Antithrombotic Management and Long-Term Outcomes of Patients with Atrial Fibrillation. Insights from CRAFT Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Primary and Secondary Endpoints
2.3. Assessment of Bleeding Risk Scores
2.4. Assessment of Thromboembolic Risk Scores
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Baseline Characteristics
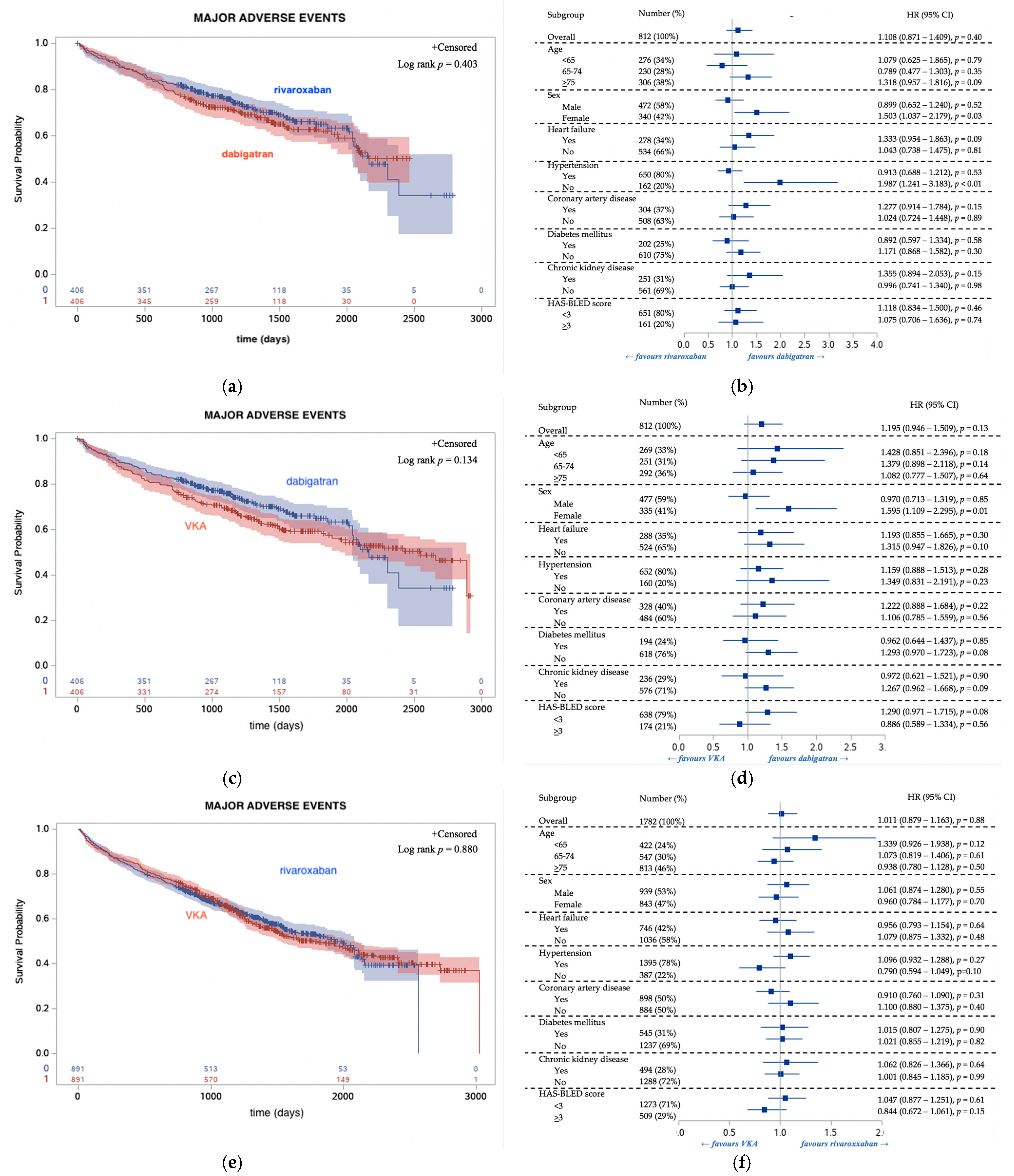
3.3. Follow Up Outcomes Regarding OAC Type
3.4. Reduced and Standard Doses of NOACs
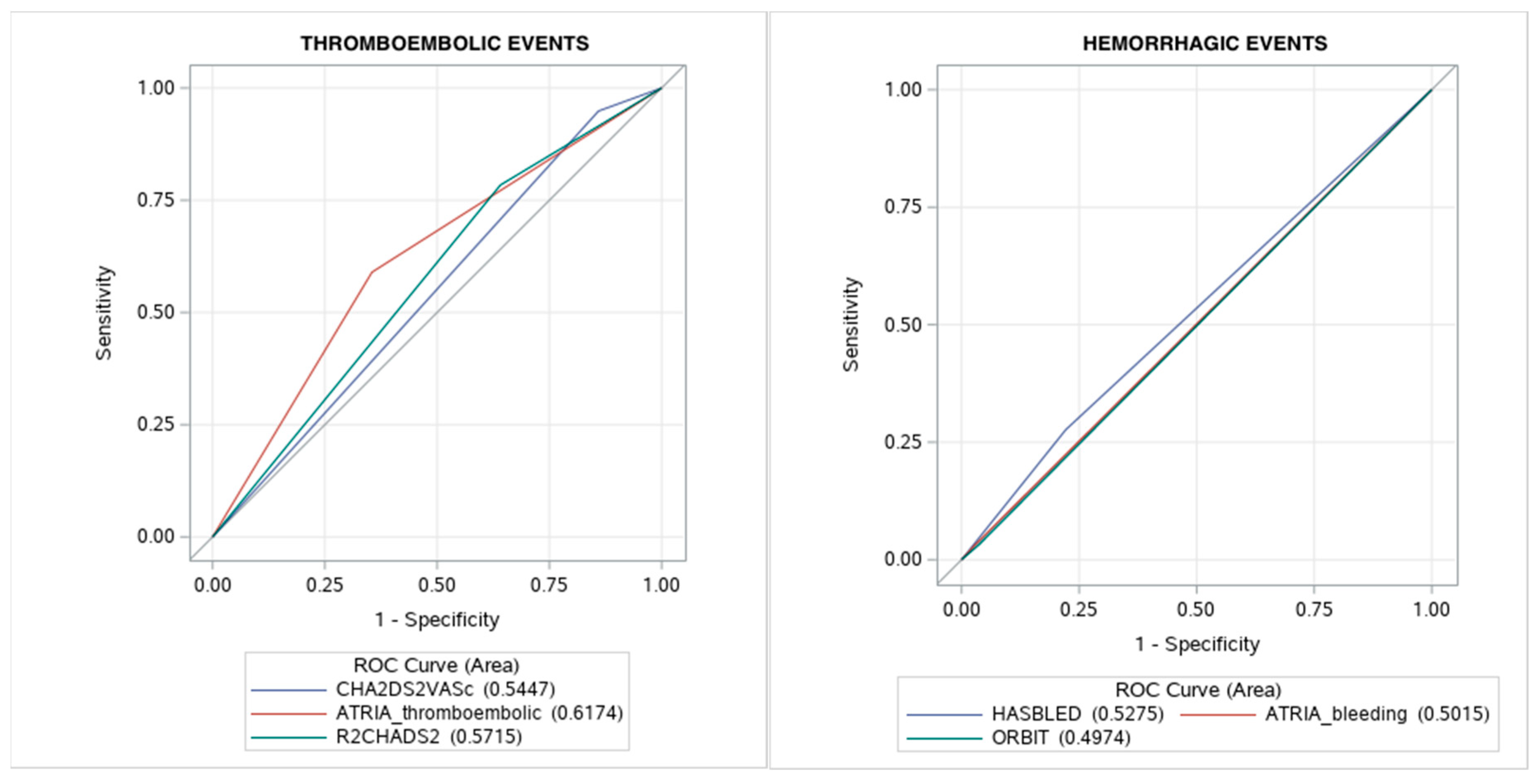
3.5. Thromboembolic and Bleeding Risk Scores
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirchhof, P.; Benussi, S.; Kotecha, D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef]
- Ntaios, G.; Papavasileiou, V.; Diener, H.C.; Makaritsis, K.; Michel, P. Nonvitamin-K-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: An updated systematic review and me-ta-analysis of randomized controlled trials. Int. J. Stroke 2017, 12, 589–596. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Bai, Y.; Shantsila, A.; Lip, G.Y. Response by Bai et al to Letter Regarding Article, “Rivaroxaban Versus Dabigatran or Warfarin in Real-World Studies of Stroke Prevention in Atrial Fibrillation: Systematic Review and Meta-Analysis”. Stroke 2017, 48, e149. [Google Scholar] [CrossRef] [PubMed]
- Balsam, P.; Ozierański, K.; Tymińska, A.; Zukowska, K.; Zaleska, M.; Szepietowska, K.; Maciejewski, K.; Peller, M.; Grabowski, M.; Lodzinski, P.; et al. Comparison of clinical characteristics of real-life atrial fibrillation patients treated with vitamin K antagonists, dabigatran, and rivaroxaban: Results from the CRAFT study. Kardiologia Polska 2018, 76, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic Therapy to Prevent Stroke in Patients Who Have Nonvalvular Atrial Fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef]
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Hsueh, Y.-H.; Izem, R.; Southworth, M.R.; Wei, Y.; Liao, J.; Goulding, M.R.; Mott, K.; et al. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Beneficiaries Treated with Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA Intern. Med. 2016, 176, 1662–1671. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; Bellolio, M.F.; McBane, R.D.; Shah, N.D.; Noseworthy, P.A. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003725. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; McBane, R.D.; Shah, N.D. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest 2016, 150, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.B.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Lip, G.Y.H. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2016, 353, i3189. [Google Scholar] [CrossRef]
