Frontal Fibrosing Alopecia: A Review
Abstract
1. Introduction
1.1. Definition and History
1.2. Aim and Methods
2. Epidemiology and Demographic Data
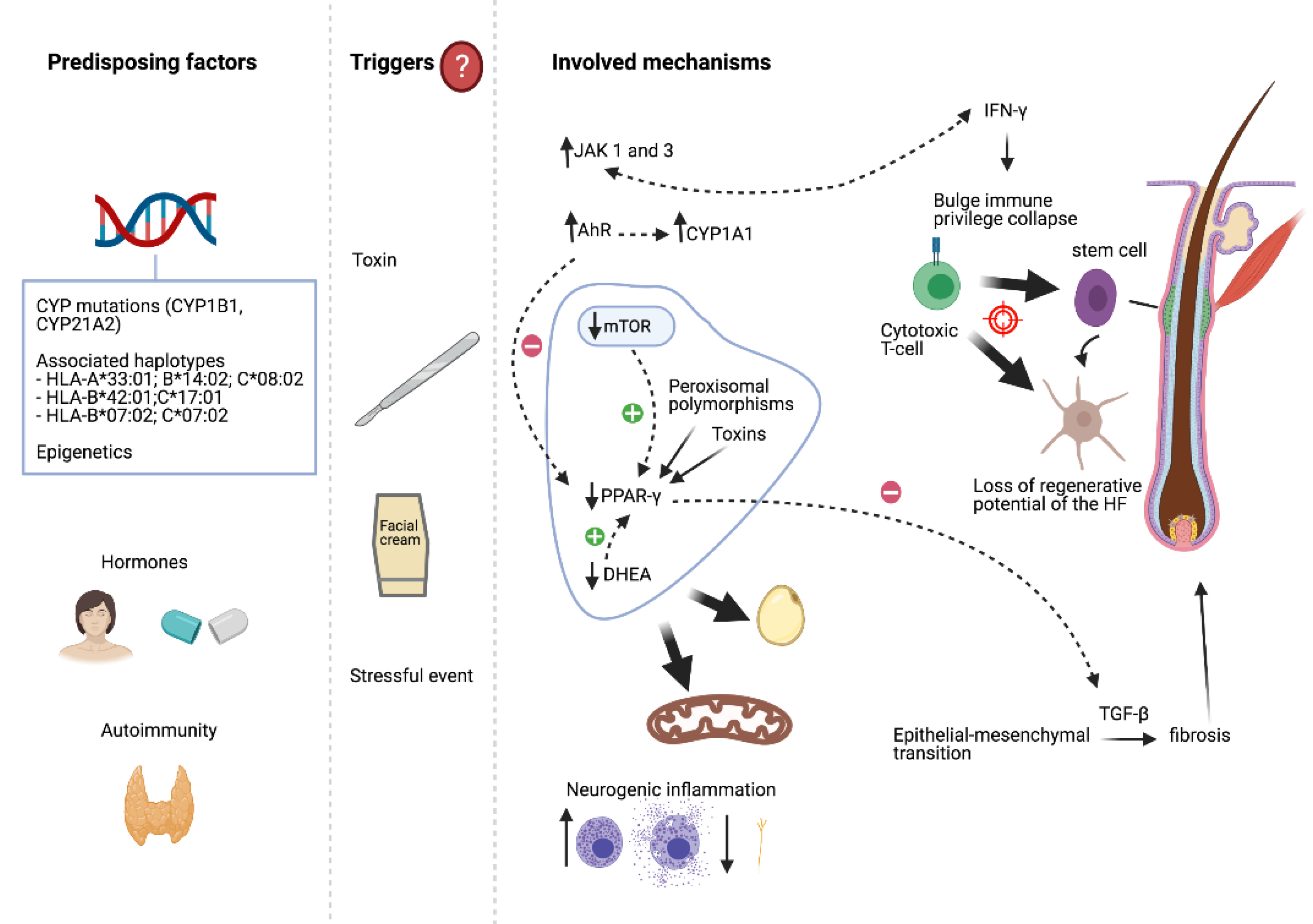
3. Aetiopathogenesis
3.1. Hormones
3.2. Associated Diseases and Autoimmunity
3.3. Genetic Factors
3.4. Surgical Procedures and Hair and Skin Care Products
3.5. Drugs, Medications, and Other Factors
4. Clinical Characteristics
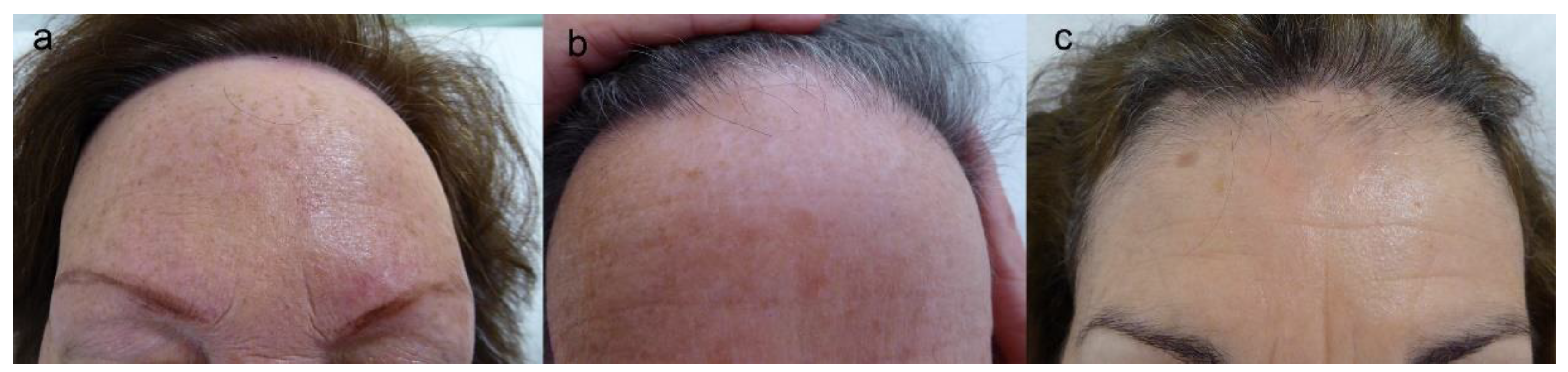
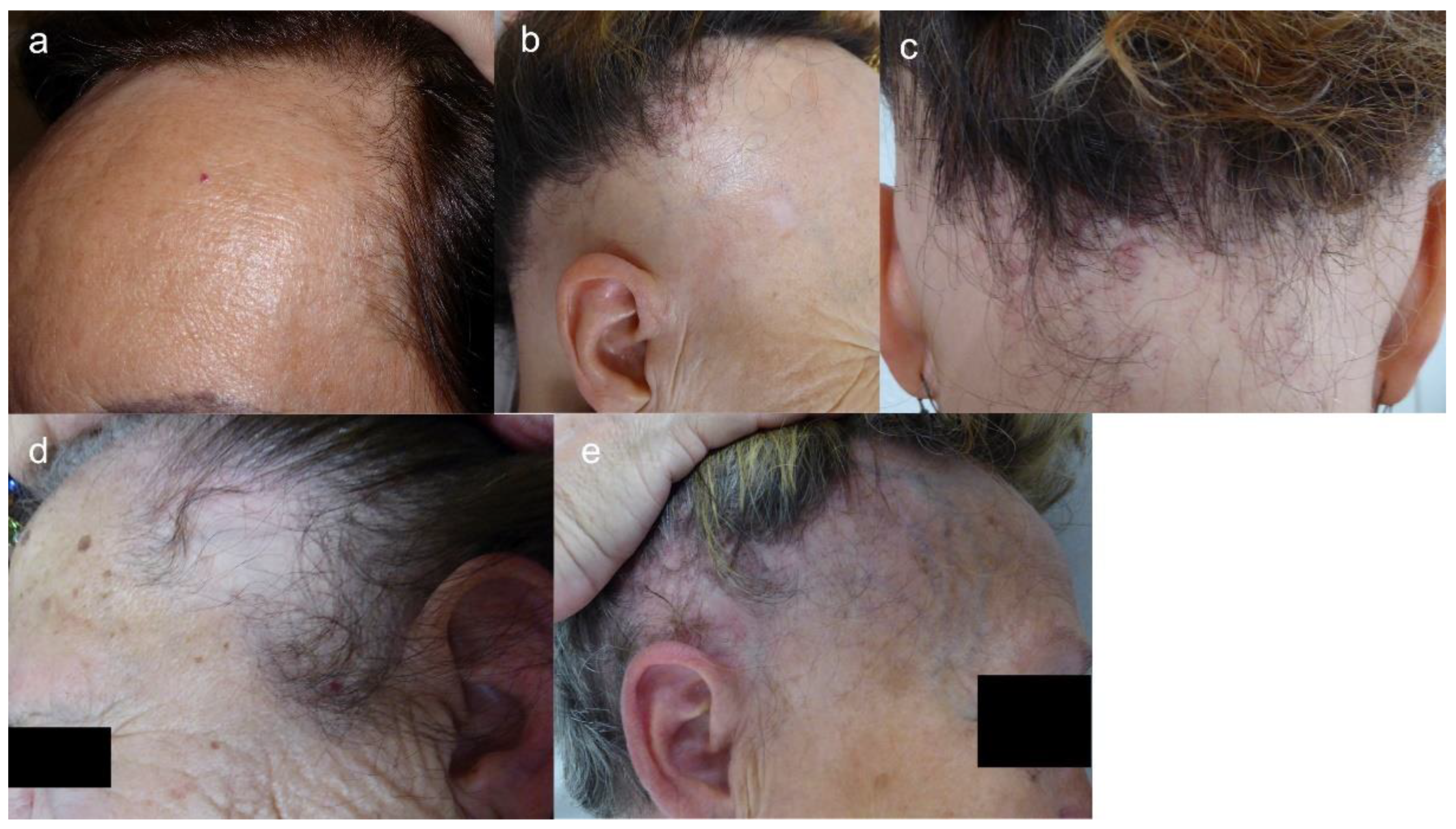
4.1. Clinical Features
4.2. Clinical Course and Prognostic Factors
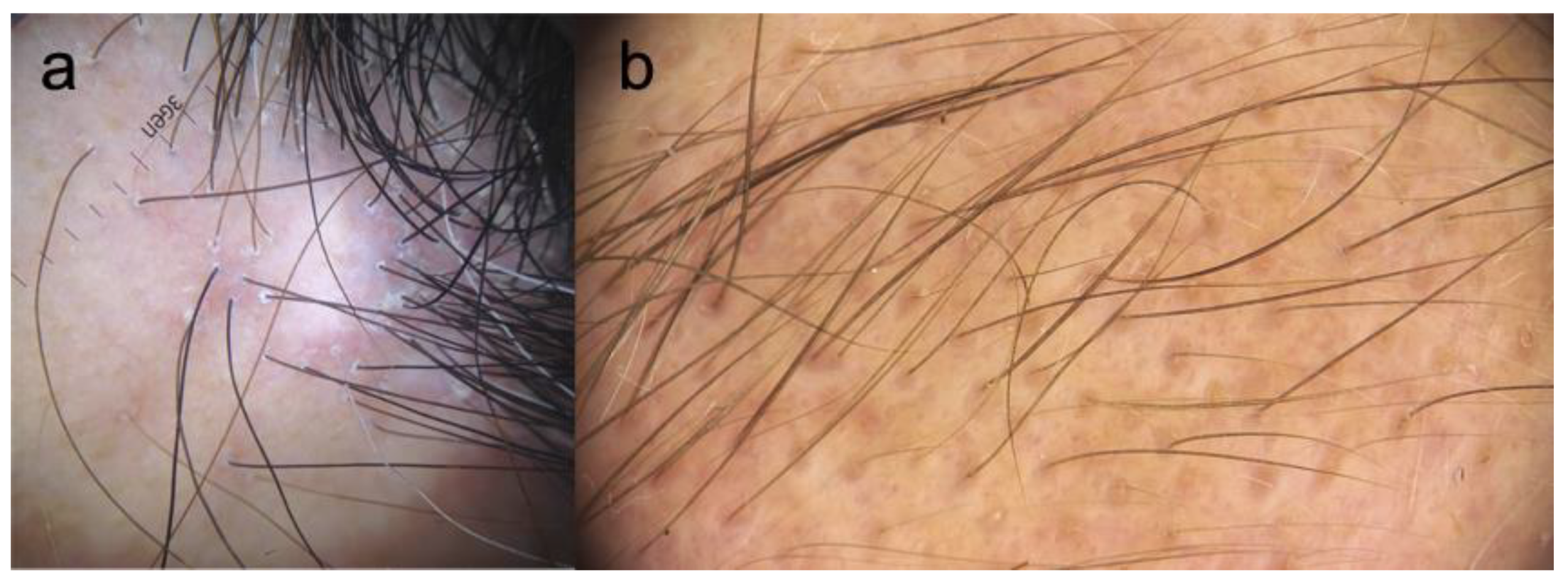
5. Trichoscopy
6. Clinical Classification and Severity Scores
7. Laboratory
8. Image Techniques
9. Histopathology
10. Are LPP and FFA the Same Disease?
11. Diagnosis
12. Differential Diagnosis
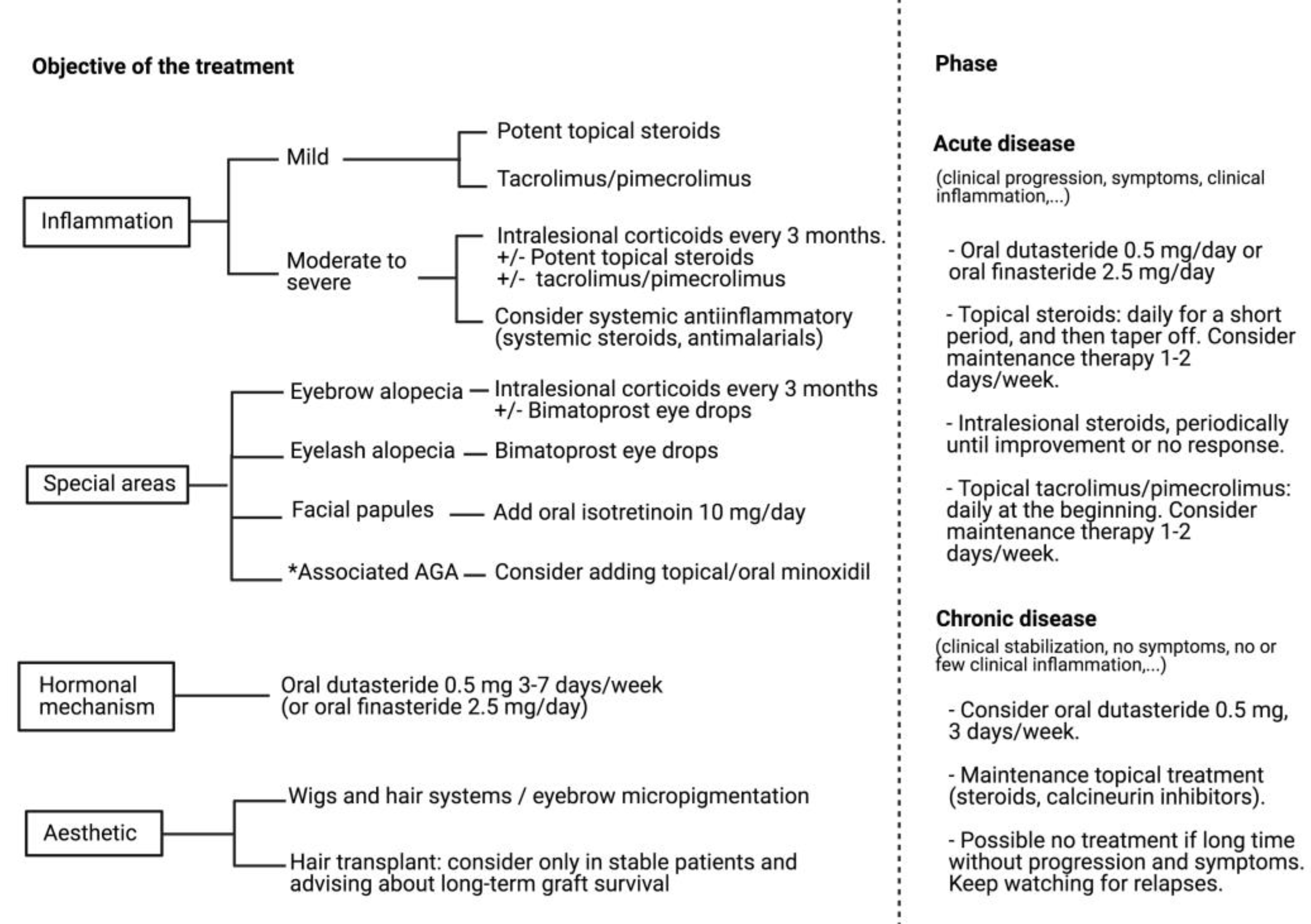
13. Treatment
13.1. Local Treatments
13.2. Systemic Treatments
13.3. Hair Transplant
14. Conclusions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kossard, S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch. Dermatol. 1994, 130, 770–774. [Google Scholar] [CrossRef]
- Kossard, S.; Lee, M.S.; Wilkinson, B. Postmenopausal frontal fibrosing alopecia: A frontal variant of lichen planopilaris. J. Am. Acad. Dermatol. 1997, 36, 59–66. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Molina-Ruiz, A.M.; Serrano-Falcón, C.; Arias-Santiago, S.; Rodrigues-Barata, A.R.; Garnacho-Saucedo, G.; Martorell-Calatayud, A.; Fernández-Crehuet, P.; Grimalt, R.; Aranegui, B.; et al. Frontal fibrosing alopecia: A multicenter review of 355 patients. J. Am. Acad. Dermatol. 2014, 70, 670–678. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Saceda-Corralo, D.; Blume-Peytavi, U.; Cucchía, J.; Dlova, N.C.; Gavazzoni-Dias, M.F.R.; Grimalt, R.; Guzmán-Sánchez, D.; Harries, M.; Ho, A.; et al. Frequency of the Types of Alopecia at Twenty-Two Specialist Hair Clinics: A Multicenter Study. Skin Appendage Disord. 2019, 5, 309–315. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M.; Iorizzo, M.; Misciali, C. Frontal fibrosing alopecia in postmenopausal women. J. Am. Acad. Dermatol. 2005, 52, 55–60. [Google Scholar] [CrossRef]
- Trager, M.H.; Lavian, J.; Lee, E.Y.; Gary, D.; Jenkins, F.; Christiano, A.M.; Bordone, L.A. Prevalence Estimates for Lichen Planopilaris and Frontal Fibrosing Alopecia in a New York City Health Care System. J. Am. Acad. Dermatol. 2021, 84, 1166–1169. [Google Scholar] [CrossRef]
- Stockmeier, M.; Kunte, C.; Sander, C.A.; Wolff, H. Kossard frontal fibrosing alopecia in a man. Hautarzt 2002, 53, 409–411. [Google Scholar] [CrossRef]
- Kossard, S.; Shiell, R.C. Frontal fibrosing alopecia developing after hair transplantation for androgenetic alopecia. Int. J. Dermatol. 2005, 44, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Porriño-Bustamante, M.L.; García-Lora, E.; Buendía-Eisman, A.; Arias-Santiago, S. Familial frontal fibrosing alopecia in two male families. Int. J. Dermatol. 2019, 58, e178–e180. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, C.F.; Wilson, N.J.; Jones, S.K. Frontal fibrosing alopecia associated with cutaneous lichen planus in a premenopausal woman. Australas. J. Dermatol. 2002, 43, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ramírez, D.; Camacho Martínez, F. Frontal fibrosing alopecia: A survey in 16 patients. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ramírez, D.; Ferrándiz, L.; Camacho, F.M. Diagnostic and therapeutic assessment of frontal fibrosing alopecia. Actas Dermosifiliogr. 2007, 98, 594–602. [Google Scholar] [CrossRef]
- MacDonald, A.; Clark, C.; Holmes, S. Frontal fibrosing alopecia: A review of 60 cases. J. Am. Acad. Dermatol. 2012, 67, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Messenger, A.G. Frontal fibrosing alopecia: Clinical presentations and prognosis. Br. J. Dermatol. 2009, 160, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Pakornphadungsit, K.; Leerunyakul, K.; Khunkhet, S.; Sriphojanart, T.; Rojhirunsakool, S. Frontal fibrosing alopecia in Asians: A retrospective clinical study. Int. J. Dermatol. 2020, 59, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dlova, N.C.; Jordaan, H.F.; Skenjane, A.; Khoza, N.; Tosti, A. Frontal fibrosing alopecia: A clinical review of 20 black patients from South Africa. Br. J. Dermatol. 2013, 169, 939–941. [Google Scholar] [CrossRef]
- Samrao, A.; Chew, A.L.; Price, V. Frontal fibrosing alopecia: A clinical review of 36 patients. Br. J. Dermatol. 2010, 163, 1296–1300. [Google Scholar] [CrossRef]
- Kanti, V.; Constantinou, A.; Reygagne, P.; Vogt, A.; Kottner, J.; Blume-Peytavi, U. Frontal fibrosing alopecia: Demographic and clinical characteristics of 490 cases. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Porriño-Bustamante, M.L.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. A Cross-sectional Study of Rosacea and Risk Factors in Women with Frontal Fibrosing Alopecia. Acta Derm. Venereol. 2019, 99, 1099–1104. [Google Scholar] [CrossRef]
- Alegre-Sánchez, A.; Saceda-Corralo, D.; Bernárdez, C.; Molina-Ruiz, A.M.; Arias-Santiago, S.; Vañó-Galván, S. Frontal fibrosing alopecia in male patients: A report of 12 cases. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e112–e114. [Google Scholar] [CrossRef]
- Sato, M.; Saga, K.; Takahashi, H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J. Dermatol. 2008, 35, 729–731. [Google Scholar] [CrossRef]
- Inui, S.; Nakajima, T.; Shono, F.; Itami, S. Dermoscopic findings in frontal fibrosing alopecia: Report of four cases. Int. J. Dermatol. 2008, 47, 796–799. [Google Scholar] [CrossRef]
- Nakamura, M.; Tokura, Y. Expression of Snail1 in the fibrotic dermis of postmenopausal frontal fibrosing alopecia: Possible involvement of an epithelial-mesenchymal transition and a review of the Japanese patients. Br. J. Dermatol. 2010, 162, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Panchaprateep, R.; Ruxrungtham, P.; Chancheewa, B.; Asawanonda, P. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: A retro-prospective cohort study. J. Dermatol. 2020, 47, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.J.; Meyer, K.; Chaudhry, I.; Kloepper, E.; Poblet, E.; Griffiths, C.E.; Paus, R. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J. Pathol. 2013, 231, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Katoulis, A.C.; Diamanti, K.; Damaskou, V.; Pouliakis, A.; Bozi, E.; Koufopoulos, N.; Rigopoulos, D.; Ioannides, D.; Panayiotides, I.G. Decreased melanocyte counts in the upper hair follicle in frontal fibrosing alopecia compared to lichen planopilaris: A retrospective histopathologic study. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef]
- Mobini, N.; Tam, S.; Kamino, H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: Lichen planopilaris as the prototype. J. Cutan. Pathol. 2005, 32, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.J.; Paus, R. Scarring alopecia and the PPAR-gamma connection. J. Investig. Dermatol. 2009, 129, 1066–1070. [Google Scholar] [CrossRef]
