PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder
Abstract
1. Introduction
2. Alcohol and Inflammation
2.1. Liver
2.2. Brain
2.3. Gut-Liver-Brain Axis
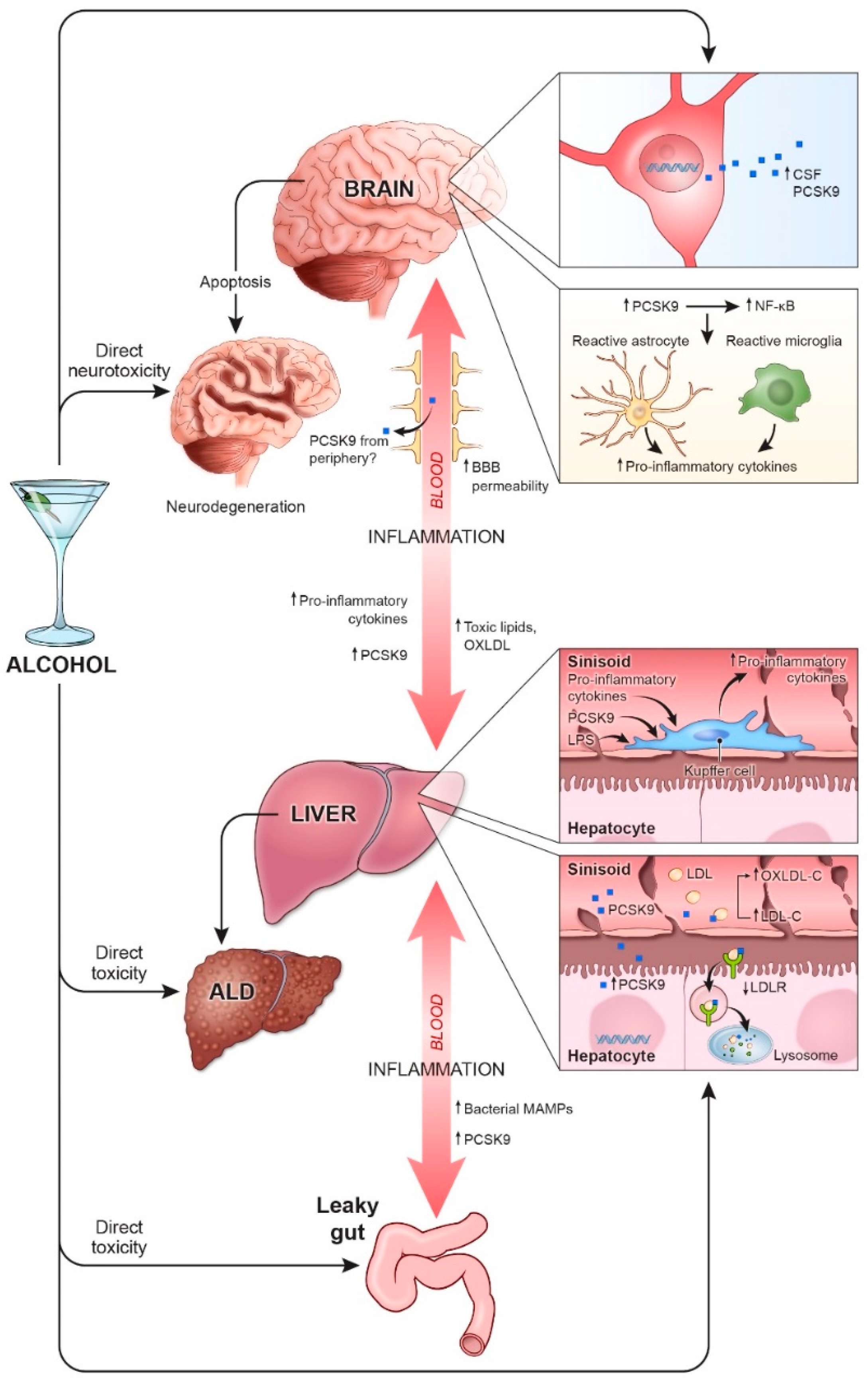
3. PCSK9, Alcohol, and Inflammation
3.1. Liver
3.2. Brain
3.3. Gut-Liver-Brain Axis
4. PCSK9 Inhibitors as a Potential Therapeutic Target for AUD and ALD
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention Excessive Drinking is Draining the U.S. Economy. Available online: https://www.cdc.gov/features/costsofdrinking/index.html#:~:text=Excessive%20alchol%20use%20is%20known,to%20losses%20in%20workplace%20productivity (accessed on 16 April 2021).
