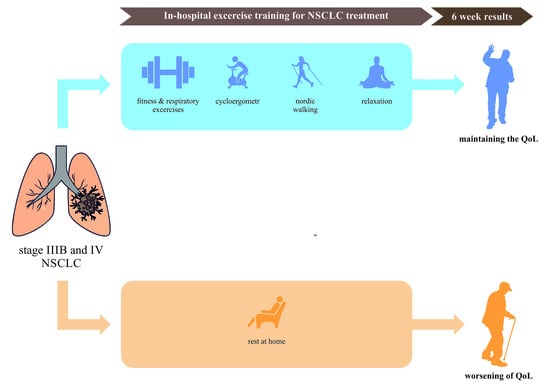
Short-Term Changes in Quality of Life in Patients with Advanced Lung Cancer during In-Hospital Exercise Training and Chemotherapy Treatment: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcomes
2.2.1. St. George’s Respiratory Questionnaire
2.2.2. The Short Form (36) Health Survey
2.2.3. Functional Assessment of Cancer Therapy-Lung
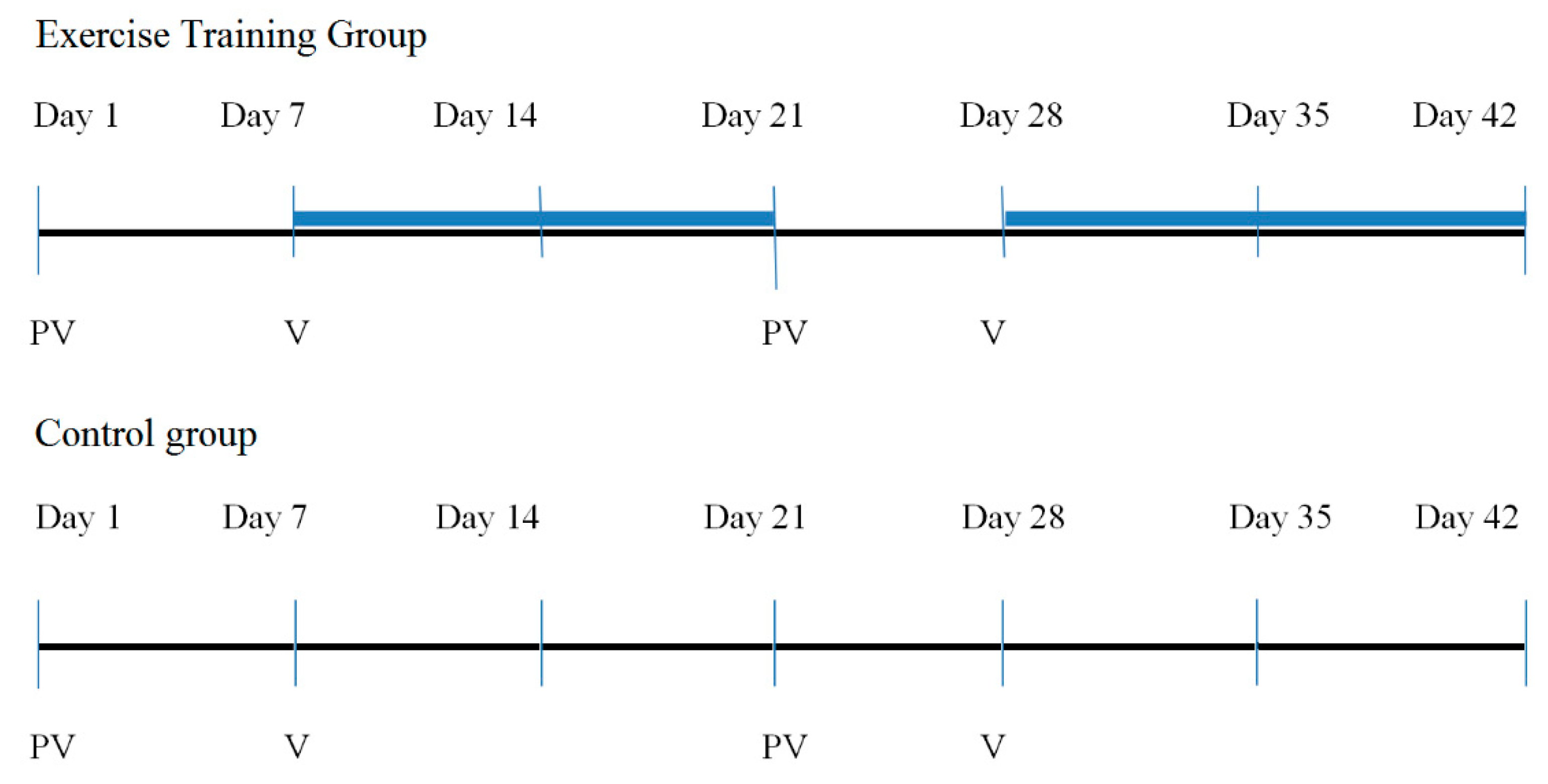
2.3. Interventions
2.4. Statistical Analysis
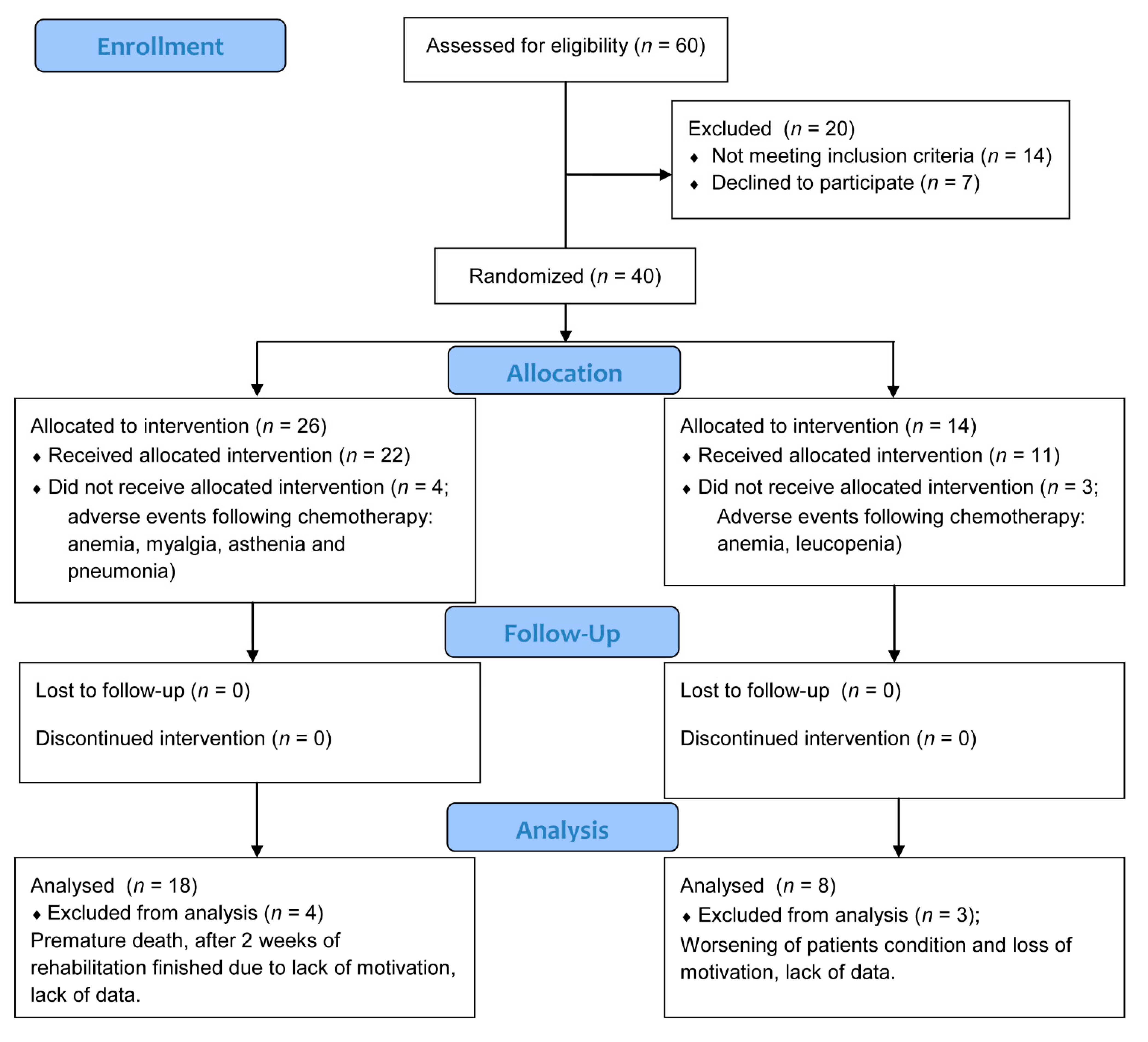
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronesi, G.; Baldwin, D.R.; Henschke, C.I.; Ghislandi, S.; Iavicoli, S.; Oudkerk, M.; De Koning, H.J.; Shemesh, J.; Field, J.K.; Zulueta, J.J.; et al. Recommendations for Implementing Lung Cancer Screening with Low-Dose Computed Tomography in Europe. Cancers 2020, 12, 1672. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- John, L.D. Self-Care Strategies Used by Patients with Lung Cancer to Promote Quality of Life. Oncol. Nurs. Forum 2010, 37, 339–347. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Polanski, J.; Jankowska-Polanska, B.; Rosinczuk, J.; Chabowski, M.; Szymanska-Chabowska, A. Quality of life of patients with lung cancer. OncoTargets Ther. 2016, 9, 1023–1028. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Cramb, S.M.; Baade, P.D.; Youlden, D.R.; Nwogu, C.; Reid, M.E. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J. Thorac. Oncol. 2016, 11, 1653–1671. [Google Scholar] [CrossRef]
- Braun, D.P.; Gupta, D.; Staren, E.D. Quality of life assessment as a predictor of survival in non-small cell lung cancer. Bmc Cancer 2011, 11, 353. [Google Scholar] [CrossRef]
- Sommer, M.S.; Vibe-Petersen, J.; Staerkind, M.B.; Langer, S.W.; Larsen, K.R.; Trier, K.; Christensen, M.; Clementsen, P.F.; Missel, M.; Christensen, K.B.; et al. Early initiated postoperative rehabilitation enhances quality of life in patients with operable lung cancer: Secondary outcomes from a randomized trial. Lung Cancer 2020, 146, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Crevenna, R.; Marosi, C.; Schmidinger, M.; Fialka-Moser, V. Neuromuscular electrical stimulation for a patient with metastatic lung cancer—A case report. Support. Care Cancer 2006, 14, 970–973. [Google Scholar] [CrossRef]
