Perioperative ABO Blood Group Isoagglutinin Titer and the Risk of Acute Kidney Injury after ABO-Incompatible Living Donor Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Technique, Anesthesia, and Desensitization Protocols
2.3. Data Collection
2.4. Statistical Analysis
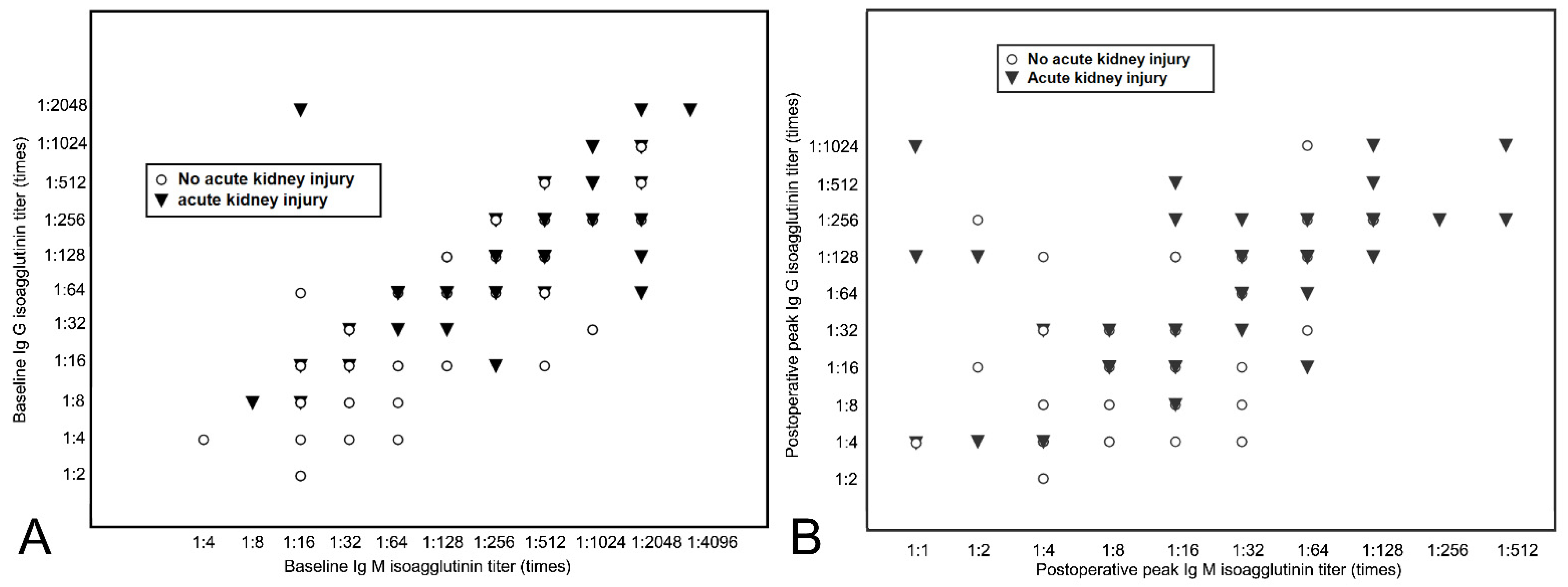
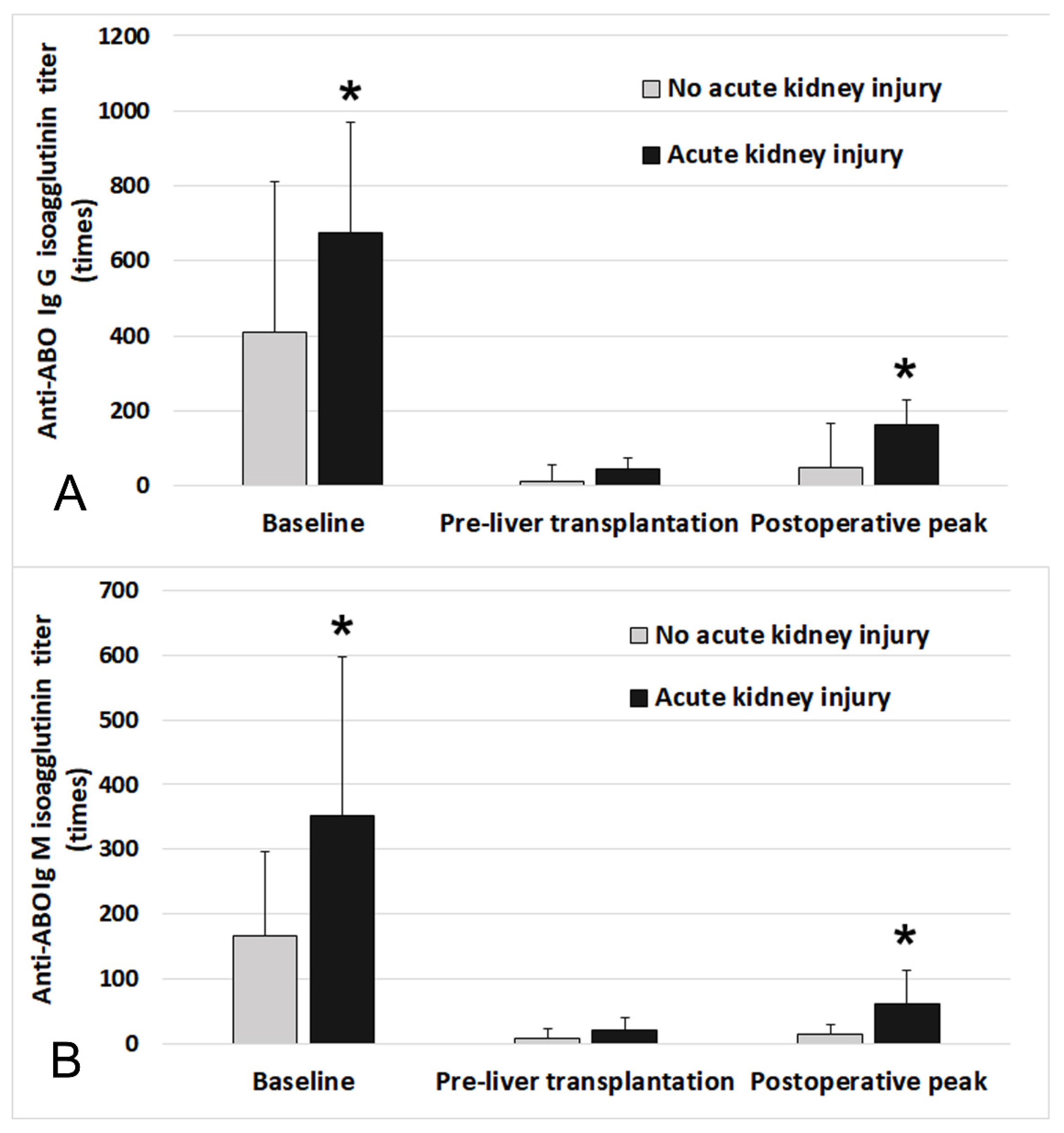
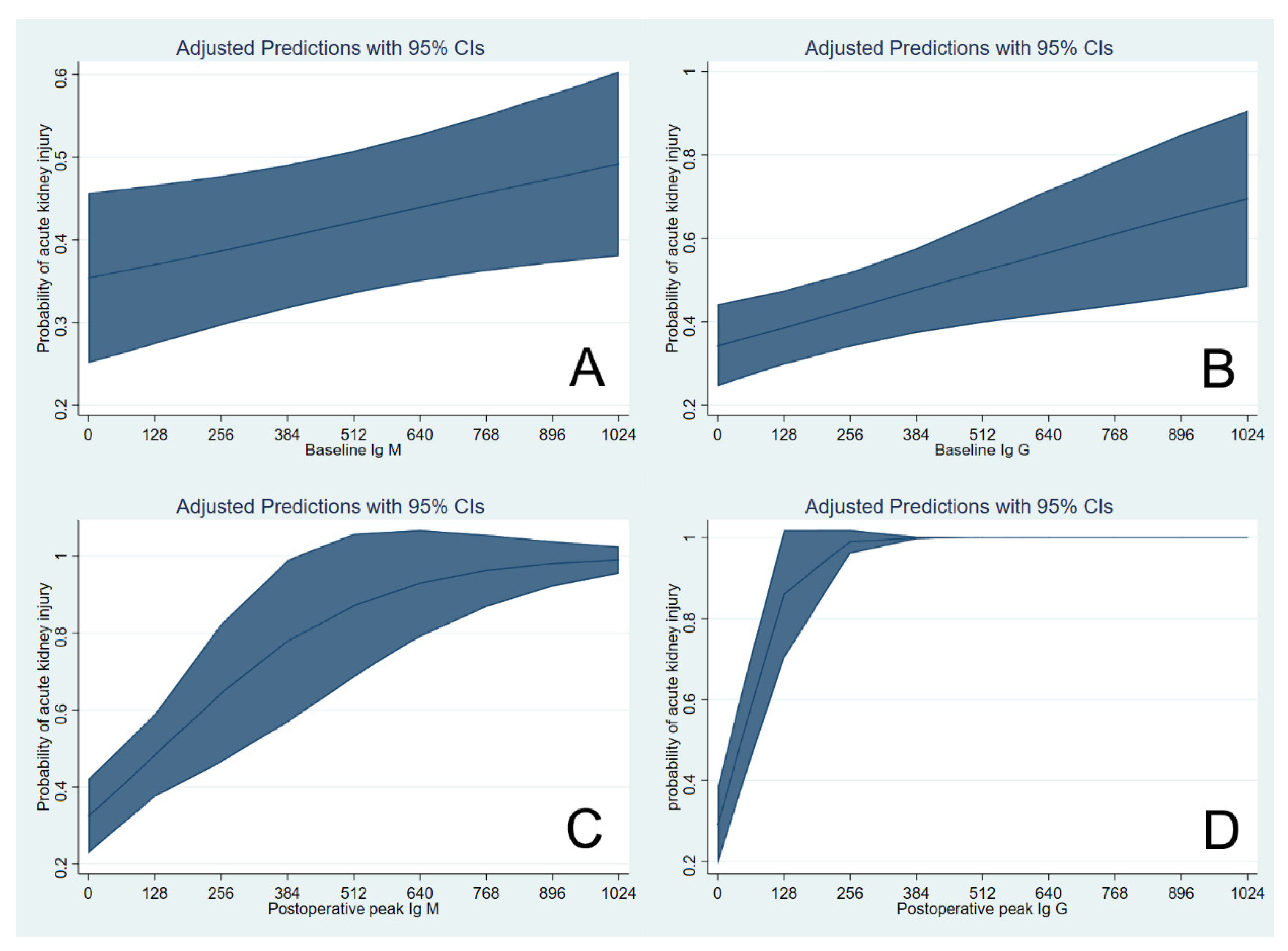
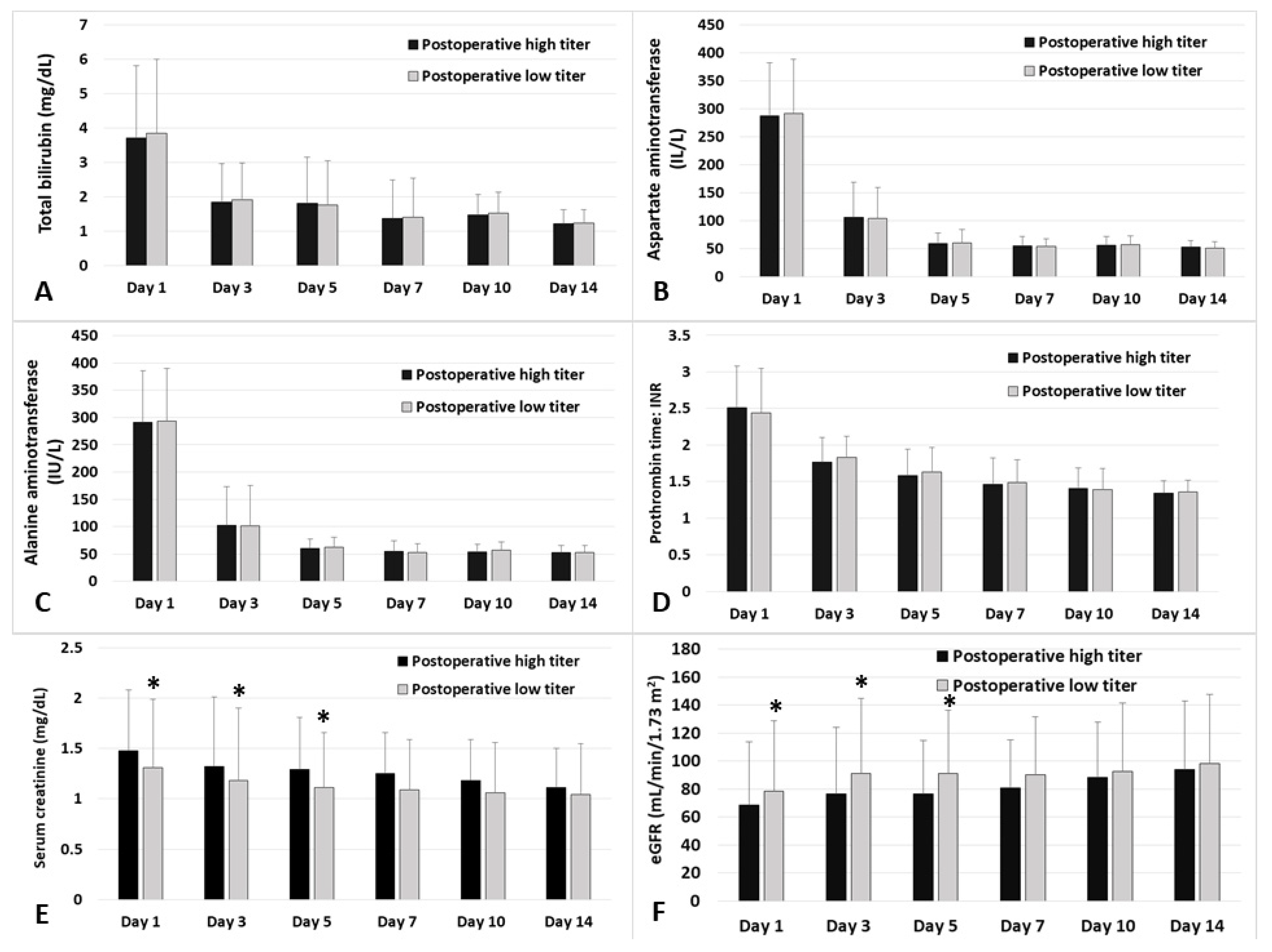
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Planinsic, R.; Boucek, C.; Sakai, T.; Chang, C.-C.H.; Kellum, J.A. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 2015, 114, 919–926. [Google Scholar] [CrossRef]
- Park, M.H.; Shim, H.S.; Kim, W.H.; Kim, H.-J.; Kim, D.J.; Lee, S.-H.; Kim, C.S.; Gwak, M.S.; Kim, G.S. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS ONE 2015, 10, e0136230. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, M.; Umeda, Y.; Sadamori, H.; Nagasaka, T.; Takaki, A.; Matsuda, H.; Shinoura, S.; Yoshida, R.; Nobuoka, D.; Satoh, D.; et al. Risk factors for acute renal injury in living donor liver transplantation: Evaluation of the RIFLE criteria. Transpl. Int. 2013, 26, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, Y.; Xia, Q.; Wang, S.; Qiu, Y.; Che, M.; Dai, H.; Qian, J.; Ni, Z.; Axelsson, J.; et al. Strong Impact of Acute Kidney Injury on Survival After Liver Transplantation. Transplant. Proc. 2010, 42, 3634–3638. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Soyama, A.; Takatsuki, M.; Hidaka, M.; Muraoka, I.; Kanematsu, T.; Eguchi, S. Acute kidney injury following living donor liver transplantation. Clin. Transplant. 