- Grant, B.F.; Chou, S.P.; Saha, T.D.; Pickering, R.P.; Kerridge, B.T.; Ruan, W.J.; Huang, B.; Jung, J.; Zhang, H.; Fan, A.; et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry 2017, 74, 911–923. [Google Scholar] [CrossRef]
- Lohoff, F.W. Pharmacotherapies and personalized medicine for alcohol use disorder: A review. Pharmacogenomics 2020, 21, 1117–1138. [Google Scholar] [CrossRef]
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr. Psychiatry Rep. 2019, 21, 10. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Heilig, M.; Perez, A.; Probst, C.; Rehm, J. Alcohol use disorders. Lancet 2019, 394, 781–792. [Google Scholar] [CrossRef]
- Yeh, M.M.; Brunt, E.M. Pathological features of fatty liver disease. Gastroenterology 2014, 147, 754–764. [Google Scholar] [CrossRef]
- European Association for the Study of Liver. EASL clinical practical guidelines: Management of alcoholic liver disease. J. Hepatol. 2012, 57, 399–420. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Janjua, N.Z.; Chong, M.; Grebely, J.; Aspinall, E.J.; Innes, H.; Valerio, H.M.; Hajarizadeh, B.; Hayes, P.C.; Krajden, M.; et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J. Hepatol. 2018, 68, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Oscar-Berman, M.; Shagrin, B.; Evert, D.L.; Epstein, C. Impairments of brain and behavior: The neurological effects of alcohol. Alcohol Health Res. World 1997, 21, 65–75. [Google Scholar] [PubMed]
- Harper, C.; Matsumoto, I. Ethanol and brain damage. Curr. Opin. Pharmacol. 2005, 5, 73–78. [Google Scholar] [CrossRef]
- Harper, C.G.; Kril, J.J. Neuropathology of alcoholism. Alcohol Alcohol 1990, 25, 207–216. [Google Scholar] [CrossRef]
- Rosenbloom, M.J.; Pfefferbaum, A. Magnetic resonance imaging of the living brain: Evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Res. Health 2008, 31, 362–376. [Google Scholar] [PubMed]
- Crews, F.T.; Nixon, K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 2009, 44, 115–127. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Harris, R.A.; Pfefferbaum, A. Alcohol’s Effects on Brain and Behavior. Alcohol Res. Health 2010, 33, 127–143. [Google Scholar]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, X.; Cederbaum, A.I. Cytokines in alcoholic liver disease. Arch. Toxicol. 2012, 86, 1337–1348. [Google Scholar] [CrossRef]
- Crews, F.T. Alcohol-related neurodegeneration and recovery: Mechanisms from animal models. Alcohol Res. Health 2008, 31, 377–388. [Google Scholar] [PubMed]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Knell, A.J. Liver function and failure: The evolution of liver physiology. J. R. Coll. Physicians Lond. 1980, 14, 205–208. [Google Scholar]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar]
- Edenberg, H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 2007, 30, 5–13. [Google Scholar]
- Lieber, C.S. New pathway of ethanol metabolism in the liver. Gastroenterology 1970, 59, 930–937. [Google Scholar] [CrossRef]
- Lieber, C.S.; Decarli, L.M. Hepatotoxicity of ethanol. J. Hepatol. 1991, 12, 394–401. [Google Scholar] [CrossRef]
- Lieber, C.S. Hepatic, metabolic and toxic effects of ethanol: 1991 update. Alcoholism 1991, 15, 573–592. [Google Scholar] [CrossRef]
- You, M.; Crabb, D.W. Recent advances in alcoholic liver disease II. minireview: Molecular mechanisms of alcoholic fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1–G6. [Google Scholar] [CrossRef]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.-X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P. The biology of peroxisome proliferator-activated receptors. Relatsh. Lipid Metab. Insul. Sensit. 2004, 53, S43–S50. [Google Scholar]
- Galli, A.; Pinaire, J.; Fischer, M.; Dorris, R.; Crabb, D.W. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J. Biol. Chem. 2001, 276, 68–75. [Google Scholar] [PubMed]