- Tanaka, K.; Akechi, T.; Okuyama, T.; Nishiwaki, Y.; Uchitomi, Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. J. Pain Symptom Manage. 2002, 23, 417–423. [Google Scholar] [CrossRef]
- Janssen, S.M.J.; Abbink, J.J.; Lindeboom, R.; Vlieland, T.P.M.V. Outcomes of Pulmonary Rehabilitation After Treatment for Non-Small Cell Lung Cancer Stages I to IIIa An Observational Study. J. Cardiopulm. Rehabil. Prev. 2017, 37, 65–71. [Google Scholar] [CrossRef]
- Spruit, M.A.; Janssen, P.P.; Willemsen, S.C.P.; Hochstenbag, M.M.H.; Wouters, E.F.M. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: A pilot study. Lung Cancer 2006, 52, 257–260. [Google Scholar] [CrossRef]
- Jastrzebski, D.; Maksymiak, M.; Kostorz, S.; Bezubka, B.; Osmanska, I.; Mlynczak, T.; Rutkowska, A.; Baczek, Z.; Ziora, D.; Kozielski, J. Pulmonary Rehabilitation in Advanced Lung Cancer Patients During Chemotherapy. Adv. Exp. Med. Biol. 2015, 861, 57–64. [Google Scholar] [CrossRef]
- Rutkowska, A.; Jastrzebski, D.; Rutkowski, S.; Zebrowska, A.; Stanula, A.; Szczegielniak, J.; Ziora, D.; Casaburi, R. Exercise Training in Patients with Non-Small Cell Lung Cancer During In-Hospital Chemotherapy Treatment: A Randomized Controlled Trial. J. Cardiopulm. Rehabil. Prev. 2019, 39, 127–133. [Google Scholar] [CrossRef]
- Rajarajeswaran, P.; Vishnupriya, R. Exercise in cancer. Indian J. Med. Paediatr. Oncol. 2009, 30, 61–70. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Goldberg, S.; Vogel, P.D.; Sullivan, M.; Pirl, W.F.; Lynch, T.J.; Christiani, D.C.; Smith, M.R. A structured exercise program for patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 595–601. [Google Scholar] [CrossRef]
- Shannon, V.R. Role of pulmonary rehabilitation in the management of patients with lung cancer. Curr. Opin. Pulm. Med. 2010, 16, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Cavalheri, V.; Granger, C.L. Exercise training as part of lung cancer therapy. Respirology 2020, 25, 80–87. [Google Scholar] [CrossRef]
- Bowen, T.S.; Cannon, D.T.; Murgatroyd, S.R.; Birch, K.M.; Witte, K.K.; Rossiter, H.B. The intramuscular contribution to the slow oxygen uptake kinetics during exercise in chronic heart failure is related to the severity of the condition. J. Appl. Physiol. 2012, 112, 378–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koelwyn, G.J.; Jones, L.W.; Hornsby, W.; Eves, N.D. Exercise therapy in the management of dyspnea in patients with cancer. Curr. Opin. Support. Palliat. Care 2012, 6, 129–137. [Google Scholar] [CrossRef]
