Clinical History and Detectable Troponin Concentrations below the 99th Percentile for Risk Stratification of Patients with Chest Pain and First Normal Troponin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Hs-cTnT Assay
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient Population, Management, and Follow-Up
3.2. Undetectable Hs-cTnT Concentrations
3.3. Clinical Data and hs-cTnT
3.4. Subgroup Analysis
4. Discussion
4.1. Undetectable hs-cTnT
4.2. Clinical Data
4.3. Subgroup Analysis
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hess, E.P.; Brison, R.J.; Perry, J.J.; Calder, L.A.; Thiruganasambandamoorthy, V.; Agarwal, D.; Sadosty, A.T.; Silvilotti, M.L.; Jaffe, A.S.; Montori, V.M.; et al. Development of a Clinical Prediction Rule for 30-Day Cardiac Events in Emergency Department Patients With Chest Pain and Possible Acute Coronary Syndrome. Ann. Emerg. Med. 2012, 59, 115–125.e1. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial In-farction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Sanchis, J.; Bodí, V.; Núñez, J.; Bertomeu-González, V.; Gómez, C.; Bosch, M.J.; Consuegra, L.; Bosch, X.; Chorro, F.J.; Llàcer, À. New Risk Score for Patients with Acute Chest Pain, Non-St-Segment Deviation, and Normal Troponin Concentrations. J. Am. Coll. Cardiol. 2005, 46, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bularga, A.; Lee, K.K.; Stewart, S.; Ferry, A.V.; Chapman, A.R.; Marshall, L.; Strachan, F.E.; Cruickshank, A.; Maguire, D.; Berry, C.; et al. High-Sensitivity Troponin and the Application of Risk Strati-fication Thresholds in Patients with Suspected Acute Coronary Syndrome. Circulation 2019, 140, 1557–1568. [Google Scholar] [CrossRef]
- Pickering, J.W.; Than, M.P.; Cullen, L.; Aldous, S.; Avest, E.T.; Body, R.; Carlton, E.W.; Collinson, P.; Dupuy, A.M.; Ekelund, U.; et al. Rapid Rule-out of Acute Myocardial Infarction with a Single High-Sensitivity Cardiac Troponin T Measurement Below the Limit of Detection: A Col-laborative Meta-Analysis. Ann. Intern. Med. 2017, 166, 715. [Google Scholar] [CrossRef] [PubMed]
- Twerenbold, R.; Neumann, J.T.; Sörensen, N.A.; Ojeda, F.; Karakas, M.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; Giménez, M.R.; Puelacher, C.; et al. Prospective Validation of the 0/1-H Algorithm for Early Diagnosis of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Alquézar-Arbé, A.; Ordóñez-Llanos, J.; Bardají, A. High-sensitivity Cardiac Troponin for the Evaluation of Patients With Suspected ACS: A True or a False Friend? Revista Española de Cardiología 2019, 72, 445–448. [Google Scholar] [CrossRef]
- Giménez, M.R.; Wildi, K.; Wussler, D.; Koechlin, L.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; Sedlmayer, R.; Puelacher, C.; Zimmermann, T.; et al. Early kinetics of cardiac troponin in suspected acute myocardial infarction. Revista Española de Cardiología 2020. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Hear. J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.W. The Need to Improve Derivation and Description of Algorithms to Rule-Out Patients With Possible Myocardial Infarction. Circulation 2019, 139, 1351–1353. [Google Scholar] [CrossRef]
- Greenslade, J.H.; Nayer, R.; Parsonage, W.; Doig, S.; Young, J.; Pickering, J.W.; Than, M.; Hammett, C.; Cullen, L. Validating the Man-chester Acute Coronary Syndromes (Macs) and Troponin-Only Manchester Acute Coronary Syndromes (T-Macs) Rules for the Prediction of Acute Myocardial Infarction in Patients Presenting to the Emergency Department with Chest Pain. Emerg. Med. J. 2017, 34, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Vigen, R.; Diercks, D.B.; Hashim, I.A.; Pandey, A.; Zhong, L.; Kutscher, P.; Fernandez, F.; Yu, A.; Bertulfo, B.; Molberg, K.; et al. Association of a Novel Protocol for Rapid Exclusion of Myocardial Infarction with Resource Use in a USA Safety Net Hospital. JAMA 2020, 3, e203359. [Google Scholar]
- Sanchis, J.; Valero, E.; Blas, S.G.; Barba, E.; Pernias, V.; Mi-ñana, G.; Brasó, J.; Fernandez-Cisnal, A.; Gonzalez, J.; Noceda, J.; et al. Undetectable High-Sensitivity Troponin in Combination with Clinical Assessment for Risk Stratification of Patients with Chest Pain and Normal Troponin at Hospital Arrival. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Mair, J.; Giannitsis, E.; Mueller, C.; Lindahl, B.; Blankenberg, S.; Huber, K.; Plebani, M.; Biasucci, L.M.; Tubaro, M.; et al. How to Use High-Sensitivity Cardiac Troponins in Acute Cardiac Care. Eur. Heart J. 2012, 33, 2252–2257. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.; Müller, M. Proc: An Open-Source Package for R and S+ to Analyze and Compare Roc Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- survIDINRI: IDI and NRI for Comparing Competing Risk Prediction Models with Censored Survival Data; Version R package version 1.1-1. Available online: https://cran.r-project.org/web/packages/survIDINRI/survIDINRI.pdf (accessed on 5 April 2021).