- Nanji, A.A.; Dannenberg, A.J.; Jokelainen, K.; Bass, N.M. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J. Pharmacol. Exp. Ther. 2004, 310, 417–424. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Charlet, K.; Jung, J.; Lee, J.; Muench, C.; Luo, A.; Longley, M.; Lohoff, F.W. Lipid profile dysregulation predicts alcohol withdrawal symptom severity in individuals with alcohol use disorder. Alcohol 2020, 86, 93–101. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Charlet, K.; Jung, J.; Lee, J.; Muench, C.; Luo, A.; Longley, M.; Mauro, K.L.; Lohoff, F.W. Association of high-intensity binge drinking with lipid and liver function enzyme levels. JAMA Netw. Open 2019, 2, e195844. [Google Scholar] [CrossRef]
- Chrostek, L.; Supronowicz, L.; Panasiuk, A.; Cylwik, B.; Gruszewska, E.; Flisiak, R. The effect of the severity of liver cirrhosis on the level of lipids and lipoproteins. Clin. Exp. Med. 2014, 14, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Breier, C.; Lisch, H.J.; Braunsteiner, H. Lipoproteins, HDL-apolipoproteins, activities of hepatic lipase and lecithin-cholesterol acyltransferase in the plasma of patients with post-alcoholic end-stage liver cirrhosis. Klin. Wochenschr. 1983, 61, 929–931. [Google Scholar] [CrossRef]
- Ahaneku, J.E.; Taylor, G.O.; Olubuyide, I.O.; Agbedana, E.O. Abnormal lipid and lipoprotein patterns in liver cirrhosis with and without hepatocellular carcinoma. J. Pak. Med. Assoc. 1992, 42, 260–263. [Google Scholar]
- Som, K.; Swaika, B.C.; Pramanik, S.; Chakraborty, P.; Gantait, K. Lipid profile in alcoholic and non alcoholic patients of chronic liver disease—A comparative and analytical study in a rural-based tertiary care centre. J. Assoc. Physicians India 2019, 67, 22–24. [Google Scholar] [PubMed]
- Huang, S.; Li, J.; Shearer, G.C.; Lichtenstein, A.H.; Zheng, X.; Wu, Y.; Jin, C.; Wu, S.; Gao, X. Longitudinal study of alcohol consumption and HDL concentrations: A community-based study. Am. J. Clin. Nutr. 2017, 105, 905–912. [Google Scholar] [CrossRef]
- Kanel, G.C.; Radvan, G.; Peters, R.L. High-density lipoprotein cholesterol and liver disease. Hepatology 1983, 3, 343–348. [Google Scholar] [CrossRef]
- Arvind, A.; Osganian, S.A.; Cohen, D.E.; Corey, K.E. Lipid and lipoprotein metabolism in liver disease. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Habib, A.; Mihas, A.A.; Abou-Assi, S.G.; Williams, L.M.; Gavis, E.; Pandak, W.M.; Heuman, D.M. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clin. Gastroenterol. Hepatol. 2005, 3, 286–291. [Google Scholar] [CrossRef]
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010, 16, 1304–1313. [Google Scholar] [CrossRef]
- Rao, R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009, 50, 638–644. [Google Scholar] [CrossRef]
- Bode, C.; Kugler, V.; Bode, J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987, 4, 8–14. [Google Scholar] [CrossRef]
- Adachi, Y.; Moore, L.E.; Bradford, B.U.; Gao, W.; Thurman, R.G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 1995, 108, 218–224. [Google Scholar] [CrossRef]
- Hritz, I.; Mandrekar, P.; Velayudham, A.; Catalano, D.; Dolganiuc, A.; Kodys, K.; Kurt-Jones, E.; Szabo, G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 2008, 48, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Yamashina, S.; Kono, H.; Schemmer, P.; Rivera, C.A.; Enomoto, A.; Nishiura, T.; Nishimura, T.; Brenner, D.A.; Thurman, R.G. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology 1999, 29, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Bautista, A.P. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 2002, 27, 17–21. [Google Scholar] [CrossRef]