- Wiskemann, J.; Hummler, S.; Diepold, C.; Keil, M.; Abel, U.; Steindorf, K.; Beckhove, P.; Ulrich, C.M.; Steins, M.; Thomas, M. Positive study: Physical exercise program in non-operable lung cancer patients undergoing palliative treatment. BMC Cancer 2016, 16, 499. [Google Scholar] [CrossRef]
- Belani, C.P.; Pereira, J.R.; von Pawel, J.; Pluzanska, A.; Gorbounova, V.; Kaukel, E.; Mattson, K.V.; Ramlau, R.; Szczesna, A.; Fidias, P.; et al. Effect of chemotherapy for advanced non-small cell lung cancer on patients’ quality of life. A randomized controlled trial. Lung Cancer 2006, 53, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Taylor-Stokes, G.; Roughley, A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer 2013, 81, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.; Damor, H. Validity of St. George’s respiratory questionnaire in lung cancer population. Int. J. Adv. Res. Ideas Innov. Technol. 2020, 5, 13–18. [Google Scholar]
- Ware, J.E., Jr.; Gandek, B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Moller, A.; Sartipy, U. Associations between changes in quality of life and survival after lung cancer surgery. J. Thorac. Oncol. 2012, 7, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.F.; Bonomi, A.E.; Lloyd, S.R.; Tulsky, D.S.; Kaplan, E.; Bonomi, P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995, 12, 199–220. [Google Scholar] [CrossRef]
- Hill, K.; Jenkins, S.C.; Cecins, N.; Philippe, D.L.; Hillman, D.R.; Eastwood, P.R. Estimating maximum work rate during incremental cycle ergometry testing from six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2008, 89, 1782–1787. [Google Scholar] [CrossRef]
- Morris, S.B. Estimating effect sizes from pretest-posttest-control group designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- Cohen, D. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 1988. [Google Scholar] [CrossRef]
- Wang, X.; Cao, H. A meta-analysis of comprehensive care on quality of life in patients with lung cancer. J. Cancer Res. Ther. 2015, 11 (Suppl. 1), C112–C114. [Google Scholar] [CrossRef]