- Sandoval, Y.; Nowak, R.; deFilippi, C.R.; Christenson, R.H.; Peacock, W.F.; McCord, J.; Limkakeng, A.T.; Sexter, A.; Apple, F.S. Myo-cardial Infarction Risk Stratification with a Single Measurement of High-Sensitivity Troponin I. J. Am. Coll. Cardiol. 2019, 74, 271–282. [Google Scholar] [CrossRef]
- Body, R.; Morris, N.; Collinson, P. Single test rule-out of acute myocardial infarction using the limit of detection of a new high-sensitivity troponin I assay. Clin. Biochem. 2020, 78, 4–9. [Google Scholar] [CrossRef]
- Wereski, R.; Chapman, A.R.; Lee, K.K.; Smith, S.W.; Lowe, D.J.; Gray, A.; Mills, N.L. High-Sensitivity Cardiac Troponin Concentrations at Presentation in Patients With ST-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2020, 5, 1302–1304. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Anand, A.; Sandoval, Y.; Lee, K.K.; Smith, S.W.; Adamson, P.D.; Chapman, A.R.; Langdon, T.; Sandeman, D.; Vaswani, A.; et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: A cohort study. Lancet 2015, 386, 2481–2488. [Google Scholar] [CrossRef]
- Mills, N.L.; Omland, T. Cardiac Troponin to Guide the Use of Noninva-sive Testing in Patients Ruled out for Myocardial Infarction. Circulation 2019, 139, 1655–1657. [Google Scholar] [CrossRef]
- Farmakis, D.; Mueller, C.; Apple, F.S. High-sensitivity cardiac troponin assays for cardiovascular risk stratification in the general population. Eur. Hear. J. 2020, 41, 4050–4056. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Passino, C.; Aspromonte, N.; Piepoli, M.F.; Migliardi, M.; Perrone, M.; Fortunato, A.; Padoan, A.; Testa, A.; et al. Evidence on clinical relevance of cardiovascular risk evaluation in the general population using cardio-specific biomarkers. Clin. Chem. Lab. Med. 2021, 59, 79–90. [Google Scholar] [CrossRef]
- Antman, E.M.; Cohen, M.; Bernink, P.J.L.M.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI Risk Score for Unstable Angina/Non–St Elevation Mi: A Method for Prognostication and Therapeutic Decision Making. JAMA 2000, 284, 835. [Google Scholar] [CrossRef]
- Six, A.J.; Backus, B.E.; Kelder, J.C. Chest pain in the emergency room: Value of the HEART score. Neth. Hear. J. 2008, 16, 191–196. [Google Scholar] [CrossRef]