- Fujimoto, M.; Uemura, M.; Nakatani, Y.; Tsujita, S.; Hoppo, K.; Tamagawa, T.; Kitano, H.; Kikukawa, M.; Ann, T.; Ishii, Y.; et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: Relation to severity of liver disturbance. Alcohol Clin. Exp. Res. 2000, 24, 48s–54s. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, A.; Moreno, C.; Gustot, T.; Maréchal, R.; Degré, D.; Demetter, P.; de Nadai, P.; Geerts, A.; Quertinmont, E.; Vercruysse, V.; et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology 2009, 49, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef]
- Gao, B.; Seki, E.; Brenner, D.A.; Friedman, S.; Cohen, J.I.; Nagy, L.; Szabo, G.; Zakhari, S. Innate immunity in alcoholic liver disease. Am. J. Physiol. 2011, 300, G516–G525. [Google Scholar] [CrossRef] [PubMed]
- Lewohl, J.M.; Wang, L.; Miles, M.F.; Zhang, L.; Dodd, P.R.; Harris, R.A. Gene expression in human alcoholism: Microarray analysis of frontal cortex. Alcohol Clin. Exp. Res. 2000, 24, 1873–1882. [Google Scholar] [CrossRef]
- Liu, J.; Lewohl, J.M.; Harris, R.A.; Iyer, V.R.; Dodd, P.R.; Randall, P.K.; Mayfield, R.D. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology 2006, 31, 1574–1582. [Google Scholar] [CrossRef]
- McBride, W.J.; Kimpel, M.W.; McClintick, J.N.; Ding, Z.M.; Hauser, S.R.; Edenberg, H.J.; Bell, R.L.; Rodd, Z.A. Changes in gene expression within the ventral tegmental area following repeated excessive binge-like alcohol drinking by alcohol-preferring (P) rats. Alcohol 2013, 47, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.M.; Flink, S.C.; Vanderlinden, L.A.; Israel, Y.; Tampier, L.; Colombo, G.; Kiianmaa, K.; Bell, R.L.; Printz, M.P.; Flodman, P.; et al. The sequenced rat brain transcriptome—Its use in identifying networks predisposing alcohol consumption. FEBS J. 2015, 282, 3556–3578. [Google Scholar] [CrossRef]
- Baxter-Potter, L.N.; Henricks, A.M.; Berger, A.L.; Bieniasz, K.V.; Lugo, J.M.; McLaughlin, R.J. Alcohol vapor exposure differentially impacts mesocorticolimbic cytokine expression in a sex-, region-, and duration-specific manner. Neuroscience 2017, 346, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, T.; Bazov, I.; Watanabe, H.; Hauser, K.F.; Bakalkin, G. Transcriptional control of maladaptive and protective responses in alcoholics: A role of the NF-κB system. Brain Behav. Immun. 2011, 25, S29–S38. [Google Scholar] [CrossRef]
- Blanco, A.M.; Vallés, S.L.; Pascual, M.; Guerri, C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J. Immunol. 2005, 175, 6893–6899. [Google Scholar] [CrossRef]
- Montesinos, J.; Gil, A.; Guerri, C. Nalmefene prevents alcohol-induced neuroinflammation and alcohol drinking preference in adolescent female mice: Role of TLR4. Alcohol Clin. Exp. Res. 2017, 41, 1257–1270. [Google Scholar] [CrossRef]
- Leclercq, S.; Stärkel, P.; Delzenne, N.M.; de Timary, P. The gut microbiota: A new target in the management of alcohol dependence? Alcohol 2019, 74, 105–111. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Dong, X.; Hu, T.; Wang, L.; Li, J.; Liu, X.; Sun, J. Fecal microbiota transplantation from healthy donors reduced alcohol-induced anxiety and depression in an animal model of chronic alcohol exposure. Chin. J. Physiol. 2018, 61, 360–371. [Google Scholar]
- Logrip, M.L. Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol 2015, 49, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.V.; Myers, S.A.; Zhang, J.; Kharebava, G.; McClain, C.J.; Kim, H.Y.; Whittemore, S.R.; Gobejishvili, L.; Barve, S. Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation. Neuropharmacology 2017, 125, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.; Truitt, J.; Merriman, M.; Ponomareva, O.; Jameson, K.; Ferguson, L.B.; Mayfield, R.D.; Harris, R.A. Localization of PPAR isotypes in the adult mouse and human brain. Sci. Rep. 2016, 6, 27618. [Google Scholar] [CrossRef]
