLCT (6y) | [64] |
| c.4453A>T (c.5428G>C) | 23 (25) | 1485 (1810) | p.Lys1485Ter (p.Asp1810His) | Between RNase IIIa and IIIb (RNase IIIb) | Pathogenic | SLCT-bilateral (12y, 14y) | [46] |
| c.4923_4926delTTTG (c.5438A>G) | 23 (25) | 1641 (1813) | p. Cys1641Ter (p.Glu1813Gly) | Between RNase IIIa and IIIb (RNase IIIb) | Pathogenic | SLCT (17), MNG, Pineal Cyst, Pituitary Cyst | [42] |
| c.5018_5021delTCAA (c.5125G>A) | 23 (24) | 1673 (1709) | p. Ile1673ThrfsTer31 (p.Asp1709Asn) | Between RNase IIIa and IIIb (RNase IIIb) | Pathogenic | SLCT, MNG | [35] |
| c.5122_5128delGGAGATG | 24 | 1708 | p. Gly1708ArgfsTer7 | RNase IIIb | Pathogenic | SLCT-bilateral (17y, 27y) | [56] |
| c.4207-41_5364+1034del (c.5437G>C) | 23-24 (25) | 1403-1788 (1813) | p. Thr1403_Glu1788del (p.Glu1813Gln) | RNase IIIb (RNase IIIb) | Pathogenic | SLCT (13y), MNG (15y) | [65] |
| c.5364+1187T>G | Intron 24 | 1788-1789 | p. Glu1788_ L1789insValTer | RNase IIIb | Likely Pathogenic | MNG (12y), SLCT (13y), LC (14y) | [66] |
| c.5437G>C (c.4626_4626delG) (Left ovarian) (LOH) (Right ovarian) | 25 (23) | 1813 (1542) | p. Glu1813Gln (p.Gln1542HisfsTer18) | RNase IIIb (Between RNase IIIa and IIIb) | Pathogenic | RC (1.2mo), LC (2.5y),|PinB (7.7y), SLCT-bilateral (13.4y, 15.7y), CBME (17.2y), Nasal-polyps (15.1y), Thyroid-DTC (10.6y) | [31] |
| c.5441C>T | 25 | 1814 | p. Ser1814Leu | RNase IIIb | Pathogenic | Thyroid-Nodule (13y), Thyroid-DTC (18y), SLCT-bilateral (7y, 18y) | [67] |
| c.5441C>T (c.5125G>A) | 25 (24) | 1814 (1709) | p. Ser1814Leu (p.Asp1709Asn) | RNase IIIb (RNase IIIb) | Pathogenic | SLCT (11.5y), MNG (12y) | [68] |
| c.5504_5507delATCC (c.5439G>T) | 25 (25) | 1835 (1813) | p. Tyr1835SerfsTer2 (p.Glu1813Asp) | RNase IIIb (RNase IIIb) | Pathogenic | cERMS (13y), SLCT (13y), Thyroid-PTC (13y), MNG | [69] |
| Full gene deletion | all | - | - | all | Pathogenic | SLCT, WT-NOS | [70] |
| Full gene deletion | all | - | - | all | Pathogenic | cERMS (13y), SLCT (14y), Thyroid-Nodule (13y) | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Paolis, E.; Paragliola, R.M.; Concolino, P. Spectrum of DICER1 Germline Pathogenic Variants in Ovarian Sertoli–Leydig Cell Tumor. J. Clin. Med. 2021, 10, 1845. https://doi.org/10.3390/jcm10091845
De Paolis E, Paragliola RM, Concolino P. Spectrum of DICER1 Germline Pathogenic Variants in Ovarian Sertoli–Leydig Cell Tumor. Journal of Clinical Medicine. 2021; 10(9):1845. https://doi.org/10.3390/jcm10091845
Chicago/Turabian StyleDe Paolis, Elisa, Rosa Maria Paragliola, and Paola Concolino. 2021. "Spectrum of DICER1 Germline Pathogenic Variants in Ovarian Sertoli–Leydig Cell Tumor" Journal of Clinical Medicine 10, no. 9: 1845. https://doi.org/10.3390/jcm10091845
APA StyleDe Paolis, E., Paragliola, R. M., & Concolino, P. (2021). Spectrum of DICER1 Germline Pathogenic Variants in Ovarian Sertoli–Leydig Cell Tumor. Journal of Clinical Medicine, 10(9), 1845. https://doi.org/10.3390/jcm10091845