- Katoulis, A.C.; Diamanti, K.; Sgouros, D.; Liakou, A.I.; Bozi, E.; Tzima, K.; Panayiotides, I.; Rigopoulos, D. Frontal fibrosing alopecia: Is the melanocyte of the upper hair follicle the antigenic target? Int. J. Dermatol. 2018, 57, e37–e38. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Valdebran, M.; Bergfeld, W.; Conic, R.Z.; Piliang, M.; Atanaskova Mesinkovska, N. Hypopigmentation in frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2017, 76, 1184–1186. [Google Scholar] [CrossRef][Green Version]
- King, A.D.; Lam, L.; Goh, C. Onset of frontal fibrosing alopecia during inhibition of Th1/17 Pathways with ustekinumab. Dermatol. Online J. 2019, 25, 13030/qt8nw631wq. [Google Scholar] [PubMed]
- Karnik, P.; Tekeste, Z.; McCormick, T.S.; Gilliam, A.C.; Price, V.H.; Cooper, K.D.; Mirmirani, P. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J. Investig. Dermatol. 2009, 129, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.G.; Festuccia, W.T.; Hounde, V.P.; St-Pierre, P.; Brûlé, S.; Turcotte, V.; Côté, M.; Bellmann, K.; Marette, A.; Deshaies, Y. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion. J. Lipid Res. 2012, 53, 1117–1125. [Google Scholar] [CrossRef]
- Dicle, O.; Celik-Ozenci, C.; Sahin, P.; Pfannes, E.K.B.; Vogt, A.; Altinok, B.N.; Blume-Peytavi, U. Differential expression of mTOR signaling pathway proteins in lichen planopilaris and frontal fibrosing alopecia. Acta Histochem. 2018, 120, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, N.K. DHEA and frontal fibrosing alopecia: Molecular and physiopathological mechanisms. An. Bras. Dermatol. 2016, 91, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.J.; Jiménez, F.; Izeta, A.; Hardman, J.; Panicker, S.P.; Poblet, E.; Paus, R. Lichen Planopilaris and Frontal Fibrosing Alopecia as Model Epithelial Stem Cell Diseases. Trends Mol. Med. 2018, 24, 435–448. [Google Scholar] [CrossRef]
- Harries, M.; Hardman, J.; Chaudhry, I.; Poblet, E.; Paus, R. Profiling the human hair follicle immune system in lichen planopilaris and frontal fibrosing alopecia: Can macrophage polarization differentiate these two conditions microscopically? Br. J. Dermatol. 2020, 183, 537–547. [Google Scholar] [CrossRef]
- Ham, S.A.; Kang, E.S.; Lee, H.; Hwang, J.S.; Yoo, T.; Paek, K.S.; Park, C.; Kim, J.H.; Lim, D.S.; Seo, H.G. PPARδ inhibits UVB-induced secretion of MMP-1 through MKP-7-mediated suppression of JNK signaling. J. Investig. Dermatol. 2013, 133, 2593–2600. [Google Scholar] [CrossRef]
- Alves de Medeiros, A.K.; Speeckaert, R.; Desmet, E.; Van Gele, M.; De Schepper, S.; Lambert, J. JAK3 as an Emerging Target for Topical Treatment of Inflammatory Skin Diseases. PLoS ONE 2016, 11, e0164080. [Google Scholar] [CrossRef]
- Harries, M.J.; Wong, S.; Farrant, P. Frontal Fibrosing Alopecia and Increased Scalp Sweating: Is Neurogenic Inflammation the Common Link? Skin Appendage Disord. 2016, 1, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Harries, M. The Immunopathobiology of Lichen Planopilaris. Ph.D. Thesis, University of Manchester, Manchester, UK, 2011. [Google Scholar]
- Doche, I.; Wilcox, G.L.; Ericson, M.; Valente, N.S.; Romiti, R.; McAdams, B.D.; Hordinsky, M.K. Evidence for neurogenic inflammation in lichen planopilaris and frontal fibrosing alopecia pathogenic mechanism. Exp. Dermatol. 2020, 29, 282–285. [Google Scholar] [CrossRef]
- Noakes, R. Frontal Fibrosing Alopecia. An Example of Disrupted Aryl Hydrocarbon Receptor-Mediated Immunological Homeostasis in the Skin? Clin. Cosmet. Investig. Dermatol. 2020, 13, 479–484. [Google Scholar] [CrossRef]
- Doche, I.; Pagliari, C.; Hordinsky, M.K.; Wilcox, G.L.; Rivitti-Machado, M.C.M.; Romiti, R.; Valente, N.Y.S.; Shaik, J.A.; Saldanha, M.; Sotto, M.N. Overexpression of the aryl hydrocarbon receptor in frontal fibrosing alopecia and lichen planopilaris: A potential pathogenic role for dioxins?: An investigational study of 38 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e326–e329. [Google Scholar] [CrossRef] [PubMed]
- Tziotzios, C.; Ainali, C.; Holmes, S.; Cunningham, F.; Lwin, S.M.; Palamaras, I.; Bhargava, K.; Rymer, J.; Stefanato, C.M.; Kirkpatrick, N.; et al. Tissue and Circulating MicroRNA Co-expression Analysis Shows Potential Involvement of miRNAs in the Pathobiology of Frontal Fibrosing Alopecia. J. Investig. Dermatol. 2017, 137, 2440–2443. [Google Scholar] [CrossRef]
- Hu, H.M.; Zhang, S.B.; Lei, X.H.; Deng, Z.L.; Guo, W.X.; Qiu, Z.F.; Liu, S.; Wang, X.Y.; Zhang, H.; Duan, E.K. Estrogen leads to reversible hair cycle retardation through inducing premature catagen and maintaining telogen. PLoS ONE 2012, 7, e40124. [Google Scholar] [CrossRef]
- Tziotzios, C.; Stefanato, C.M.; Fenton, D.A.; Simpson, M.A.; McGrath, J.A. Frontal fibrosing alopecia: Reflections and hypotheses on aetiology and pathogenesis. Exp. Dermatol. 2016, 25, 847–852. [Google Scholar] [CrossRef]
- Buendía-Castaño, D.; Saceda-Corralo, D.; Moreno-Arrones, O.M.; Fonda-Pascual, P.; Alegre-Sánchez, A.; Pindado-Ortega, C.; Fernández-González, P.; Vañó-Galván, S. Hormonal and Gynecological Risk Factors in Frontal Fibrosing Alopecia: A Case-Control Study. Skin Appendage Disord. 2018, 4, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Banka, N.; Mubki, T.; Bunagan, M.J.; McElwee, K.; Shapiro, J. Frontal fibrosing alopecia: A retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int. J. Dermatol. 2014, 53, 1324–1330. [Google Scholar] [CrossRef]
- Lobato-Berezo, A.; March-Rodríguez, A.; Deza, G.; Bertolín-Colilla, M.; Pujol, R.M. Frontal fibrosing alopecia after antiandrogen hormonal therapy in a male patient. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e291–e292. [Google Scholar] [CrossRef] [PubMed]
- Bernárdez, C.; Molina-Ruiz, A.M.; Vañó-Galván, S.; Urech, M.; Saceda-Corralo, D.; Moreno-Arrones, O.M.; Requena, L.; Camacho, F.M. Sex hormone status in premenopausal women with frontal fibrosing alopecia: A multicentre review of 43 patients. Clin. Exp. Dermatol. 2017, 42, 921–923. [Google Scholar] [CrossRef]
- Ranasinghe, G.C.; Piliang, M.P.; Bergfeld, W.F. Prevalence of hormonal and endocrine dysfunction in patients with lichen planopilaris (LPP): A retrospective data analysis of 168 patients. J. Am. Acad. Dermatol. 2017, 76, 314–320. [Google Scholar] [CrossRef]
- Nasiri, S.; Dadkhahfar, S.; Mansouri, P.; Rahmani-Khah, E.; Mozafari, N. Evaluation of serum level of sex hormones in women with frontal fibrosing alopecia in comparison to healthy controls. Dermatol. Ther. 2020, e13842. [Google Scholar] [CrossRef]
- Sasannia, M.; Saki, N.; Aslani, F.S. Comparison of Serum Level of Sex Hormones in Patients with Frontal Fibrosing Alopecia with Control Group. Int. J. Trichology 2020, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pindado-Ortega, C.; Saceda-Corralo, D.; Buendía-Castaño, D.; Fernández-González, P.; Moreno-Arrones, O.M.; Fonda-Pascual, P.; Rodrigues-Barata, A.R.; Jaén-Olasolo, P.; Vañó-Galván, S. Frontal fibrosing alopecia and cutaneous comorbidities: A potential relationship with rosacea. J. Am. Acad. Dermatol. 2018, 78, 596–597. [Google Scholar] [CrossRef]