- Ferguson, L.B.; Most, D.; Blednov, Y.A.; Harris, R.A. PPAR agonists regulate brain gene expression: Relationship to their effects on ethanol consumption. Neuropharmacology 2014, 86, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bastías, O.; Karahanian, E. Neuroinflammation produced by heavy alcohol intake is due to loops of interactions between Toll-like 4 and TNF receptors, peroxisome proliferator-activated receptors and the central melanocortin system: A novel hypothesis and new therapeutic avenues. Neuropharmacology 2018, 128, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Matyas, C.; Haskó, G.; Liaudet, L.; Trojnar, E.; Pacher, P. Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications. Nat. Rev. Cardiol. 2021, 18, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L. Role of inflammation in neurodegenerative diseases. Curr. Opin. Neurol. 2005, 18, 315–321. [Google Scholar] [CrossRef]
- Ubogu, E.E.; Cossoy, M.B.; Ransohoff, R.M. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol. Sci. 2006, 27, 48–55. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol 2012, 302, G168–G175. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Farhadi, A.; Jakate, S.M.; Tang, Y.; Shaikh, M.; Keshavarzian, A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009, 43, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Choudhary, S.; Holmes, E.W.; Yong, S.; Banan, A.; Jakate, S.; Fields, J.Z. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J. Pharmacol. Exp. Ther. 2001, 299, 442–448. [Google Scholar] [PubMed]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.; Tzortzis, G.; Burnet, P.W. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Cao, Q.; Mak, K.M.; Lieber, C.S. Cytochrome P4502E1 primes macrophages to increase TNF-alpha production in response to lipopolysaccharide. Am. J. Physiol. 2005, 289, G95–G107. [Google Scholar]
- Crews, F.T.; Sarkar, D.K.; Qin, L.; Zou, J.; Boyadjieva, N.; Vetreno, R.P. Neuroimmune function and the consequences of alcohol exposure. Alcohol Res. 2015, 37, 331–341, 344–351. [Google Scholar]
- Elwing, J.E.; Lustman, P.J.; Wang, H.L.; Clouse, R.E. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom. Med. 2006, 68, 563–569. [Google Scholar] [CrossRef]
- Filipović, B.; Marković, O.; Đurić, V.; Filipović, B. Cognitive changes and brain volume reduction in patients with nonalcoholic fatty liver disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9638797. [Google Scholar] [CrossRef]
- Lambert, G.; Sjouke, B.; Choque, B.; Kastelein, J.J.; Hovingh, G.K. The PCSK9 decade. J. Lipid Res. 2012, 53, 2515–2524. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Breslow, J.L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. USA 2004, 101, 7100–7105. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Moon, Y.A.; Horton, J.D. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 2004, 279, 50630–50638. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Pertsemlidis, A.; Kotowski, I.K.; Graham, R.; Garcia, C.K.; Hobbs, H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005, 37, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- May, C.L.; Kourimate, S.; Langhi, C.; Chétiveaux, M.; Jarry, A.; Comera, C.; Collet, X.; Kuipers, F.; Krempf, M.; Cariou, B.; et al. Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chretien, M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef]
- Zaid, A.; Roubtsova, A.; Essalmani, R.; Marcinkiewicz, J.; Chamberland, A.; Hamelin, J.; Tremblay, M.; Jacques, H.; Jin, W.; Davignon, J.; et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): Hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 2008, 48, 646–654. [Google Scholar] [CrossRef]
- Garçon, D.; Moreau, F.; Ayer, A.; Dijk, W.; Prieur, X.; Arnaud, L.; Roubtsova, A.; Seidah, N.; Prat, A.; Cariou, B.; et al. Circulating rather than intestinal PCSK9 (proprotein convertase subtilisin kexin type 9) regulates postprandial lipemia in mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2084–2094. [Google Scholar] [CrossRef]