- Hernandez, I.; Zhang, Y.; Saba, S. Comparison of the Effectiveness and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Newly Diagnosed Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Keshishian, A.; Kamble, S.; Pan, X.; Mardekian, J.; Horblyuk, R.; Hamilton, M. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. Thromb. Haemost. 2016, 116, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Larsen, T.B.; Skjøth, F.; Rasmussen, L.H. Indirect Comparisons of New Oral Anticoagulant Drugs for Efficacy and Safety When Used for Stroke Prevention in Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 738–746. [Google Scholar] [CrossRef]
- Wang, K.-L.; Lopes, R.D.; Patel, M.R.; Büller, H.R.; Tan, D.S.-Y.; Chiang, C.-E.; Giugliano, R.P. Efficacy and safety of reduced-dose non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Eur. Hear. J. 2018, 40, 1492–1500. [Google Scholar] [CrossRef]
- Liu, M.-S.; Liao, Y.; Li, G.-Q. Glomerular Filtration Rate is Associated with Hemorrhagic Transformation in Acute Ischemic Stroke Patients without Thrombolytic Therapy. Chin. Med J. 2018, 131, 1639–1644. [Google Scholar] [CrossRef]
- Lip, G.Y.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Larsen, T.B. The HAS-BLED, ATRIA, and ORBIT Bleeding Scores in Atrial Fibrillation Patients Using Non-Vitamin K Antagonist Oral Anticoagulants. Am. J. Med. 2018, 131, 574.e13–574.e27. [Google Scholar] [CrossRef]
- Abumuaileq, R.R.; Abu-Assi, E.; Lopez-Lopez, A. Comparison between CHA2DS2-VASc and the new R2CHADS2 and ATRIA scores at predicting thromboembolic event in non-anticoagulated and anticoagulated patients with non-valvular atri-al fibrillation. BMC Cardiovasc. Disord. 2015, 15, 156. [Google Scholar] [CrossRef]
- Roldán, V.; Marín, F.; Manzano-Fernández, S.; Gallego, P.; Vílchez, J.A.; Valdés, M.; Vicente, V.; Lip, G.Y. The HAS-BLED Score Has Better Prediction Accuracy for Major Bleeding Than CHADS2 or CHA2DS2-VASc Scores in Anticoagulated Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 62, 2199–2204. [Google Scholar] [CrossRef]