- Imhof, R.L.; Chaudhry, H.M.; Larkin, S.C.; Torgerson, R.R.; Tolkachjov, S.N. Frontal Fibrosing Alopecia in Women: The Mayo Clinic Experience with 148 Patients, 1992–2016. Mayo Clin. Proc. 2018, 93, 1581–1588. [Google Scholar] [CrossRef]
- Del Rei, M.; Pirmez, R.; Sodré, C.T.; Tosti, A. Coexistence of frontal fibrosing alopecia and discoid lupus erythematosus of the scalp in 7 patients: Just a coincidence? J. Eur. Acad. Dermatol. Venereol. 2016, 30, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Eginli, A.N.; Bagayoko, C.W.; McMichael, A.J. A Case of Frontal Fibrosing Alopecia in a Patient with Primary Biliary Cirrhosis and Polymyalgia Rheumatica. Skin Appendage Disord. 2016, 2, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Amate, X.; Riquelme-McLoughlin, C.; Morgado-Carrasco, D.; Rojano-Fritz, L.; Iranzo-Fernández, P. Report of two cases of mucous membrane pemphigoid with frontal fibrosing alopecia: A variant of lichen planus pemphigoides or an incidental finding? Clin. Exp. Dermatol. 2020, 45, 727–731. [Google Scholar] [CrossRef]
- McSweeney, S.M.; Christou, E.A.A.; Dand, N.; Boalch, A.; Holmes, S.; Harries, M.; Palamaras, I.; Cunningham, F.; Parkins, G.; Kaur, M.; et al. Frontal fibrosing alopecia: A descriptive cross-sectional study of 711 cases in female patients from the UK. Br. J. Dermatol. 2020, 183, 1136–1138. [Google Scholar] [CrossRef]
- Fertig, R.M.; Hu, S.; Maddy, A.J.; Balaban, A.; Aleid, N.; Aldahan, A.; Tosti, A. Medical comorbidities in patients with lichen planopilaris, a retrospective case-control study. Int. J. Dermatol. 2018, 57, 804–809. [Google Scholar] [CrossRef]
- Trager, M.H.; Lavian, J.; Lee, E.Y.; Gary, D.; Jenkins, F.; Christiano, A.M.; Bordone, L.A. Medical Comorbidities and Gender Distribution among Patients with Lichen Planopilaris and Frontal Fibrosing Alopecia: A Retrospective Cohort Study. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef]
- Katoulis, A.C.; Diamanti, K.; Sgouros, D.; Liakou, A.I.; Alevizou, A.; Bozi, E.; Damaskou, V.; Panayiotides, I.; Rigopoulos, D. Frontal fibrosing alopecia and vitiligo: Coexistence or true association? Skin Appendage Disord. 2016, 2, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Zabielinski, M.; Aber, C.; Miteva, M.; Tosti, A. Frontal fibrosing alopecia in a patient with common variable immunodeficiency. Br. J. Dermatol. 2012, 166, 689–690. [Google Scholar] [CrossRef]
- Trüeb, R.M.; Torricelli, R. Lichen planopilaris simulating postmenopausal frontal fibrosing alopecia (Kossard). Hautarzt 1998, 49, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.L.; Bashir, S.J.; Wain, E.M.; Fenton, D.A.; Stefanato, C.M. Expanding the spectrum of frontal fibrosing alopecia: A unifying concept. J. Am. Acad. Dermatol. 2010, 63, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, M.; Hohendorf-Ansari, P.; Trüeb, R.M. Nail Involvement in Frontal Fibrosing Alopecia. Int. J. Trichology 2015, 7, 64–66. [Google Scholar] [CrossRef]
- Mervis, J.S.; Borda, L.J.; Miteva, M. Facial and Extrafacial Lesions in an Ethnically Diverse Series of 91 Patients with Frontal Fibrosing Alopecia Followed at a Single Center. Dermatology 2019, 235, 112–119. [Google Scholar] [CrossRef]
- Feldmann, R.; Harms, M.; Saurat, J.H. Postmenopausal frontal fibrosing alopecia. Hautarzt 1996, 47, 533–536. [Google Scholar] [CrossRef]
- Dlova, N.C. Frontal fibrosing alopecia and lichen planus pigmentosus: Is there a link? Br. J. Dermatol. 2013, 168, 439–442. [Google Scholar] [CrossRef]
- Munera-Campos, M.; Castillo, G.; Ferrándiz, C.; Carrascosa, J.M. Actinic lichen planus triggered by drug photosensitivity. Photodermatol. Photoimmunol. Photomed. 2019, 35, 124–126. [Google Scholar] [CrossRef]
- Saha, A.; Seth, J.; Das, A.; Dhar, S. Graham-Little-Piccardi Syndrome: A Lens Through beyond What is Known. Int. J. Trichology 2016, 8, 173–175. [Google Scholar] [CrossRef]
- Katoulis, A.C.; Diamanti, K.; Sgouros, D.; Liakou, A.I.; Bozi, E.; Avgerinou, G.; Panayiotides, I.; Rigopoulos, D. Is there a pathogenetic link between frontal fibrosing alopecia, androgenetic alopecia and fibrosing alopecia in a pattern distribution? J. Eur. Acad. Dermatol. Venereol. 2018, 32, e218–e220. [Google Scholar] [CrossRef]
- Ormaechea-Pérez, N.; López-Pestaña, A.; Zubizarreta-Salvador, J.; Jaka-Moreno, A.; Panés-Rodríguez, A.; Tuneu-Valls, A. Frontal Fibrosing Alopecia in Men: Presentations in 12 Cases and a Review of the Literature. Actas Dermosifiliogr. 2016, 107, 836–844. [Google Scholar] [CrossRef]
- Porriño-Bustamante, M.L.; Arias-Santiago, S.; Buendía-Eisman, A. Concomitant occurrence of frontal fibrosing alopecia and trichotemnomania: The importance of trichoscopy. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 112–115. [Google Scholar]
- Nemazee, L.; Harries, M. Frontal fibrosing alopecia sparing a vascular naevus: The Renbök phenomenon. Clin. Exp. Dermatol. 2020. [Google Scholar] [CrossRef]
- Viglizzo, G.; Verrini, A.; Rongioletti, F. Familial Lassueur-Graham-Little-Piccardi syndrome. Dermatology 2004, 208, 142–144. [Google Scholar] [CrossRef]
- Copeman, P.W.; Tan, R.S.; Timlin, D.; Samman, P.D. Familial lichen planus. Another disease or a distinct people? Br. J. Dermatol. 1978, 98, 573–577. [Google Scholar] [CrossRef]
- Junqueira Ribeiro Pereira, A.F.; Vincenzi, C.; Tosti, A. Frontal fibrosing alopecia in two sisters. Br. J. Dermatol. 2010, 162, 1154–1155. [Google Scholar] [CrossRef]
- Porriño-Bustamante, M.L.; López-Nevot, M.; Aneiros-Fernández, J.; García-Lora, E.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. Familial frontal fibrosing alopecia: A cross-sectional study of 20 cases from nine families. Australas. J. Dermatol. 2019, 60, e113–e118. [Google Scholar] [CrossRef]
- Chan, D.V.; Kartono, F.; Ziegler, R.; Abdulwahab, N.; DiPaola, N.; Flynn, J.; Wong, H.K. Absence of HLA-DR1 positivity in 2 familial cases of frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2014, 71, e208–e210. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.M.; Antolín, S.C.; Sambucety, P.S.; González, E.S.; Ruíz de Morales, J.M.; Prieto, M. Frontal fibrosing alopecia and lichen planopilaris in HLA-identical mother and daughter. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 162–165. [Google Scholar] [CrossRef]
- Missio, D.M.; Dias, M.F.R.G.; Trüeb, R.M. Familial Cicatricial Alopecia: Report of Familial Frontal Fibrosing Alopecia and Fibrosing Alopecia in a Pattern Distribution. Int. J. Trichology 2017, 9, 130–134. [Google Scholar] [CrossRef]
- Porriño-Bustamante, M.L.; López-Nevot, M.; Aneiros-Fernández, J.; Casado-Ruiz, J.; García-Linares, S.; Pedrinacci-Rodríguez, S.; García-Lora, E.; Martín-Casares, M.A.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. Study of Human Leukocyte Antigen (HLA) in 13 cases of familial frontal fibrosing alopecia: CYP21A2 gene p.V281L mutation from congenital adrenal hyperplasia linked to HLA class I haplotype HLA-A*33:01; B*14:02; C*08:02 as a genetic marker. Australas. J. Dermatol. 2019, 60, e195–e200. [Google Scholar] [CrossRef] [PubMed]