- Feingold, K.R.; Moser, A.H.; Shigenaga, J.K.; Patzek, S.M.; Grunfeld, C. Inflammation stimulates the expression of PCSK9. Biochem. Biophys. Res. Commun. 2008, 374, 341–344. [Google Scholar] [CrossRef]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, S.; Zhou, S.; Kore, R.A.; Mu, S.; Deng, X.; Fan, Y.; Mehta, J.L. NLRP3 inflammasome via IL-1β regulates PCSK9 secretion. Theranostics 2020, 10, 7100–7110. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.-T.; Li, T.-H.; Wang, Z.; Wei, D.-H.; Liu, L.-S.; Zheng, X.-L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, S.; Xu, B.; Guo, H.; Zhang, S.; Cai, Y. Inhibition of proprotein convertase subtilisin/kexin type 9 ameliorates liver fibrosis via mitigation of intestinal endotoxemia. Inflammation 2020, 43, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Feder, S.; Wiest, R.; Weiss, T.S.; Aslanidis, C.; Schacherer, D.; Krautbauer, S.; Liebisch, G.; Buechler, C. Proprotein convertase subtilisin/kexin type 9 (PCSK9) levels are not associated with severity of liver disease and are inversely related to cholesterol in a cohort of thirty eight patients with liver cirrhosis. Lipids Health Dis. 2021, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Rodrigues, R.M.; Wang, X.; Seo, W.; Ma, J.; Hwang, S.; Fu, Y.; Trojnár, E.; Mátyás, C.; Zhao, S.; et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J. Clin. Invest. 2021, 131, e141513. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, S.; Bartesaghi, S.; Rametta, R.; Marra, F.; Margherita Mancina, R.; Pihlajamäki, J.; Kakol-Palm, D.; Andréasson, A.C.; Dongiovanni, P.; Ludovica Fracanzani, A.; et al. PCSK9 rs11591147 R46L loss-of-function variant protects against liver damage in individuals with NAFLD. Liver Int. 2021, 41, 321–332. [Google Scholar] [CrossRef]
- Lohoff, F.W.; Sorcher, J.L.; Rosen, A.D.; Mauro, K.L.; Fanelli, R.R.; Momenan, R.; Hodgkinson, C.A.; Vendruscolo, L.F.; Koob, G.F.; Schwandt, M.; et al. Methylomic profiling and replication implicates deregulation of PCSK9 in alcohol use disorder. Mol. Psychiatry 2018, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Yao, T.; Song, Z.Y. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: Down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol. Clin. Exp. Res. 2010, 34, 471–478. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Singh, A.B.; Cao, A.; Liu, J. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1α protein expression through the ubiquitin-proteasome degradation pathway. J. Biol. Chem. 2015, 290, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Shende, V.R.; Wu, M.; Singh, A.B.; Dong, B.; Kan, C.F.; Liu, J. Reduction of circulating PCSK9 and LDL-C levels by liver-specific knockdown of HNF1α in normolipidemic mice. J. Lipid Res. 2015, 56, 801–809. [Google Scholar] [CrossRef]
- Poirier, S.; Prat, A.; Marcinkiewicz, E.; Paquin, J.; Chitramuthu, B.P.; Baranowski, D.; Cadieux, B.; Bennett, H.P.; Seidah, N.G. Implication of the proprotein convertase NARC-1/PCSK9 in the development of the nervous system. J. Neurochem 2006, 98, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Rousselet, E.; Marcinkiewicz, J.; Kriz, J.; Zhou, A.; Hatten, M.E.; Prat, A.; Seidah, N.G. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J. Lipid Res. 2011, 52, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, G.; Baysarowich, J.; Kavana, M.; Addona, G.H.; Bierilo, K.K.; Mudgett, J.S.; Pavlovic, G.; Sitlani, A.; Renger, J.J.; et al. PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain. J. Lipid Res. 2010, 51, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Apaijai, N.; Moisescu, D.M.; Palee, S.; McSweeney, C.M.; Saiyasit, N.; Maneechote, C.; Boonnag, C.; Chattipakorn, N.; Chattipakorn, S.C. Pretreatment with PCSK9 inhibitor protects the brain against cardiac ischemia/reperfusion injury through a reduction of neuronal inflammation and amyloid beta aggregation. J. Am. Heart Assoc. 2019, 8, e010838. [Google Scholar] [CrossRef]