- Thigpen, J.L.; Dillon, C.; Forster, K.B.; Henault, L.; Quinn, E.K.; Tripodis, Y.; Berger, P.B.; Hylek, E.M.; Limdi, N.A. Validity of International Classification of Disease Codes to Identify Ischemic Stroke and Intracranial Hemorrhage Among Individuals With Associated Diagnosis of Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
| Thromboembolic Risk Scores | Bleeding Risk Scores | |||||
|---|---|---|---|---|---|---|
| Variable | CHA2DS2-VASc | ATRIA (Thromboembolic Risk) | R2CHADS2 | HAS-BLED | ATRIA (Bleeding Risk) | ORBIT |
| Heart failure | 1 | 1 | 1 | |||
| Hypertension | 1 | 1 | 1 | 1 | 1 | |
| Age > 85 years | 2 | 6 or 9 (if stroke) | 1 | 1 | 2 | 1 |
| Age 75–84 years | 5 or 7 (if stroke) | |||||
| Age 65–74 years | 1 | 3 or 7 (if stroke) | ||||
| Diabetes mellitus | 1 | 1 | 1 | |||
| Ischemic stroke/TIA | 2 | 8 | 2 | 1 | ||
| Vascular disease | 1 | |||||
| Female sex | 1 | 1 | ||||
| eGFR < 60 mL/min/1.73 m2 | 2 | 1 | ||||
| eGFR < 45 mL/min/1.73 m2 | 1 | |||||
| eGFR < 30 mL/min/1.73 m2 | 1 | 3 | ||||
| Liver impairment | 1 | |||||
| Labile INR | 1 | |||||
| Excess alcohol usage | 1 | |||||
| Drugs (antiplatelet drugs, NSAIDs) | 1 | |||||
| Antiplatelet drugs | 1 | |||||
| Prior bleeding | 1 | 1 | 2 | |||
| Low hemoglobin | 3 | 2 | ||||
| Results | ||||||
| Low risk | 0 | 0–5 | 0 | 0–1 | 0–3 | 0–2 |
| Intermediate risk | 1 | 6 | 1 | 2 | 4 | 3 |
| High risk | 2–9 | 7–14 a | 2–8 | 3–9 | 5–10 | 4–7 |
| Variable | Rivaroxaban (n = 891) | Dabigatran (n = 406) | VKA (n = 1686) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, median (IQR) | 74 (65–81) | 69 (62–78) | 68 (61–78) | <0.01 |
| Females, n (%) | 420 (47%) | 166 (41%) | 638 (38%) | <0.01 |
| Atrial Fibrillation Type, n (%) | ||||
| Paroxysmal | 467 (56%) n = 831 | 208 (53%) n = 391 | 822 (51%) n = 1622 | 0.02 |
| Long-standing persistent | 8 (1.0%) n = 831 | 10 (2.6%) n = 391 | 81 (5.0%) n = 1622 | <0.01 |
| Persistent | 99 (12%) n = 831 | 78 (20%) n = 391 | 203 (13%) n = 1622 | <0.01 |
| Permanent | 257 (31%) n = 831 | 95 (24%) n = 391 | 516 (32%) n = 1622 | <0.01 |
| Comorbidities, n (%) | ||||
| Heart failure | 381 (43%) | 149 (37%) n = 404 | 655 (39%) n = 1683 | 0.07 |
| Hypertension | 689 (77%) n = 890 | 326 (80%) | 1348 (80%) n = 1683 | 0.24 |
| Coronary artery disease | 447 (15%) | 150 (39%) | 759 (45%) | <0.01 |
| Diabetes mellitus | 274 (31%) n = 887 | 94 (23%) n = 405 | 486 (29%) n = 1678 | 0.02 |