- Tziotzios, C.; Petridis, C.; Dand, N.; Ainali, C.; Saklatvala, J.R.; Pullabhatla, V.; Onoufriadis, A.; Pramanik, R.; Baudry, D.; Lee, S.H.; et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat. Commun. 2019, 10, 1150. [Google Scholar] [CrossRef]
- Ramos, P.M.; Garbers, L.E.F.M.; Silva, N.S.B.; Castro, C.F.B.; Andrade, H.S.; Souza, A.S.; Castelli, E.C.; Miot, H.A. A large familial cluster and sporadic cases of frontal fibrosing alopecia in Brazil reinforce known human leucocyte antigen (HLA) associations and indicate new HLA susceptibility haplotypes. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2409–2413. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Belmonte, M.R.; Navarro-López, V.; Ramírez-Boscà, A.; Martínez-Andrés, M.A.; Molina-Gil, C.; González-Nebreda, M.; Asín-Lorca, M. Case series of familial frontal fibrosing alopecia and a review of the literature. J. Cosmet. Dermatol. 2015, 14, 64–69. [Google Scholar] [CrossRef]
- Da Silva Libório, R.; Trüeb, R.M. Case Report of Connubial Frontal Fibrosing Alopecia. Int. J. Trichology 2018, 10, 76–79. [Google Scholar] [CrossRef]
- Tziotzios, C.; Fenton, D.A.; Stefanato, C.M.; McGrath, J.A. Familial frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2015, 73, e37. [Google Scholar] [CrossRef]
- Crisóstomo, M.R.; Crisóstomo, M.C.; Crisóstomo, M.G.; Gondim, V.J.; Benevides, A.N. Hair loss due to lichen planopilaris after hair transplantation: A report of two cases and a literature review. An. Bras. Dermatol. 2011, 86, 359–362. [Google Scholar] [CrossRef]
- Chiang, Y.Z.; Tosti, A.; Chaudhry, I.H.; Lyne, L.; Farjo, B.; Farjo, N.; Cadore de Farias, D.; Griffiths, C.E.M.; Paus, R.; Harries, M.J. Lichen planopilaris following hair transplantation and face-lift surgery. Br. J. Dermatol. 2012, 166, 666-370. [Google Scholar] [CrossRef]
- Taguti, P.; Dutra, H.; Trüeb, R.M. Lichen Planopilaris Caused by Wig Attachment: A Case of Koebner Phenomenon in Frontal Fibrosing Alopecia. Int. J. Trichology 2018, 10, 172–174. [Google Scholar] [CrossRef]
- Aldoori, N.; Dobson, K.; Holden, C.R.; McDonagh, A.J.; Harries, M.; Messenger, A.G. Frontal fibrosing alopecia: Possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br. J. Dermatol. 2016, 175, 762–767. [Google Scholar] [CrossRef]
- Debroy Kidambi, A.; Dobson, K.; Holmes, S.; Carauna, D.; Del Marmol, V.; Vujovic, A.; Kaur, M.R.; Takwale, A.; Farrant, P.; Champagne, C.; et al. Frontal fibrosing alopecia in men: An association with facial moisturizers and sunscreens. Br. J. Dermatol. 2017, 177, 260–261. [Google Scholar] [CrossRef]
- Moreno-Arrones, O.M.; Saceda-Corralo, D.; Rodrigues-Barata, A.; Castellanos-González, M.; Fernández-Pugnaire, M.A.; Grimalt, R.; Hermosa-Gelbard, A.; Bernárdez, C.; Molina-Ruiz, A.M.; Ormaechea-Pérez, N.; et al. Risk factors associated with frontal fibrosing alopecia: A multicentre case-control study. Clin. Exp. Dermatol. 2019, 44, 404–410. [Google Scholar] [CrossRef]
- Cranwell, W.C.; Sinclair, R. Frontal fibrosing alopecia: Regrowth following cessation of sunscreen on the forehead. Australas. J. Dermatol. 2019, 60, 60–61. [Google Scholar] [CrossRef]
- Imhof, R.L.; Larkin, S.C.; Cantwell, H.M.; Torgerson, R.R.; Tolkachjov, S.N. The association of frontal fibrosing alopecia with skin and hair care products: A survey-based case series of 56 patients seen at Mayo Clinic. J. Am. Acad. Dermatol. 2021, 84, 532–534. [Google Scholar] [CrossRef]
- Callander, J.; Frost, J.; Stone, N. Ultraviolet filters in hair-care products: A possible link with frontal fibrosing alopecia and lichen planopilaris. Clin. Exp. Dermatol. 2018, 43, 69–70. [Google Scholar] [CrossRef]
- Brunet-Possenti, F.; Deschamps, L.; Colboc, H.; Somogyi, A.; Medjoubi, K.; Bazin, D.; Descamps, V. Detection of titanium nanoparticles in the hair shafts of a patient with frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e442–e443. [Google Scholar] [CrossRef] [PubMed]
- Aerts, O.; Bracke, A.; Goossens, A.; Meuleman, V.; Lambert, J. Titanium dioxide nanoparticles and frontal fibrosing alopecia: Cause or consequence? J. Eur. Acad. Dermatol. Venereol. 2019, 33, e45–e46. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.T.; Chen, Z.Q.; Kolivras, A.; Tosti, A. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: A pilot study of 20 patients. Br. J. Dermatol. 2019, 181, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Nieto, M.A.; Gatica-Ortega, M.E.; Sánchez-Herreros, C.; Vergara-Sánchez, A.; Martínez-Mariscal, J.; De Eusebio-Murillo, E. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermat. 2020. [Google Scholar] [CrossRef]
- Rudnicka, L.; Rokni, G.R.; Lotti, T.; Wollina, U.; Fölster-Holst, R.; Katsambas, A.; Goren, A.; Di Lernia, V.G.; Rathod, D.; Mirabi, A.; et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: An international multi-center study. Dermatol. Ther. 2020, 33, e13560. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.B.; Donati, A.; Contin, L.A.; Kakizaki, P.; Machado, C.J.; Brito, F.F.; Claudino, D.; Moraes, P.; Guerra, J.; Pires, M.C. Photopatch and patch testing in 63 frontal fibrosing alopecia patients: A case series. Br. J. Dermatol. 2018, 179, 1402–1403. [Google Scholar] [CrossRef]
- Robinson, G.; McMichael, A.; Wang, S.Q.; Lim, H.W. Sunscreen and Frontal Fibrosing Alopecia: A Review. J. Am. Acad. Dermatol. 2020, 82, 723–728. [Google Scholar] [CrossRef]
- Dhana, A.; Gumedze, F.; Khumalo, N.P. Regarding ‘Frontal fibrosing alopecia: Possible association with leave-on facial skincare products and sunscreens; a questionnaire study’. Br. J. Dermatol. 2017, 176, 836–837. [Google Scholar] [CrossRef]
- Ramos, P.M.; Anzai, A.; Duque-Estrada, B.; Farias, D.C.; Melo, D.F.; Mulinari-Brenner, F.; Pinto, G.M.; Abraham, L.S.; Santos, L.D.N.; Pirmez, R.; et al. Risk Factors for Frontal Fibrosing Alopecia: A case-control study in a multiracial population. J. Am. Acad. Dermatol. 2021, 84, 712–718. [Google Scholar] [CrossRef]
- Fonda-Pascual, P.; Saceda-Corralo, D.; Moreno-Arrones, O.M.; Alegre-Sanchez, A.; Vaño-Galvan, S. Frontal fibrosing alopecia and environment: May tobacco be protective? J. Eur. Acad. Dermatol. Venereol. 2017, 31, e98–e99. [Google Scholar] [CrossRef]
- Moreno-Arrones, O.M.; Saceda-Corralo, D.; Rodrigues-Barata, A.; Castellanos-González, M.; Fernández-Pugnaire, M.A.; Grimalt, R.; Hermosa-Gelbard, A.; Bernárdez, C.; Molina-Ruiz, A.M.; Ormaechea-Pérez, N.; et al. Factors influencing frontal fibrosing alopecia severity: A multicentre cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e315–e316. [Google Scholar] [CrossRef]
- Frioui, R.; Rabhi, F.; Gargouri, F.; Jaber, K.; Dhaoui, A. Nilotinib-induced keratosis pilaris associated with cicatricial alopecia resembling frontal fibrosing alopecia. Dermatol. Ther. 2020, 34, e14579. [Google Scholar] [CrossRef]