- Wang, L.; Hou, H.; Zi, D.; Habib, A.; Tan, J.; Sawmiller, D. Novel apoE receptor mimetics reduce LPS-induced microglial inflammation. Am. J. Transl. Res. 2019, 11, 5076–5085. [Google Scholar]
- Mansour, H.A.; Hassan, W.A.; Georgy, G.S. Neuroinflammatory reactions in sickness behavior induced by bacterial infection: Protective effect of minocycline. J. Biochem. Mol. Toxicol. 2018, 32. [Google Scholar] [CrossRef]
- Sanchez-Marin, L.; Pavon, F.J.; Decara, J.; Suarez, J.; Gavito, A.; Castilla-Ortega, E.; Rodriguez de Fonseca, F.; Serrano, A. Effects of intermittent alcohol exposure on emotion and cognition: A potential role for the endogenous cannabinoid system and neuroinflammation. Front. Behav. Neurosci. 2017, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Lohoff, F.W. Lipid-lowering drug effects beyond the cardiovascular system: Relevance for neuropsychiatric disorders. Int. J. Neuropsychopharmacol. 2018, 21, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Favero, C.; Ceresa, A.; Vigna, L.; Conti, D.M.; Pesatori, A.C.; Racagni, G.; Corsini, A.; Ferri, N.; Sirtori, C.R.; et al. Depression and cardiovascular risk-association among Beck Depression Inventory, PCSK9 levels and insulin resistance. Cardiovasc. Diabetol. 2020, 19, 187. [Google Scholar] [CrossRef]
- Lee, J.S.; Rosoff, D.; Luo, A.; Longley, M.; Phillips, M.; Charlet, K.; Muench, C.; Jung, J.; Lohoff, F.W. PCSK9 is Increased in cerebrospinal fluid of individuals with alcohol use disorder. Alcohol Clin. Exp. Res. 2019, 43, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Troutt, J.S.; Konrad, R.J. PCSK9 is present in human cerebrospinal fluid and is maintained at remarkably constant concentrations throughout the course of the day. Lipids 2014, 49, 445–455. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.; Derdak, Z.; Wands, J.R. Alcohol, insulin resistance and the liver–brain axis. J. Gastroenterol. Hepatol. 2012, 27, 33–41. [Google Scholar] [CrossRef]
- Morelli, M.B.; Wang, X.; Santulli, G. Functional role of gut microbiota and PCSK9 in the pathogenesis of diabetes mellitus and cardiovascular disease. Atherosclerosis 2019, 289, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Wu, Q.; Peng, J.; Pan, L.H.; Ren, Z.; Liu, H.T.; Jiang, Z.S.; Wang, G.X.; Tang, Z.H.; Liu, L.S. Hyperlipidemia-induced apoptosis of hippocampal neurons in apoE(-/-) mice may be associated with increased PCSK9 expression. Mol. Med. Rep. 2017, 15, 712–718. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Shi, J.; Jiang, Q.; Wang, H.; Li, X.; Hao, D. Inhibition of proprotein convertase subtilisin/kexin type 9 attenuates neuronal apoptosis following focal cerebral ischemia via apolipoprotein E receptor 2 downregulation in hyperlipidemic mice. Int. J. Mol. Med. 2018, 42, 2098–2106. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zhao, S.P.; Chu, L.X.; Ye, H.J. Pioglitazone reduces tumor necrosis factor-alpha serum concentration and mRNA expression of adipose tissue in hypercholesterolemic rabbits. Int. J. Cardiol. 2010, 138, 151–156. [Google Scholar] [CrossRef]
- Sun, J.; Sukhova, G.K.; Wolters, P.J.; Yang, M.; Kitamoto, S.; Libby, P.; MacFarlane, L.A.; Mallen-St Clair, J.; Shi, G.P. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat. Med. 2007, 13, 719–724. [Google Scholar] [CrossRef]
- PRALUENT (Alirocumab) [Package Insert]. Sanofi-Aventis: Bridgewater, NJ, USA, 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125559Orig1s000lbledt.pdf (accessed on 1 April 2021).
- REPATHA (Evolocumab) [Package Insert]. Amgen Inc.: Thousand Oaks, CA, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf (accessed on 1 April 2021).