- Nestelberger, T.; Boeddinghaus, J.; Wussler, D.; Twerenbold, R.; Badertscher, P.; Wildi, K.; Miró, Ò.; López, B.; Martin-Sanchez, F.J.; Muzyk, P.; et al. Predicting Major Adverse Events in Patients With Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 74, 842–854. [Google Scholar] [CrossRef]
- Than, M.P.; Pickering, J.W.; Sandoval, Y.; Shah, A.S.; Tsanas, A.; Apple, F.S.; Blankenberg, S.; Cullen, L.; Mueller, C.; Neumann, J.T.; et al. Machine Learning to Predict the Likelihood of Acute Myocardial Infarction. Circulation 2019, 140, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Twerenbold, R.; Badertscher, P.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Puelacher, C.; Sabti, Z.; Gimenez, M.R.; Tschirky, S.; Lavallaz, J.D.F.D.; et al. 0/1-Hour Triage Algorithm for Myocardial Infarction in Patients With Renal Dysfunction. Circulation 2018, 137, 436–451. [Google Scholar] [CrossRef] [PubMed]
| Reported Risk Factors | |
| Age (years) | 56 ± 16 |
| Males | 2380 (53%) |
| Current smokers | 1142 (26%) |
| Hypertension | 1812 (41%) |
| Hypercholesterolemia | 1911 (43%) |
| Diabetes mellitus | 603 (14%) |
| Family history of early ischemic heart disease | 143 (3.2%) |
| Reported Patient History | |
| Previous myocardial infarction | 523 (12%) |
| Previous percutaneous coronary intervention | 448 (10%) |
| Previous coronary bypass surgery | 59 (1.3%) |
| Previous admission for heart failure | 111 (2.5%) |
| Peripheral artery disease | 60 (1.3%) |
| Previous stroke | 141 (3.2%) |
| Chest Pain Characteristics at Presentation | |
| Effort-related chest pain in the previous week | 481 (11%) |
| Recurrent chest pain in the last 24 h | 289 (6.5%) |
| Physical Measures at Presentation | |
| Admission systolic blood pressure (mmHg) | 139 ± 21 |
| Admission diastolic blood pressure (mmHg) | 80 ± 14 |
| Admission heart rate (beats/minute) | 79 ± 15 |
| Electrocardiogram at Presentation | |
| ST-segment depression ≥ 0.5 mm | 155 (3.5%) |
| T-wave inversion ≥ 1 mm | 296 (6.6%) |
| Admission atrial fibrillation (ventricular rate < 100 beats/min) | 165 (3.7%) |
| Left bundle branch block | 67 (1.5%) |
| Permanent pacemaker | 27 (0.6%) |
| Troponin | |
| Admission hs-cTnT (ng/L) | 6.9 ± 2.5 |
| Admission undetectable hs-cTnT (<5 ng/L) | 1847 (41%) |