- Rudnicka, L.; Rakowska, A. The increasing incidence of frontal fibrosing alopecia. In search of triggering factors. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1579–1580. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Mendese, G.; Goldberg, L.J. Scarring alopecia of the sideburns: A unique presentation of frontal fibrosing alopecia in men. Arch. Dermatol. 2012, 148, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.K.; Tan, E.; Shapiro, J. Update on primary cicatricial alopecias. J. Am. Acad. Dermatol. 2005, 53, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Mirmirani, P.; Zimmerman, B. Cocking the eyebrows to find the missing hairline in frontal fibrosing alopecia: A useful clinical maneuver. J. Am. Acad. Dermatol. 2016, 75, e63–e64. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.; Bergfeld, W. Wood’s Light Examination for Assessment in Frontal Fibrosing Alopecia: A Manoeuvre to Enhance the Hairline. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef]
- Moreno-Arrones, O.M.; Saceda-Corralo, D.; Fonda-Pascual, P.; Rodrigues-Barata, A.R.; Buendía-Castaño, D.; Alegre-Sánchez, A.; Pindado-Ortega, C.; Molins, M.; Perosanz, D.; Segurado-Miravalles, G.; et al. Frontal fibrosing alopecia: Clinical and prognostic classification. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Grassi, S.; Fortuna, M.C.; Garelli, V.; Pranteda, G.; Caro, G.; Carlesimo, M. Unusual patterns of presentation of frontal fibrosing alopecia: A clinical and trichoscopic analysis of 98 patients. J. Am. Acad. Dermatol. 2017, 77, 172–174. [Google Scholar] [CrossRef][Green Version]
- Goldman, C.; Diaz, A.; Miteva, M. A Novel Atypical Presentation of Frontal Fibrosing Alopecia Involving the Frontoparietal Scalp. Skin Appendage Disord. 2020, 6, 250–253. [Google Scholar] [CrossRef]
- Tosti, A.; Miteva, M.; Torres, F. Lonely hair: A clue to the diagnosis of frontal fibrosing alopecia. Arch. Dermatol. 2011, 147, 1240. [Google Scholar] [CrossRef]
- Pirmez, R.; Duque-Estrada, B.; Abraham, S.L.; Pinto, G.M.; de Farias, D.C.; Kelly, Y.; Doche, I. It’s not all traction: The pseudo ‘fringe sign’ in frontal fibrosing alopecia. Br. J. Dermatol. 2015, 173, 1336–1338. [Google Scholar] [CrossRef]
- Brandi, N.; Starace, M.; Alessandrini, A.; Bruni, F.; Piraccini, B.M. The doll hairline: A clue for the diagnosis of frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2017, 77, e127–e128. [Google Scholar] [CrossRef]
- Donovan, J.C.; Samrao, A.; Ruben, B.S.; Price, V.H. Eyebrow regrowth in patients with frontal fibrosing alopecia treated with intralesional triamcinolone acetonide. Br. J. Dermatol. 2010, 163, 1142–1144. [Google Scholar] [CrossRef]
- Anzai, A.; Donati, A.; Valente, N.Y.; Romiti, R.; Tosti, A. Isolated eyebrow loss in frontal fibrosing alopecia: Relevance of early diagnosis and treatment. Br. J. Dermatol. 2016, 175, 1099–1101. [Google Scholar] [CrossRef]
- Waśkiel-Burnat, A.; Rakowska, A.; Kurzeja, M.; Czuwara, J.; Sikora, M.; Olszewska, M.; Rudnicka, L. The value of dermoscopy in diagnosing eyebrow loss in patients with alopecia areata and frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 213–219. [Google Scholar] [CrossRef]
- Salido-Vallejo, R.; Garnacho-Saucedo, G.; Moreno-Gimenez, J.C.; Camacho-Martinez, F.M. Beard involvement in a man with frontal fibrosing alopecia. Indian J. Dermatol. Venereol. Leprol. 2014, 80, 542–544. [Google Scholar] [CrossRef]
- Armenores, P.; Shirato, K.; Reid, C.; Sidhu, S. Frontal fibrosing alopecia associated with generalized hair loss. Australas. J. Dermatol. 2010, 51, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Dina, Y.; Okoye, G.A.; Aguh, C. The Timing and Distribution of Non-Scalp Hair Loss in Patients with Lichen Planopilaris and Frontal Fibrosing Alopecia: A Survey-Based Study. J. Am. Acad. Dermatol. 2018. [Google Scholar] [CrossRef]
- Fertig, R.; Farias, D.; Tosti, A. Postcast hypertrichosis in a patient with frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e53–e54. [Google Scholar] [CrossRef] [PubMed]
- Ladizinski, B.; Bazakas, A.; Selim, M.A.; Olsen, E.A. Frontal fibrosing alopecia: A retrospective review of 19 patients seen at Duke University. J. Am. Acad. Dermatol. 2013, 68, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Chedraoui, A.; Ghosn, S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome. J. Am. Acad. Dermatol. 2007, 57, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- López-Pestaña, A.; Tuneu, A.; Lobo, C.; Ormaechea, N.; Zubizarreta, J.; Vildosola, S.; Del Alcázar, E. Facial lesions in frontal fibrosing alopecia (FFA): Clinicopathological features in a series of 12 cases. J. Am. Acad. Dermatol. 2015, 73, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Kłosowicz, A.; Thompson, C.; Tosti, A. Erythematous Papules Involving the Eyebrows in a Patient with a History of Rosacea and Hair Loss. Skin Appendage Disord. 2020, 6, 190–193. [Google Scholar] [CrossRef]
- Donati, A.; Molina, L.; Doche, I.; Valente, N.S.; Romiti, R. Facial papules in frontal fibrosing alopecia: Evidence of vellus follicle involvement. Arch. Dermatol. 2011, 147, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.F.; Duarte, A.F.; Haneke, E.; Correia, O. Yellow facial papules associated with frontal fibrosing alopecia: A distinct histologic pattern and response to isotretinoin. J. Am. Acad. Dermatol. 2017, 77, 764–766. [Google Scholar] [CrossRef]
- Pirmez, R.; Donati, A.; Valente, N.S.; Sodré, C.T.; Tosti, A. Glabellar red dots in frontal fibrosing alopecia: A further clinical sign of vellus follicle involvement. Br. J. Dermatol. 2014, 170, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Sachse, M.; Rose, C.; Wagner, G. Follicular red dots of the hip in frontal fibrosing alopecia—Do we have to look twice? J. Dtsch. Dermatol. Ges. 2017, 15, 327–328. [Google Scholar] [CrossRef]
- Billero, V.; Oberlin, K.E.; Miteva, M. Red Dots in a Net-Like Pattern on the Upper Chest: A Novel Clinical Observation in Frontal Fibrosing Alopecia and Fibrosing Alopecia in Pattern Distribution. Skin Appendage Disord. 2018, 4, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Pirmez, R.; Duque-Estrada, B.; Donati, A.; Campos-do-Carmo, G.; Valente, N.S.; Romiti, R.; Sodré, C.T.; Tosti, A. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br. J. Dermatol. 2016, 175, 1387–1390. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Rodrigues-Barata, A.R.; Urech, M.; Jiménez-Gómez, N.; Saceda-Corralo, D.; Paoli, J.; Cuevas, J.; Jaén, P. Depression of the frontal veins: A new clinical sign of frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2015, 72, 1087–1088. [Google Scholar] [CrossRef]
- Nanda, S.; De Bedout, V.; Hirt, P.A.; Castillo, D.E.; Mesquita, T.; Scott, L.; Miteva, M. Increased Preauricular Wrinkles in Frontal Fibrosing Alopecia Compared to Age-Matched Controls: A Prospective Study of 64 Patients. Skin Appendage Disord. 2020, 6, 11–13. [Google Scholar] [CrossRef]
- Defo, D.; Naouri, M.; Martin, L.; Estève, E. Hair darkening close to a patch of frontal fibrosing alopecia. Ann. Dermatol. Venereol. 2006, 133, 799–801. [Google Scholar] [CrossRef]