2012, 26, E530–E535. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Singhapricha, T.; Hu, K.-Q.; Hong, J.C.; Steadman, R.H.; Busuttil, R.W.; Xia, V.W. Postliver Transplant Acute Renal Injury and Failure by the RIFLE Criteria in Patients With Normal Pretransplant Serum Creatinine Concentrations: A Matched Study. Transplantation 2011, 91, 348–353. [Google Scholar] [CrossRef]
- Lebron Gallardo, M.; Herrera Gutierrez, M.E.; Seller Perez, G.; Curiel Balsera, E.; Fernandez Ortega, J.F.; Quesada Garcia, G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. 2004, 10, 1379–1385. [Google Scholar] [CrossRef]
- Jun, I.G.; Kwon, H.M.; Jung, K.W.; Moon, Y.J.; Shin, W.J.; Song, J.G.; Hwang, G.S. The Impact of Postreperfusion Syndrome on Acute Kidney Injury in Living Donor Liver Transplantation: A Propensity Score Analysis. Anesth. Analg. 2018, 127, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.-G.; Lee, B.; Kim, S.-O.; Shin, W.-J.; Bang, J.-Y.; Song, J.-G.; Song, G.-W.; Lee, S.-G.; Hwang, G.-S. Comparison of acute kidney injury between ABO-compatible and ABO-incompatible living donor liver transplantation: A propensity matching analysis. Liver Transplant. 2016, 22, 1656–1665. [Google Scholar] [CrossRef]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Sakai, T.; Donaldson, J.B.; Winger, D.G.; Kellum, J.A. Acute kidney injury after orthotopic liver transplantation using living donor versus deceased donor grafts: A propensity score-matched analysis. Liver Transplant. 2015, 21, 1179–1185. [Google Scholar] [CrossRef]