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.; Honarpour, N.; Wang, H.; Liu, T.; Wasserman, S.M.; Scott, R.; Sever, P.S.; Pedersen, T.R. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with elevated risk trial. Am. Heart J. 2016, 173, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Bessac, L.; Berdan, L.G.; Bhatt, D.L.; Bittner, V.; Diaz, R.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; Jukema, J.W.; et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: Rationale and design of the ODYSSEY outcomes trial. Am. Heart J. 2014, 168, 682–689. [Google Scholar] [CrossRef]
- Ruscica, M.; Tokgözoğlu, L.; Corsini, A.; Sirtori, C.R. PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 2019, 288, 146–155. [Google Scholar] [CrossRef]
- Swiger, K.J.; Martin, S.S. PCSK9 inhibitors and neurocognitive adverse events: Exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf. 2015, 38, 519–526. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Mach, F.; Zavitz, K.; Kurtz, C.; Im, K.; Kanevsky, E.; Schneider, J.; Wang, H.; Keech, A.; Pedersen, T.R.; et al. Cognitive function in a randomized trial of evolocumab. N. Engl. J. Med. 2017, 377, 633–643. Available online: http://dx.doi.org/10.1056/NEJMoa1701131 (accessed on 1 April 2021). [CrossRef] [PubMed]
- Robinson, J.G.; Rosenson, R.S.; Farnier, M.; Chaudhari, U.; Sasiela, W.J.; Merlet, L.; Miller, K.; Kastelein, J.J. Safety of very low low-density lipoprotein cholesterol levels with alirocumab: Pooled data from randomized trials. J. Am. Coll. Cardiol. 2017, 69, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Mukhopadhyay, P.; Matyas, C.; Trojnar, E.; Paloczi, J.; Yang, Y.R.; Blank, B.A.; Savage, C.; Sorokin, A.V.; Mehta, N.N.; et al. PCSK9 inhibition as a novel therapeutic target for alcoholic liver disease. Sci. Rep. 2019, 9, 17167. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Muñoz Estrella, A.; Sourlas, A.; Silverio, D.; Hilario, E.; Montan, P.D.; Guzman, E. Inclisiran: A new promising agent in the management of hypercholesterolemia. Diseases 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.T.; Turner, T.; Visseren, F.L.J.; et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Mechanism of Action | Clinical Trial Identifier | Study Population | Primary Objectives | Primary Completion Date |
|---|---|---|---|---|---|
| Alirocumab | Monoclonal antibody | NCT03067844 | 294 patients with acute myocardial infarction undergoing percutaneous coronary intervention and receiving statin therapy | To determine the effect of alirocumab on plaque volume after 52 weeks | September 2021 |
| NCT03207945 | 140 individuals with HIV with known CVD or risk factors | To determine whether alirocumab can improve arterial inflammation and endothelial function in the setting of HIV infection | November 2021 | ||
| Evolocumab | Monoclonal antibody | NCT02624869 | 163 subjects ages 10–17 with homozygous or heterozygous familial hypercholesterolemia | To evaluate safety and efficacy of evolocumab in pediatric subjects | June 2021 |
| NCT02867813 | 5037 participants with clinically evidence atherosclerotic CVD on statin therapy who completed the FOURIER study | To assess the long-term safety of evolocumab in subjects who completed the FOURIER study | December 2021 | ||
| IBI306 | Monoclonal antibody | NCT04031742 | 30 Chinese participants with homozygous familial hypercholesterolemia | To evaluate safety and efficacy of IBI306 | January 2021 |
| NCT04759534 | 148 Chinese participants with heterozygous familial hypercholesterolemia | To evaluate safety and efficacy of IBI306 | June 2021 | ||
| Inclisiran sodium | Small interfering RNA | NCT03705234 | 15000 participants with CVD | To determine if inclisiran lowers the risk of heart attacks and strokes | December 2024 |
| Lerodalcibep (LIB003) | Recombinant fusion protein of PCSK9-binding domain and human serum albumin | NCT04034485 | 70 patients with homozygous familial hypercholesterolemia receiving lipid-lowering therapy | To compare safety, tolerability, and serum LDL-C levels of LIB003 and evolocumab | May 2022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; O’Connell, E.M.; Pacher, P.; Lohoff, F.W. PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder. J. Clin. Med. 2021, 10, 1758. https://doi.org/10.3390/jcm10081758
Lee JS, O’Connell EM, Pacher P, Lohoff FW. PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder. Journal of Clinical Medicine. 2021; 10(8):1758. https://doi.org/10.3390/jcm10081758
Chicago/Turabian StyleLee, Ji Soo, Emma M. O’Connell, Pal Pacher, and Falk W. Lohoff. 2021. "PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder" Journal of Clinical Medicine 10, no. 8: 1758. https://doi.org/10.3390/jcm10081758
APA StyleLee, J. S., O’Connell, E. M., Pacher, P., & Lohoff, F. W. (2021). PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder. Journal of Clinical Medicine, 10(8), 1758. https://doi.org/10.3390/jcm10081758