- Pastor-Nieto, M.A.; Vañó-Galván, S.; Gómez-Zubiaur, A.; Jiménez-Blázquez, E.; Moreno-Arrones, O.M.; Melgar-Molero, V. Localized gray hair repigmentation (canities reversal) in patients with frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.F.; Barreto, T.M.; Faro, G.B.A.; Machado, C.J.; Donati, A. Occipital hairline involvement in frontal fibrosing alopecia: Frequency, clinical presentation and trichoscopy findings in a series of twenty patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e405–e407. [Google Scholar] [CrossRef]
- Saceda-Corralo, D.; Pindado-Ortega, C.; Moreno-Arrones, O.; Fernández-González, P.; Rodrigues-Barata, A.R.; Jaén-Olasolo, P.; Vañó-Galván, S. Health-Related Quality of Life in Patients with Frontal Fibrosing Alopecia. JAMA Dermatol. 2018, 154, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Papanikou, S.; Xydeas-Kikemenis, A.; Nicolaidou, E.; Chatziioannou, A.; Rigopoulos, D.; Stratigos, A.; Chasapi, V. Social Status May Interfere in the Prognosis of Frontal Fibrosing Alopecia in Female Patients: An Observational Study. Skin Appendage Disord. 2019, 5, 355–358. [Google Scholar] [CrossRef]
- Rudnicka, L.; Olszewska, M.; Rakowska, A.; Slowinska, M. Trichoscopy update 2011. J. Dermatol. Case Rep. 2011, 5, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Callender, V.D.; Reid, S.D.; Obayan, O.; Mcclellan, L.; Sperling, L. Diagnostic Clues to Frontal Fibrosing Alopecia in Patients of African Descent. J. Clin. Aesthet. Dermatol. 2016, 9, 45–51. [Google Scholar]
- Tosti, A. Dermoscopy of the Hair and Nails, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 53–56. [Google Scholar]
- Thompson, C.T.; Martínez-Velasco, M.A.; Tosti, A. Yellow dots in frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e75–e76. [Google Scholar] [CrossRef]
- Saceda-Corralo, D.; Moreno-Arrones, O.M.; Rubio-Lambraña, M.; Gil-Redondo, R.; Bernárdez, C.; Hermosa-Gelbard, A.; Jaén-Olasolo, P.; Vañó-Galván, S. Trichoscopic features of mild frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e205–e207. [Google Scholar] [CrossRef]
- Cervantes, J.; Miteva, M. Distinct Trichoscopic Features of the Sideburns in Frontal Fibrosing Alopecia Compared to the Frontotemporal Scalp. Skin Appendage Disord. 2018, 4, 50–54. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Micali, G.; Tosti, A. Absence of vellus hair in the hairline: A videodermatoscopic feature of frontal fibrosing alopecia. Br. J. Dermatol. 2013, 169, 473–474. [Google Scholar] [CrossRef]
- Toledo-Pastrana, T.; Hernández, M.J.; Camacho Martínez, F.M. Perifollicular erythema as a trichoscopy sign of progression in frontal fibrosing alopecia. Int. J. Trichology 2013, 5, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Saceda-Corralo, D.; Pindado-Ortega, C.; Moreno-Arrones, O.M.; Ortega-Quijano, D.; Fernández-Nieto, D.; Jiménez-Cauhe, J.; Vañó-Galván, S. Association of Inflammation With Progression of Hair Loss in Women With Frontal Fibrosing Alopecia. JAMA Dermatol. 2020, 156, 700–702. [Google Scholar] [CrossRef]
- Fernández-Crehuet, P.; Rodrigues-Barata, A.R.; Vañó-Galván, S.; Serrano-Falcón, C.; Molina-Ruiz, A.M.; Arias-Santiago, S.; Martorell-Calatayud, A.; Grimalt, R.; Garnacho-Saucedo, G.; Serrano, S.; et al. Trichoscopic features of frontal fibrosing alopecia: Results in 249 patients. J. Am. Acad. Dermatol. 2015, 72, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Vincenzi, C.; Tosti, A. Pili Torti as a Sign of Eyebrow Involvement in Frontal Fibrosing Alopecia. Skin Appendage Disord. 2019, 5, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Pirmez, R.; Vincenzi, C.; Fabbrocini, G.; Romiti, R.; Tosti, A. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: A study of 151 cases. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef]
- Saceda-Corralo, D.; Moreno-Arrones, O.M.; Fonda-Pascual, P.; Pindado-Ortega, C.; Hermosa-Gelbard, A.; Rodrigues-Barata, A.R.; Vañó-Galván, S. Steroid-Induced Changes Noted On Trichoscopy Of Patients With Frontal Fibrosing Alopecia. J. Am. Acad. Dermatol. 2018, 79, 956–957. [Google Scholar] [CrossRef]
- Rodrigues-Barata, A.R.; Moreno-Arrones, O.M.; Corralo-Saceda, D.; Vañó-Galván, S. The “Starry Night Sky Sign” Using Ultraviolet-Light-Enhanced Trichoscopy: A New Sign That May Predict Efficacy of Treatment in Frontal Fibrosing Alopecia. Int. J. Trichology 2018, 10, 241–243. [Google Scholar] [CrossRef]
- Holmes, S.; Ryan, T.; Young, D.; Harries, M. British Hair and Nail Society. Frontal Fibrosing Alopecia Severity Index (FFASI): A validated scoring system for assessing frontal fibrosing alopecia. Br. J. Dermatol. 2016, 175, 203–207. [Google Scholar] [CrossRef]
- Saceda-Corralo, D.; Moreno-Arrones, O.; Fonda-Pascual, P.; Pindado-Ortega, C.; Buendía-Castaño, D.; Alegre-Sánchez, A.; Segurado-Miravelles, G.; Rodrigues-Barata, A.R.; Jaén-Olasolo, P.; Vañó-Galván, S. Development and validation of the Frontal Fibrosing Alopecia Severity Score. J. Am. Acad. Dermatol. 2018, 78, 522–529. [Google Scholar] [CrossRef]
- Vazquez-Herrera, N.E.; Eber, A.E.; Martínez-Velasco, M.A.; Perper, M.; Cervantes, J.; Verne, S.H.; Magno, R.J.; Nouri, K.; Tosti, A. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 318–322. [Google Scholar] [CrossRef]
- Kurzeja, M.; Czuwara, J.; Walecka, I.; Olszewska, M.; Rudnicka, L. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: A preliminary study. Skin Res. Technol. 2020. [Google Scholar] [CrossRef]
- Porriño-Bustamante, M.L.; Fernández-Pugnaire, M.A.; Castellote-Caballero, L.; Arias-Santiago, S. Colour Doppler ultrasound study in patients with frontal fibrosing alopecia. Skin Res. Technol. 2021. [Google Scholar] [CrossRef]
- Mirmirani, P.; Willey, A.; Headington, J.T.; Stenn, K.; McCalmont, T.H.; Price, V.H. Primary cicatricial alopecia: Histopathologic findings do not distinguish clinical variants. J. Am. Acad. Dermatol. 2005, 52, 637–643. [Google Scholar] [CrossRef]
- Martínez-Velasco, M.A.; Vázquez-Herrera, N.E.; Misciali, C.; Vincenzi, C.; Maddy, A.J.; Asz-Sigall, D.; Tosti, A. Frontal Fibrosing Alopecia Severity Index: A Trichoscopic Visual Scale That Correlates Thickness of Peripilar Casts with Severity of Inflammatory Changes at Pathology. Skin Appendage Disord. 2018, 4, 277–280. [Google Scholar] [CrossRef]
- Poblet, E.; Jiménez, F.; Pascual, A.; Piqué, E. Frontal fibrosing alopecia versus lichen planopilaris: A clinicopathological study. Int. J. Dermatol. 2006, 45, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Katoulis, A.C.; Damaskou, V.; Diamanti, K.; Pouliakis, A.; Mortaki, D.; Zacharatou, A.; Bozi, E.; Sgouros, D.; Panayiotides, I.G. Eyebrow involvement in frontal fibrosing alopecia: A clinicopathologic cohort study for the reversibility of hair loss. J. Am. Acad. Dermatol. 2020, 82, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Miteva, M.; Sabiq, S. A New Histologic Pattern in 6 Biopsies From Early Frontal Fibrosing Alopecia. Am. J. Dermatopathol. 2019, 41, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.A.; Imadojemu, S.; Beer, K.; Seykora, J.T. Inflammatory features of frontal fibrosing alopecia. J. Cutan. Pathol. 2017, 44, 672–676. [Google Scholar] [CrossRef]