- Henke, C.C.; Cabri, J.; Fricke, L.; Pankow, W.; Kandilakis, G.; Feyer, P.C.; de Wit, M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support. Care Cancer 2014, 22, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.L.; Yu, C.J.; Shih, J.Y.; Yang, P.C.; Wu, Y.T. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support. Care Cancer 2012, 20, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Dittus, K.L.; Gramling, R.E.; Ades, P.A. Exercise interventions for individuals with advanced cancer: A systematic review. Prev. Med. 2017, 104, 124–132. [Google Scholar] [CrossRef]
- Larsson, M.; Ljung, L.; Johansson, B.B. Health-related quality of life in advanced non-small cell lung cancer: Correlates and comparisons to normative data. Eur. J. Cancer Care 2012, 21, 642–649. [Google Scholar] [CrossRef]
- Dagnelie, P.C.; Pijls-Johannesma, M.C.G.; Lambin, P.; Beijer, S.; De Ruysscher, D.; Kempen, G.I.J.M. Impact of fatigue on overall quality of life in lung and breast cancer patients selected for high-dose radiotherapy. Ann. Oncol. 2007, 18, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, H.; Yang, P. Long-term survivorship in lung cancer: A review. Chest 2006, 129, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Cavalheri, V.; Burtin, C.; Formico, V.R.; Nonoyama, M.L.; Jenkins, S.; Spruit, M.A.; Hill, K. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst. Rev. 2019, 6, CD009955. [Google Scholar] [CrossRef]
- Brocki, B.C.; Andreasen, J.; Nielsen, L.R.; Nekrasas, V.; Gorst-Rasmussen, A.; Westerdahl, E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery—A randomized controlled trial. Lung Cancer 2014, 83, 102–108. [Google Scholar] [CrossRef]
- Riedl, D.; Giesinger, J.M.; Wintner, L.M.; Loth, F.L.; Rumpold, G.; Greil, R.; Nickels, A.; Licht, T.; Holzner, B. Improvement of quality of life and psychological distress after inpatient cancer rehabilitation: Results of a longitudinal observational study. Wien. Klin. Wochenschr. 2017, 129, 692–701. [Google Scholar] [CrossRef]
- Li, X.H.; Zhu, J.L.; Hong, C.; Zeng, L.; Deng, L.M.; Jin, L.Y. Effects of systematic rehabilitation programs on quality of life in patients undergoing lung resection. Mol. Clin. Oncol. 2013, 1, 200–208. [Google Scholar] [CrossRef][Green Version]
- Rutkowski, S.; Kiper, P.; Cacciante, L.; Cieslik, B.; Mazurek, J.; Turolla, A.; Szczepanska-Gieracha, J. Use of virtual reality-based training in different fields of rehabilitation: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00121. [Google Scholar] [CrossRef] [PubMed]