- Lee, H.-C.; Bin Yoon, S.; Yang, S.-M.; Kim, W.H.; Ryu, H.-G.; Jung, C.-W.; Suh, K.-S.; Lee, K.H. Prediction of Acute Kidney Injury after Liver Transplantation: Machine Learning Approaches vs. Logistic Regression Model. J. Clin. Med. 2018, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Paramesh, A.S.; Roayaie, S.; Doan, Y.; E Schwartz, M.; Emre, S.; Fishbein, T.; Florman, S.; E Gondolesi, G.; Krieger, N.; Ames, S.; et al. Post-liver transplant acute renal failure: Factors predicting development of end-stage renal disease. Clin. Transplant. 2004, 18, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Trinh, E.; Alam, A.; Tchervenkov, J.; Cantarovich, M. Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin. Transplant. 2017, 31, e12863. [Google Scholar] [CrossRef]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Kaewput, W.; Thamcharoen, N.; Bathini, T.; Watthanasuntorn, K.; Lertjitbanjong, P.; Sharma, K.; Salim, S.A.; Ungprasert, P.; Wijarnpreecha, K.; et al. Incidence and Impact of Acute Kidney Injury after Liver Transplantation: A Meta-Analysis. J. Clin. Med. 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, N.; Satomi, S. ABO-Incompatible Living Donor Liver Transplantation: New Insights into Clinical Relevance. Transplantation 2008, 85, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kwon, C.H.D.; Joh, J.-W.; Kang, E.-S.; Park, J.B.; Lee, J.H.; Kim, S.J.; Paik, S.W.; Lee, S.-K.; Kim, D.W. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J. Hepatol. 2013, 59, 1215–1222. [Google Scholar] [CrossRef]