| Time from chest pain onset to hs-cTnT determination (minutes) * | 270 (162 to 655) |
| Second hs-cTnT determination | 1438 (32%) |
| Other Admission Blood Tests | |
| Hemoglobin (g/dL) | 14.1 ± 1.5 |
| Creatinine (mg/dL) | 0.85 ± 0.2 |
| Management at the Index Episode | |
| Hospitalization at the index episode | 329 (7.4%) |
| Exercise testing | 122 (2.7%) |
| Stress cardiac magnetic resonance | 98 (2.3%) |
| Invasive coronary angiogram | 183 (4.1%) |
| One Year | NPV | PPV | S | Sp |
|---|---|---|---|---|
| Primary endpoint | 99.1 (98.5 to 99.4) | 5.9 (5.1 to 6.9) | 90.2 (84.8 to 93.8) | 42.5 (41.1 to 44.0) |
| Secondary endpoint | 98.8 (98.2 to 99.2) | 8.7 (7.7 to 9.9) | 91.3 (87.1 to 94.2) | 43.2 (41.7 to 44.7) |
| 30 days | ||||
| Primary endpoint | 99.5 (99.1 to 99.7) | 3.2 (2.6 to 3.9 | 90.3 (82.6 to 94.8) | 41.9 (40.5 to 43.4) |
| Secondary endpoint | 99.3 (98.8 to 99.6) | 5.4 (4.6 to 6.4) | 91.7 (86.3 to 95.1) | 42.5 (41.0 to 43.9) |
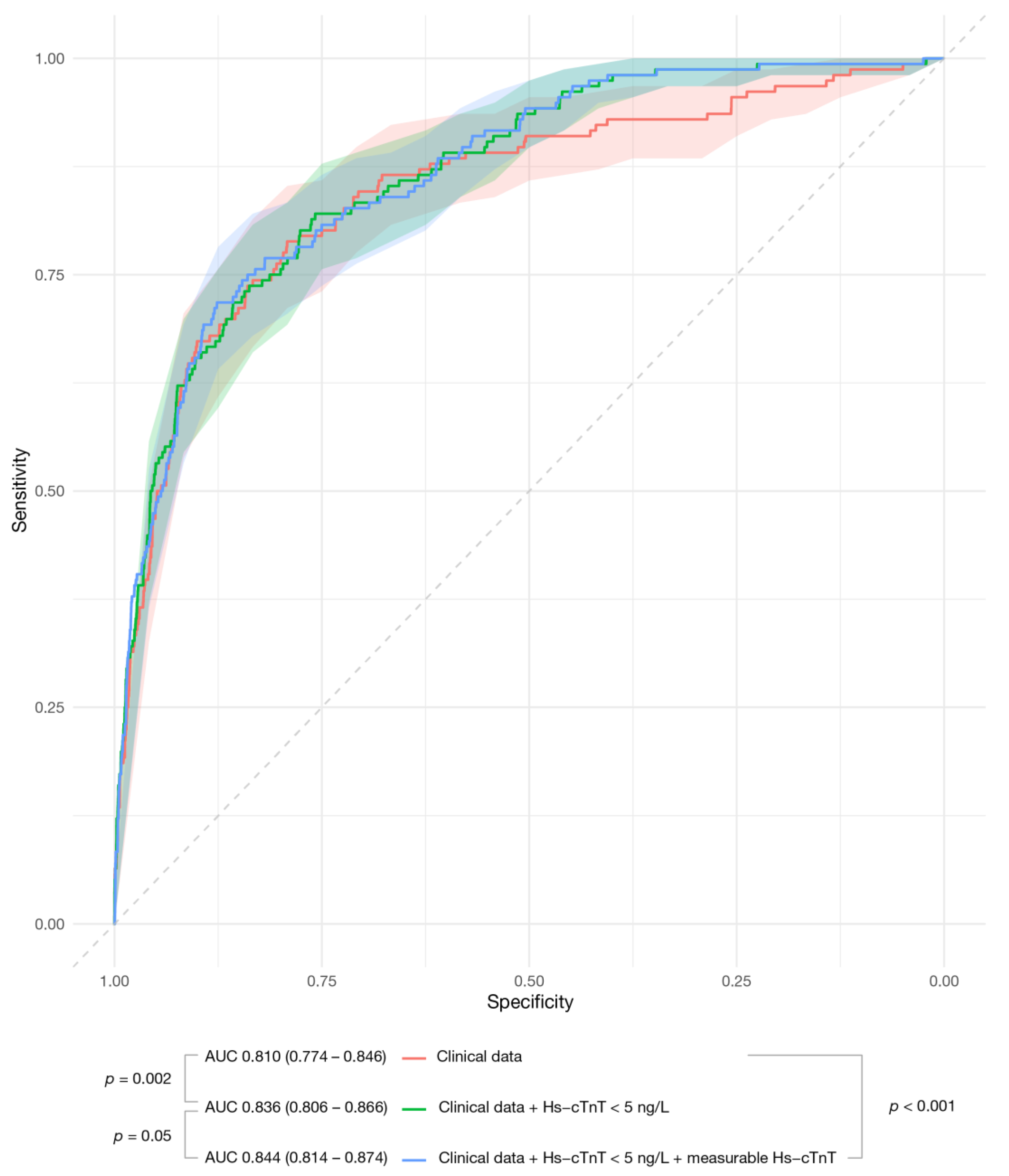
| One-Year | Clinical Data | Clinical Data + Hs-cTnT ≥ 5 ng/L | Clinical Data + Hs-cTnT ≥ 5 ng/L + Measured Hs-cTnT |
|---|---|---|---|
| Primary endpoint | 0.810 (0.775–0.846) | 0.836 (0.806–0.866) 0.002 a | 0.844 (0.814–0.874) 0.05 b |
| Secondary endpoint | 0.847 (0.820–0.875) | 0.871 (0.849–0.896) <0.0001 a | 0.876 (0.854–0.898) 0.04 b |
| 30 days | |||
| Primary endpoint | 0.817 (0.767–0.867) | 0.839 (0.798–0.880) 0.05 a | 0.840 (0.799–0.881) 0.6 b |
| Secondary endpoint | 0.847 (0.811–0.883) | 0.866 (0.837–0.895) 0.05 a | 0.866 (0.837–0.896) 0.9 b |
| Adding Hs-cTnT ≥ 5 ng/L to Clinical Data | Adding Measured hs-cTnT to Clinical Data Plus Hs-cTnT ≥ 5 ng/L | ||
|---|---|---|---|
| IDI | Continuos NRI | IDI | Continuos NRI |
| 0.0090 (−0.0001–0.019) p = 0.05 | 0.2859 (0.1867–0.3422) p = 0.001 | 0.006 (−0.001–0.0211) p = 0.1 | −0.0277 (−0.1106–0.0560) p = 0.6 |
| Clinical Data | Clinical Data + Hs-cTnT ≥ 5 ng/L | Clinical Data + Hs-cTnT ≥ 5 ng/L + Measured Hs-cTnT | |
|---|---|---|---|
| Age | |||
| <70 years (n = 3487) | 0.808 (0.762–0.853) | 0.841 (0.804–0.879) 0.008 a | 0.848 (0.811–0.885) 0.1 b |
| ≥70 years (n = 989) | 0.788 (0.709–0.864) | 0.794 (0.720–0.868) 0.003 a | 0.811 (0.741–0.880) 0.3 b |
| Gender | |||
| Female (n = 2096) | 0.853 (0.803–0.903) | 0.874 (0.831–0.917) 0.1989 a | 0.884 (0.843–0.925) 0.06 b |