- Barsasella, D.; Liu, M.F.; Malwade, S.; Galvin, C.J.; Dhar, E.; Chang, C.C.; Li, Y.J.; Syed-Abdul, S. Effects of Virtual Reality Sessions on the Quality of Life, Happiness, and Functional Fitness among the Older People: A Randomized Controlled Trial from Taiwan. Comput. Methods Programs Biomed. 2021, 200, 105892. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, M.; Criado-Alvarez, J.J.; Corregidor-Sanchez, A.I.; Martin-Conty, J.L.; Mohedano-Moriano, A.; Polonio-Lopez, B. Effects of Virtual Reality-Based Therapy on Quality of Life of Patients with Subacute Stroke: A Three-Month Follow-Up Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 2810. [Google Scholar] [CrossRef]
- Gordon, E.M.; Li, B.M.; Liu, S.; Chawla, S.P.; Liu, S.M.; Liu, S.M. RELAX: An immersion virtual reality relaxation intervention for quality of life improvement in cancer patients. J. Clin. Oncol. 2018, 36, 152. [Google Scholar] [CrossRef]
- Rutkowski, S.; Szczegielniak, J.; Szczepanska-Gieracha, J. Evaluation of the efficacy of immersive virtual reality therapy as a method supporting pulmonary rehabilitation: A randomized controlled trial. J. Clin. Med. 2021, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Hechtner, M.; Eichler, M.; Wehler, B.; Buhl, R.; Sebastian, M.; Stratmann, J.; Schmidberger, H.; Gohrbandt, B.; Peuser, J.; Kortsik, C.; et al. Quality of Life in NSCLC Survivors—A Multicenter Cross-Sectional Study. J. Thorac. Oncol. 2019, 14, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
| Exercise Training Group (n = 18) | Control Group (n = 8) | p | |
|---|---|---|---|
| Age, mean (SD) | 60.4 (7.2) | 62.2 (9.0) | >0.05 |
| Male, n (%) | 16 (89) | 8 (100) | >0.05 |
| BMI, mean (SD) | 23.9 (3.7) | 22.1 (2.2) | >0.05 |
| Comorbidties | |||
| COPD, n (%) | 10 (55) | 4 (50) | >0.05 |
| Coronary artery disease history, n (%) | 3 (17) | 1 (13) | >0.05 |
| Diabetes, n (%) | 5 (28) | 3 (37) | >0.05 |
| Smoking history | |||
| Current | 0 (0) | 0 (0) | >0.05 |
| Former | 15 (83) | 6 (75) | >0.05 |
| Never | 3 (17) | 2 (25) | >0.05 |
| Performance status [ECOG/WHO] | |||
| 0, n (%) | 3 (17) | 1 (12) | >0.05 |
| 1, n (%) | 15 (83) | 7 (88) | >0.05 |
| Diagnosis | |||