- Miteva, M.; Tosti, A. The follicular triad: A pathological clue to the diagnosis of early frontal fibrosing alopecia. Br. J. Dermatol. 2012, 166, 440–442. [Google Scholar] [CrossRef]
- Del Duca, E.; Ruano-Ruiz, J.; Pavel, A.B.; Sanyal, R.D.; Song, T.; Gay-Mimbrera, J.; Zhang, N.; Estrada, Y.D.; Peng, X.; Renert-Yuval, Y.; et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br. J. Dermatol. 2020, 183, 1083–1093. [Google Scholar] [CrossRef]
- Sleiman, R.; Kurban, M.; Abbas, O. Evaluation of the Diagnostic Value of Plasmacytoid Dendritic Cells in Differentiating the Lymphocytic Cicatricial Alopecias. Dermatology 2015, 231, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Saceda-Corralo, D.; Desai, K.; Pindado-Ortega, C.; Moreno-Arrones, O.M.; Vañó-Galván, S.; Miteva, M. Histological evidence for epidermal and dermal atrophy of the alopecic band in treatment-naïve patients with Frontal Fibrosing Alopecia. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e47–e49. [Google Scholar] [CrossRef]
- Pindado-Ortega, C.; Perna, C.; Saceda-Corralo, D.; Fernández-Nieto, D.; Jaén-Olasolo, P.; Vañó-Galván, S. Frontal fibrosing alopecia: Histopathological, immunohistochemical and hormonal study of clinically unaffected scalp areas. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e84–e85. [Google Scholar] [CrossRef]
- Doche, I.; Romiti, R.; Hordinsky, M.K.; Valente, N.S. “Normal-appearing” scalp areas are also affected in lichen planopilaris and frontal fibrosing alopecia: An observational histopathologic study of 40 patients. Exp. Dermatol. 2020, 29, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Miteva, M.; Castillo, D.; Sabiq, S. Adipose Infiltration of the Dermis, Involving the Arrector Pili Muscle, and Dermal Displacement of Eccrine Sweat Coils: New Histologic Observations in Frontal Fibrosing Alopecia. Am. J. Dermatopathol. 2019, 41, 492–497. [Google Scholar] [CrossRef]
- Pirmez, R.; Barreto, T.; Duque-Estrada, B.; Quintella, D.C.; Cuzzi, T. Facial Papules in Frontal Fibrosing Alopecia: Beyond Vellus Hair Follicle Involvement. Skin Appendage Disord. 2018, 4, 145–149. [Google Scholar] [CrossRef]
- Miteva, M. Frontal Fibrosing Alopecia Involving the Limbs Shows Inflammatory Pattern on Histology: A Review of 13 Cases. Am. J. Dermatopathol. 2020, 42, 226–229. [Google Scholar] [CrossRef]
- Miteva, M.; Goldberg, L.J. Lichenoid folliculitis in facial lichen planus pigmentosus-A clue to frontal fibrosing alopecia? J. Cutan. Pathol. 2020, 47, 983–985. [Google Scholar] [CrossRef]
- Rivera Pérez de Rada, P.; Rivera Salazar, J.; Juárez Tosina, R.; Olalla Gallardo, J.M. Eyelash loss in frontal fibrosing alopecia: Microscopic features of two cases. J. Fr. Ophtalmol. 2021, 44, 48–52. [Google Scholar] [CrossRef]
- Gálvez-Canseco, A.; Sperling, L. Lichen planopilaris and frontal fibrosing alopecia cannot be differentiated by histopathology. J. Cutan. Pathol. 2018, 45, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Goldberg, L.J. The depth of inflammation in frontal fibrosing alopecia and lichen planopilaris: A potential distinguishing feature. J. Am. Acad. Dermatol. 2017, 76, 1183–1184. [Google Scholar] [CrossRef][Green Version]
- Annessi, G.; Lombardo, G.; Gobello, T.; Puddu, P. A clinicopathologic study of scarring alopecia due to lichen planus: Comparison with scarring alopecia in discoid lupus erythematosus and pseudopelade. Am. J. Dermatopathol 1999, 21, 324–331. [Google Scholar] [CrossRef]
- Donati, A.; Gupta, A.K.; Jacob, C.; Cavelier-Balloy, B.; Reygagne, P. The Use of Direct Immunofluorescence in Frontal Fibrosing Alopecia. Skin Appendage Disord. 2017, 3, 125–128. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Saceda-Corralo, D.; Moreno-Arrones, O.; Camacho-Martinez, F.M. Updated diagnostic criteria for frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2018, 78, e21–e22. [Google Scholar] [CrossRef]
- Tolkachjov, S.N.; Chaudhry, H.M.; Imhof, R.L.; Camilleri, M.J.; Torgerson, R.R. Reply to: “Updated diagnostic criteria for frontal fibrosing alopecia”. J. Am. Acad. Dermatol. 2018, 78, e23–e24. [Google Scholar] [CrossRef] [PubMed]
- Georgala, S.; Katoulis, A.C.; Befon, A.; Danopoulou, I.; Georgala, C. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J. Am. Acad. Dermatol. 2009, 61, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Katoulis, A.; Georgala, S.; Bozi, E.; Papadavid, E.; Kalogeromitros, D.; Stavrianeas, N. Frontal fibrosing alopecia: Treatment with oral dutasteride and topical pimecrolimus. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Letulé, V.; Laniauskaite, I.; Reinholz, M.; Tietze, J.K.; Wolff, H.; Ruzicka, T.; Sattler, E.C. Frontal Fibrosing Alopecia: A Retrospective Analysis of 72 Patients from a German Academic Center. Facial Plast. Surg. 2018, 34, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, L.C.; Avila, L.; Li, X.; Lo Sicco, K.; Shapiro, J. Prognosis, treatment, and disease outcomes in frontal fibrosing alopecia: A retrospective review of 92 cases. J. Am. Acad. Dermatol. 2018, 78, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.; Bergfeld, W. Prostaglandin analogue for treatment of eyebrow loss in frontal fibrosing alopecia: Three cases with different outcomes. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e138–e140. [Google Scholar] [CrossRef]
- Navarini, A.A.; Kolios, A.G.; Prinz-Vavricka, B.M.; Haug, S.; Trüeb, R.M. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch. Dermatol. 2011, 147, 1325–1326. [Google Scholar] [CrossRef] [PubMed]
- Gerkowicz, A.; Bartosińska, J.; Wolska-Gawron, K.; Michalska-Jakubus, M.; Kwaśny, M.; Krasowska, D. Application of superluminescent diodes (sLED) in the treatment of scarring alopecia—A pilot study. Photodiagnosis Photodyn. Ther. 2019, 28, 195–200. [Google Scholar] [CrossRef]
- Özcan, D.; Tunçer Vural, A.; Özen, Ö. Platelet-rich plasma for treatment resistant frontal fibrosing alopecia: A case report. Dermatol. Ther. 2019, 32, e13072. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.C. Finasteride-mediated hair regrowth and reversal of atrophy in a patient with frontal fibrosing alopecia. JAAD Case Rep. 2015, 1, 353–355. [Google Scholar] [CrossRef]
- Rakowska, A.; Gradzińska, A.; Olszewska, M.; Rudnicka, L. Efficacy of Isotretinoin and Acitretin in Treatment of Frontal Fibrosing Alopecia: Retrospective Analysis of 54 Cases. J. Drugs Dermatol. 2017, 16, 988–992. [Google Scholar]
- Lee, J.Y.; Hong, J.S.; Lee, S.H.; Lee, A.Y. Successful treatment of frontal fibrosing alopecia with alitretinoin. Dermatol. Ther. 2019, 32, e13037. [Google Scholar] [CrossRef] [PubMed]
- Pindado-Ortega, C.; Saceda-Corralo, D.; Moreno-Arrones, O.M.; Rodrigues-Barata, A.R.; Hermosa-Gelbard, A.; Jaén-Olasolo, P.; Vañó-Galván, S. Effectiveness of dutasteride in alarge series of patients with frontal fibrosing alopecia in real clinical practice. J. Am. Acad. Dermatol. 2021, 84, 1285–1294. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Rostami, A.; Tavakopour, S.; Nili, A.; Teimourpour, A.; Farid, A.S.; Abedini, R.; Amini, M.; Daneshpazhooh, M. Oral isotretinoin combined with topical clobetasol 0.05% and tacrolimus 0.1% for the treatment of frontal fibrosing alopecia: A randomized controlled trial. J. Dermatolog Treat. 2020, 1–7. [Google Scholar] [CrossRef]