| History of TEs | 157 (18%) n = 889 | 58 (14%) n = 404 | 208 (12%) n = 1682 | <0.01 |
| History of HEs | 98 (11%) | 30 (7.4%) | 113 (6.7%) | <0.01 |
| COPD | 114 (13%) n = 890 | 28 (6.9%) n = 405 | 142 (8.4%) n = 1683 | <0.01 |
| CKD | 123 (5.4%) n = 576 | 49 (17%) n = 285 | 329 (23%) n = 1409 | 0.07 |
| Smoking | 57 (6.4%) | 32 (7.9%) n = 405 | 76 (4.5%) n = 1677 | 0.01 |
| Device therapy (PM, ICD, CRT) | 237 (27%) | 84 (21%) | 456 (27%) | 0.03 |
| Laboratory Parameters | ||||
| Hemoglobin, g/dL, median (IQR) | 14 (13–15) n = 574 | 14 (13–15) n = 285 | 14 (13–15) n = 1399 | <0.01 |
| Platelet count (thousand/mm3, median (IQR)) | 205 (172–242) n = 574 | 210 (174–248) n = 285 | 202 (166–237) n = 1403 | 0.01 |
| eGFR ≤ 14 (mL/min/1.73 m2), n (%) | 3 (0.4%) n = 831 | 0 (0%) n = 353 | 3 (0.3%); n = 1174 | <0.01 |
| eGFR 15–29 (mL/min/1.73 m2), n (%) | 18 (2.2%) n = 831 | 3 (0.9%) n = 353 | 56 (4.8%) n = 1174 | <0.01 |
| eGFR 30–49 (mL/min/1.73 m2), n (%) | 178 (21%) n = 831 | 67 (19%) n = 353 | 312 (27%) n = 1174 | <0.01 |
| eGFR ≥ 50 (mL/min/1.73 m2), n (%) | 632 (76%) n = 831 | 283 (80%) n = 353 | 803 (68%) n = 1174 | <0.01 |
| Thromboembolic and Bleeding Scores | ||||
| CHA2DS2-VASc score, median (IQR) | 4 (3–5) | 3 (2–5) | 3 (2–5) | <0.01 |
| HAS-BLED score, median (IQR) | 2 (1–3) | 2 (1–2) | 2 (1–2) | 0.06 |
| Other medications, n (%) | ||||
| Antiplatelet drugs | 94 (11%) | 34 (8.4%) | 307 (18%) | <0.01 |
| Beta-blockers | 478 (83%) n = 576 | 230 (81%) n = 285 | 1199 (85%) n = 1409 | 0.13 |
| Calcium channel blockers | 162 (28%) n = 576 | 68 (24%) n = 285 | 294 (21%) n = 1409 | <0.01 |
| Antiarrhythmic drugs | 163 (18%) | 74 (18%) n = 405 | 274 (16%) n = 1684 | 0.35 |
| RAS inhibitors | 457 (79%) n = 576 | 225 (79%) n = 285 | 1186 (83%) n = 1410 | 0.12 |
| Statins | 397 (69%) n = 576 | 172 (60%) n = 285 | 970 (69%) n = 1410 | 0.02 |
| Long Term Outcomes, n (%) | ||||
| MAEs | 373 (42%) | 126 (31%) | 729 (43%) | <0.01 |
| All-cause death | 250 (28%) | 89 (22%) | 489 (29%) | 0.02 |
| TEs | 92 (10%) | 30 (7.4%) | 151 (9.0%) | 0.22 |
| HEs | 128 (14%) | 33 (8.1%) | 284 (17%) | <0.01 |
| Major Adverse Event | All-Cause Death | Thromboembolic Events | Hemorrhagic Events | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| (A) Dabigatran Standard and Reduced Doses | ||||
| Rivaroxaban reduced (n = 131) | 2.242 (1.608–3.125) | 3.044 (1.973–4.697) | 2.340 (1.180–4.637) | 1.757 (1.020–3.026) |