- Song, G.-W.; Lee, S.-G.; Hwang, S.; Kim, K.-H.; Ahn, C.-S.; Moon, D.-B.; Ha, T.-Y.; Jung, D.-H.; Park, G.-C.; Kang, S.-H.; et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J. Hepatol. 2014, 61, 575–582. [Google Scholar] [CrossRef]
- Ikegami, T.; Taketomi, A.; Soejima, Y.; Yoshizumi, T.; Uchiyama, H.; Harada, N.; Iguchi, T.; Hashimoto, N.; Maehara, Y. Rituximab, IVIG, and Plasma Exchange Without Graft Local Infusion Treatment: A New Protocol in ABO Incompatible Living Donor Liver Transplantation. Transplantation 2009, 88, 303–307. [Google Scholar] [CrossRef]
- Song, G.W.; Lee, S.G. Living donor liver transplantation. Curr. Opin. Organ. Transpl. 2014, 19, 217–222. [Google Scholar] [CrossRef]
- Egawa, H.; Oike, F.; Buhler, L.; Shapiro, A.M.J.; Minamiguchi, S.; Haga, H.; Uryuhara, K.; Kiuchi, T.; Kaihara, S.; Tanaka, K. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation 2004, 77, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lee, S.; Hwang, S.; Kim, K.; Ahn, C.; Moon, D.; Ha, T.; Jung, D.; Park, G.; Kim, W.; et al. ABO-Incompatible Adult Living Donor Liver Transplantation Under the Desensitization Protocol With Rituximab. Arab. Archaeol. Epigr. 2015, 16, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Francoz, C.; Asrani, S.K.; Khemichian, S.; Pham, T.A.; Sung, R.S.; Genyk, Y.S.; Nadim, M.K. Acute Kidney Injury After Liver Transplantation. Transplantation 2018, 102, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Paugam-Burtz, C.; Kavafyan, J.; Merckx, P.; Dahmani, S.; Sommacale, D.; Ramsay, M.; Belghiti, J.; Mantz, J. Postreperfusion syndrome during liver transplantation for cirrhosis: Outcome and predictors. Liver Transplant. 2009, 15, 522–529. [Google Scholar] [CrossRef]
- Vives, M.; Callejas, R.; Duque, P.; Echarri, G.; Wijeysundera, D.; Hernandez, A.; Sabate, A.; Bes-Rastrollo, M.; Monedero, P. Modern hydroxyethyl starch and acute kidney injury after cardiac surgery: A prospective multicentre cohort † †This Article is accompanied by Editorial Aew304. Br. J. Anaesth. 2016, 117, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-M.; Choi, S.-N.; Yu, J.H.; Yoon, H.-K.; Kim, W.H.; Jung, C.-W.; Suh, K.-S.; Lee, K.H. Intraoperative hyponatremia is an independent predictor of one-year mortality after liver transplantation. Sci. Rep. 2018, 8, 18023. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Kashfi, A.; Cattral, M.S.; Selzner, N.; McGilvray, I.D.; Greig, P.D.; Levy, G.A.; Renner, E.L.; Grant, D.R. Live Donor Liver Transplantation in High MELD Score Recipients. Ann. Surg. 2010, 251, 153–157. [Google Scholar] [CrossRef]
- Won, D.; Choe, W.; Kim, H.-J.; Kwon, S.-W.; Han, D.-J.; Park, S.-K. Significance of isoagglutinin titer in ABO-incompatible kidney transplantation. J. Clin. Apher. 2013, 29, 243–250. [Google Scholar] [CrossRef]