| Adenocarcinoma, n (%) | 12 (67) | 5 (62) | >0.05 |
| Squamous cc, n (%) | 6 (33) | 3 (38) | >0.05 |
| TNM status | |||
| T2N2M0 | 14 (78) | 6 (75) | >0.05 |
| T2N2M1 | 4 (22) | 2 (15) | >0.05 |
| Tumor stage | |||
| IIIB, n (%) | 14 (78) | 6 (75) | >0.05 |
| IV, n (%) | 4 (22) | 2 (15) | >0.05 |
| ETG Pre | ETG Post | p * | CG Pre | CG Post | p * | Between Group Δ Post-Pre p † | Effect Size | |
|---|---|---|---|---|---|---|---|---|
| SYMPTOMS | 48.18 [27.86–60.65] | 37.53 [27.44–55.49] | <0.27 | 38.52 [6.65–55.64] | 28.36 [9.94–56.06] | <0.75 | 0.66 | −0.31 |
| ACTIVITY | 32.33 [11.21–53.53] | 38.30 [17.12–53.49] | <0.82 | 23.63 [11.87–41.42] | 29.93 [20.33–50.40] | <0.73 | 0.74 | −0.115 |
| IMPACT OF LIFE | 20.03 [8.21–41.00] | 17.43 [10.93–21.22] | <0.39 | 20.56 [9.92–25.65] | 17.25 [11.42–21.76] | <0.61 | 0.30 | −0.43 |
| GLOBAL | 24.61 [14.91–49.19] | 25.54 [17.15–31.36] | <0.35 | 26.08 [11.22–35.85] | 24.80 [10.45–30.41] | <0.50 | 0.50 | −0.171 |
| ETG Pre | ETG Post | p * | CG Pre | CG Post | p * | Between Group Δ Post-Pre p † | Effect Size | |
|---|---|---|---|---|---|---|---|---|
| PF | 47.75 [36.20–50.90] | 47.75 [38.10–52.90] | <0.36 | 52.80 [43.35–55.95] | 52.25 [37.05–54.80] | <0.07 | 0.15 | 0.199 |
| RP | 43.40 [28.00–56.20] | 42.10 [28.00–49.20] | <0.22 | 43.40 [39.75–56.90] | 47.10 [39.75–48.30] | <0.34 | 0.85 | −0.123 |
| BP | 39.65 [33.60–62.10] | 45.80 [37.50–51.60] | <0.48 | 53.25 [41.80–62.10] | 56.60 [48.20–62.10] | <0.28 | 0.93 | −0.074 |
| GH | 41.50 [33.60–46.20] | 41.05 [38.20–42.90] | <0.55 | 43.40 [37.70–48.20] | 48.20 [38.90–51.75] | <0.55 | 0.93 | −0.059 |
| VT | 51.65 [34.90–60.90] | 49.50 [42.00–58.50] | <0.81 | 55.20 [48.95–64.55] | 58.30 [34.95–61.45] | <0.26 | 0.22 | 0.506 |
| SF | 43.40 [30.00–51.10] | 41.75 [35.40–51.40] | <0.87 | 54.10 [43.20–56.80] | 51.40 [35.05–56.80] | <0.06 | 0.34 | 0.424 |
| RE | 55.30 [40.30–55.30] | 49.15 [23.70–55.30] | <0.44 | 46.15 [36.45–55.90] | 44.20 [44.20–53.95] | <0.89 | 0.93 | −0.094 |
| MH | 43.75 [34.50–52.70] | 47.70 [39.10–50.00] | <0.58 | 48.60 [38.75–57.05] | 48.60 [34.50–57.05] | <0.73 | 0.49 | 0.239 |
| PCS | 41.85 [35.30–48.90] | 42.85 [35.80–47.80] | <0.66 | 51.25 [42.55–54.05] | 50.55 [39.40–53.20] | <0.36 | 0.22 | 0.179 |
| MCS | 46.70 [40.70–52.30] | 47.25 [38.10–53.10] | <0.56 | 48.20 [42.15–57.80] | 47.25 [37.95–59.20] | <0.57 | 0.91 | 0.111 |