| Rivaroxaban standard (n = 275) | reference | reference | reference | reference |
| (B) Rivaroxaban Standard and Reduced Doses | ||||
| Dabigatran reduced (n = 177) | 2.793 (1.935–4.032) | 4.716 (2.887–7.703) | 0.737 (0.345–1.576) | 2.034 (1.019–4.060) |
| Dabigatran standard (n = 229) | reference | reference | reference | reference |
| (C) Dabigatran Standard and Rivaroxaban Standard Doses | ||||
| Rivaroxaban standard (n = 275) | 1.428 (0.985–2.071) | 1.377 (0.807–2.350) | 0.680 (0.356–1.298) | 1.922 (1.026–3.602) |
| Dabigatran standard (n = 229) | reference | reference | reference | reference |
| (D) Dabigatran Reduced and Rivaroxaban Reduced Doses | ||||
| Rivaroxaban reduced (n = 131) | 1.131 (0.817–1.567) | 0.898 (0.616–1.310) | 2.149 (0.975–4.737) | 1.606 (0.869–2.969) |
| Dabigatran reduced (n = 177) | reference | reference | reference | reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balsam, P.; Lodziński, P.; Gawałko, M.; Kraj, L.; Śliwczyński, A.; Maciejewski, C.; Krzowski, B.; Tymińska, A.; Ozierański, K.; Grabowski, M.; et al. Antithrombotic Management and Long-Term Outcomes of Patients with Atrial Fibrillation. Insights from CRAFT Trial. J. Clin. Med. 2021, 10, 1780. https://doi.org/10.3390/jcm10081780
Balsam P, Lodziński P, Gawałko M, Kraj L, Śliwczyński A, Maciejewski C, Krzowski B, Tymińska A, Ozierański K, Grabowski M, et al. Antithrombotic Management and Long-Term Outcomes of Patients with Atrial Fibrillation. Insights from CRAFT Trial. Journal of Clinical Medicine. 2021; 10(8):1780. https://doi.org/10.3390/jcm10081780
Chicago/Turabian StyleBalsam, Paweł, Piotr Lodziński, Monika Gawałko, Leszek Kraj, Andrzej Śliwczyński, Cezary Maciejewski, Bartosz Krzowski, Agata Tymińska, Krzysztof Ozierański, Marcin Grabowski, and et al. 2021. "Antithrombotic Management and Long-Term Outcomes of Patients with Atrial Fibrillation. Insights from CRAFT Trial" Journal of Clinical Medicine 10, no. 8: 1780. https://doi.org/10.3390/jcm10081780
APA StyleBalsam, P., Lodziński, P., Gawałko, M., Kraj, L., Śliwczyński, A., Maciejewski, C., Krzowski, B., Tymińska, A., Ozierański, K., Grabowski, M., Bednarski, J., & Opolski, G. (2021). Antithrombotic Management and Long-Term Outcomes of Patients with Atrial Fibrillation. Insights from CRAFT Trial. Journal of Clinical Medicine, 10(8), 1780. https://doi.org/10.3390/jcm10081780