| Male (n = 2380) | 0.800 (0.753–0.846) | 0.819 (0.778–0.860) 0.02 a | 0.822 (0.780–0.864) 0.5 b |
| Time from chest pain onset to blood sample c | |||
| ≤180 min (n = 1461) | 0.781 (0.728–0.834) | 0.805 (0.760–0.851) 0.04 a | 0.806 (0.760–0.852) 1 b |
| >180 min (n = 2822) | 0.848 (0.798–0.897) | 0.874 (0.834–0.913) 0.04 a | 0.889 (0.852–0.926) 0.03 b |
| Admission creatinine | |||
| <1.3 mg/dL (n = 4337) | 0.800 (0.761–0.838) | 0.828 (0.796–0.860) 0.002 a | 0.837 (0.805–0.869) 0.07 b |
| ≥1.3 mg/dL (n = 139) | 0.924 (0.870–0.977) | 0.928 (0.876–0.981) 0.6 a | 0.926 (0.872–0.980) 0.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Cisnal, A.; Valero, E.; García-Blas, S.; Pernias, V.; Pozo, A.; Carratalá, A.; González, J.; Noceda, J.; Miñana, G.; Núñez, J.; et al. Clinical History and Detectable Troponin Concentrations below the 99th Percentile for Risk Stratification of Patients with Chest Pain and First Normal Troponin. J. Clin. Med. 2021, 10, 1784. https://doi.org/10.3390/jcm10081784
Fernández-Cisnal A, Valero E, García-Blas S, Pernias V, Pozo A, Carratalá A, González J, Noceda J, Miñana G, Núñez J, et al. Clinical History and Detectable Troponin Concentrations below the 99th Percentile for Risk Stratification of Patients with Chest Pain and First Normal Troponin. Journal of Clinical Medicine. 2021; 10(8):1784. https://doi.org/10.3390/jcm10081784
Chicago/Turabian StyleFernández-Cisnal, Agustín, Ernesto Valero, Sergio García-Blas, Vicente Pernias, Adela Pozo, Arturo Carratalá, Jessika González, José Noceda, Gema Miñana, Julio Núñez, and et al. 2021. "Clinical History and Detectable Troponin Concentrations below the 99th Percentile for Risk Stratification of Patients with Chest Pain and First Normal Troponin" Journal of Clinical Medicine 10, no. 8: 1784. https://doi.org/10.3390/jcm10081784
APA StyleFernández-Cisnal, A., Valero, E., García-Blas, S., Pernias, V., Pozo, A., Carratalá, A., González, J., Noceda, J., Miñana, G., Núñez, J., & Sanchis, J. (2021). Clinical History and Detectable Troponin Concentrations below the 99th Percentile for Risk Stratification of Patients with Chest Pain and First Normal Troponin. Journal of Clinical Medicine, 10(8), 1784. https://doi.org/10.3390/jcm10081784