| ETG Pre | ETG Post | p * | CG Pre | CG Post | p * | Between Group Δ Post-Pre p † | Effect Size | |
|---|---|---|---|---|---|---|---|---|
| PWB | 20.5 [13.0–23.0] | 18.5 [15.0–24.0] | <0.84 | 25.5 [22.0–28.0] | 19.5 [16.5–25.0] | <0.02 | 0.02 | 0.753 |
| SWB | 22.5 [18.0–25.0] | 20.0 [18.0–24.0] | <0.40 | 25.5 [22.0–28.0] | 19.5 [16.5- 25.0] | <0.93 | 0.64 | −0.459 |
| EWB | 17.0 [8.0–22.0] | 16.5 [12.0–21.0] | <0.94 | 24.5 [19.0- 25.5] | 23.5 [18.5–25.5] | <0.34 | 0.50 | −0.524 |
| FWB | 18.5 [16.0- 21.0] | 19.5 [15.0- 24.0] | <0.56 | 17.5 [15.5–19.5] | 19.0 [15.5–22.0] | <0.44 | 0.78 | −0.251 |
| LC | 17.5 [16.0–24.0] | 22.5 [18.0–24.0] | <0.17 | 17.5 [15.5–22.0] | 22.0 [15.5–5.0] | <0.11 | 0.10 | 0.592 |
| FACT-L | 91.0 [76.0–113.0] | 92.5 [77.0–108.0] | <0.87 | 99.5 [95.5–118.0] | 106.0 [84.5–115.0] | <0.22 | 0.49 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, A.; Rutkowski, S.; Wrzeciono, A.; Czech, O.; Szczegielniak, J.; Jastrzębski, D. Short-Term Changes in Quality of Life in Patients with Advanced Lung Cancer during In-Hospital Exercise Training and Chemotherapy Treatment: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 1761. https://doi.org/10.3390/jcm10081761
Rutkowska A, Rutkowski S, Wrzeciono A, Czech O, Szczegielniak J, Jastrzębski D. Short-Term Changes in Quality of Life in Patients with Advanced Lung Cancer during In-Hospital Exercise Training and Chemotherapy Treatment: A Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(8):1761. https://doi.org/10.3390/jcm10081761
Chicago/Turabian StyleRutkowska, Anna, Sebastian Rutkowski, Adam Wrzeciono, Oliver Czech, Jan Szczegielniak, and Dariusz Jastrzębski. 2021. "Short-Term Changes in Quality of Life in Patients with Advanced Lung Cancer during In-Hospital Exercise Training and Chemotherapy Treatment: A Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 8: 1761. https://doi.org/10.3390/jcm10081761
APA StyleRutkowska, A., Rutkowski, S., Wrzeciono, A., Czech, O., Szczegielniak, J., & Jastrzębski, D. (2021). Short-Term Changes in Quality of Life in Patients with Advanced Lung Cancer during In-Hospital Exercise Training and Chemotherapy Treatment: A Randomized Controlled Trial. Journal of Clinical Medicine, 10(8), 1761. https://doi.org/10.3390/jcm10081761