- Morandi Stumpf, M.A.; do Rocio Valenga Baroni, E.; Schafranski, M.D. Frontal Fibrosing Alopecia: Successfully Treated with Methotrexate or Just the Natural Disease Progression? Acta Dermatovenerol. Croat. 2020, 28, 188–189. [Google Scholar]
- Mirmirani, P.; Karnik, P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch. Dermatol. 2009, 145, 1363–1366. [Google Scholar] [CrossRef]
- Spring, P.; Spanou, Z.; de Viragh, P.A. Lichen planopilaris treated by the peroxisome proliferator activated receptor-γ agonist pioglitazone: Lack of lasting improvement or cure in the majority of patients. J. Am. Acad. Dermatol. 2013, 69, 830–832. [Google Scholar] [CrossRef]
- Vano-Galvan, S.; Trindade de Carvalho, L.; Saceda-Corralo, D.; Rodrigues-Barata, R.; Kerkemeyer, K.L.; Sinclair, R.D.; Hermosa-Gelbard, A.; Moreno-Arrones, O.M.; Jiménez-Cauhe, J.; Bhoyrul, B. Oral minoxidil improves background hair thickness in lichen planopilaris. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef]
- Yang, C.C.; Khanna, T.; Sallee, B.; Christiano, A.M.; Bordone, L.A. Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatol. Ther. 2018, 31, e12656. [Google Scholar] [CrossRef] [PubMed]
- Trindade de Carvalho, L.; Meah, N.; Wall, D.; Sinclair, R. Recalcitrant lichen planopilaris and frontal fibrosing alopecia responding to tildrakizumab. Dermatol. Ther. 2020, 33, e13694. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; LaBelle, B. Treatment of lichen planopilaris with adalimumab in a patient with hidradenitis suppurativa and rheumatoid arthritis. JAAD Case Rep. 2020, 6, 219–221. [Google Scholar] [CrossRef]
- Unger, W.; Unger, R.; Wesley, C. The surgical treatment of cicatricial alopecia. Dermatol. Ther. 2008, 21, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, B.P.; Nusbaum, A.G. Frontal fibrosing alopecia in a man: Results of follicular unit test grafting. Dermatol. Surg. 2010, 36, 959–962. [Google Scholar] [CrossRef]
- Jiménez, F.; Poblet, E. Is hair transplantation indicated in frontal fibrosing alopecia? The results of test grafting in three patients. Dermatol. Surg. 2013, 39, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Audickaite, A.; Alam, M.; Jimenez, F. Eyebrow Hair Transplantation in Frontal Fibrosing Alopecia: Pitfalls of Short- and Long-Term Results. Dermatol. Surg. 2020, 46, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Vañó-Galván, S.; Villodres, E.; Pigem, R.; Navarro-Belmonte, M.R.; Asín-Llorca, M.; Meyer-González, T.; Rodrigues-Barata, R.; Moreno-Arrones, O.M.; Saceda-Corralo, D.; Bouhanna, P.; et al. Hair transplant in frontal fibrosing alopecia: A multicenter review of 51 patients. J. Am. Acad. Dermatol. 2019, 81, 865–866. [Google Scholar] [CrossRef]
| Autoimmune Associated Diseases |
|---|
| Thyroid disorders: Hypothyroidism (8–44.6%) Hashimoto thyroiditis (8.1%) Graves disease (1.4%) |
| Lichen planus (1.7–18.2%): Cutaneous (3–6.5%) Mucosal (3–16.7%) Pilaris (0.8–25.3%) |
| Psoriasis (7.4%) |
| Vitiligo (0.6–5.6%) |
| Inflammatory bowel disease (5.4%) |
| Lichen sclerosus (0.3–5.4%) |
| Sjögren syndrome (1.7–4.1%) |
| Discoid cutaneous lupus erythematosus (-) |
| Systemic lupus erythematosus (3.4%) |
| Coeliac disease (1.5–2.0%) |
| Pernicious anaemia (1.2–1.7%) |
| Alopecia areata (0.6–1.7%) |
| Scleroderma (1.4%) |
| Rheumatoid arthritis (1.4%) |
| Polymyalgia rheumatic (-) |
| Primary biliary cirrhosis (-) |
| Mucous membrane pemphigoid (-) |
| Pattern Name | Clinical Description |
|---|---|
| Typical Patterns | |
| Pattern I (linear) | Uniform band of frontal hairline recession in the absence of loss of hair density behind the hairline |
| Pattern II (diffuse) | Diffuse or zigzag band-like alopecia affecting the frontal hairline with significant loss of hair density behind the hairline (at least a 50% decrease in normal hair density) with a compatible trichoscopy. |
| Pattern III (pseudo-fringe-sign) | Unaffected primitive frontal or temporal hairline forming the pseudo “fringe sign.” |
| Unusual Patterns | |
| AGA-like pattern | Symmetric recession of frontotemporal hairlines, with sparing of the paramedian frontal hairline (mimicking male pattern AGA). |
| Ophiasis-like pattern | Continuous involvement of the hairline from frontal to occipital regions. |
| Cockade-like pattern | Presence of oval patches of alopecia in the temporal regions, with sparing of a band of temporal hairlines. |
| Upsilon pattern | Band-like pattern along the frontotemporal scalp extending into two symmetrical triangles along the parietal scalp. |
| Histological Features | FFA | LPP |
|---|---|---|
| Inflammatory infiltrate degree | + | ++ |
| Inflammation/fibrosis below the isthmus | ++/− | +/− |
| Basal layer damage degree | + | ++ |
| Superficial perivascular infiltrate | +/− | ++ |
| Keratinocyte necrosis in the external root sheath | ++ | + |
| Foreign body reaction | ++ | +/− |
| Involvement of interfollicular epidermis | − | ++ |
| Concentric lamellar fibroplasia | + | ++ |
| Presence of terminal catagen-telogen hairs | ++ | +/− |
| Direct immunofluorescence deposits | +/− | + |
| Epidermal thickness reduction | ++ | + |
| Macrophage polarization | +CD86, −CD163 | −CD86, +CD163 |
| Lower melanocyte count in the upper follicle | + | − |
| Major Criteria | Minor Criteria |
|---|---|
| 1. Cicatricial alopecia of the frontal, temporal, or frontotemporal scalp, in the absence of follicular keratotic papules on the body. | 1. Typical trichoscopic features (perifollicular erythema and/or follicular hyperkeratosis, lonely hair sign). |
| 2. Diffuse bilateral eyebrow alopecia. | 2. Histopathological features of FFA and LPP. |
| 3. Involvement (hair loss or perifollicular erythema) of additional FFA sites (occipital area, facial hair, sideburns, or body hair). | |
| 4. Non-inflammatory facial papules. | |
| 5. Preceding or concurrent symptoms (pruritus or pain) at the areas of involvement. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porriño-Bustamante, M.L.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. Frontal Fibrosing Alopecia: A Review. J. Clin. Med. 2021, 10, 1805. https://doi.org/10.3390/jcm10091805
Porriño-Bustamante ML, Fernández-Pugnaire MA, Arias-Santiago S. Frontal Fibrosing Alopecia: A Review. Journal of Clinical Medicine. 2021; 10(9):1805. https://doi.org/10.3390/jcm10091805
Chicago/Turabian StylePorriño-Bustamante, María Librada, María Antonia Fernández-Pugnaire, and Salvador Arias-Santiago. 2021. "Frontal Fibrosing Alopecia: A Review" Journal of Clinical Medicine 10, no. 9: 1805. https://doi.org/10.3390/jcm10091805
APA StylePorriño-Bustamante, M. L., Fernández-Pugnaire, M. A., & Arias-Santiago, S. (2021). Frontal Fibrosing Alopecia: A Review. Journal of Clinical Medicine, 10(9), 1805. https://doi.org/10.3390/jcm10091805