- Shin, S.R.; Kim, W.H.; Kim, D.J.; Shin, I.W.; Sohn, J.T. Prediction and Prevention of Acute Kidney Injury after Cardiac Surgery. Biomed. Res. Int. 2016, 2016, 2985148. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D.R. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Sanchez-Urdazpal, L.; Batts, K.P.; Gores, G.J.; Moore, S.B.; Sterioff, S.; Wiesner, R.H.; Krom, R.A.F. Increased Bile Duct Complications in Liver Transplantation Across the ABO Barrier. Ann. Surg. 1993, 218, 152–158. [Google Scholar] [CrossRef]
- Yu, J.H.; Kwon, Y.; Kim, J.; Yang, S.-M.; Kim, W.H.; Jung, C.-W.; Suh, K.-S.; Lee, K.H. Influence of Transfusion on the Risk of Acute Kidney Injury: ABO-Compatible versus ABO-Incompatible Liver Transplantation. J. Clin. Med. 2019, 8, 1785. [Google Scholar] [CrossRef]
- Kwon, H.-M.; Jun, I.-G.; Lee, J.; Moon, Y.-J.; Jung, K.-W.; Jeong, H.-W.; Park, Y.-S.; Song, J.-G.; Hwang, G.-S. Prevalent metabolic derangement and severe thrombocytopenia in ABO-incompatible liver recipients with pre-transplant plasma exchange. Sci. Rep. 2018, 8, 6679. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, H.-C.; Lim, L.; Ryu, H.-G.; Jung, C.-W. Intraoperative Oliguria with Decreased SvO2 Predicts Acute Kidney Injury after Living Donor Liver Transplantation. J. Clin. Med. 2018, 8, 29. [Google Scholar] [CrossRef]
- Kim, W.H.; Oh, H.-W.; Yang, S.-M.; Yu, J.H.; Lee, H.-C.; Jung, C.-W.; Suh, K.-S.; Lee, K.H. Intraoperative Hemodynamic Parameters and Acute Kidney Injury After Living Donor Liver Transplantation. Transplantation 2019, 103, 1877–1886. [Google Scholar] [CrossRef]
- Aksu Erdost, H.; Ozkardesler, S.; Ocmen, E.; Avkan-Oguz, V.; Akan, M.; Iyilikci, L.; Unek, T.; Ozbilgin, M.; Meseri Dalak, R.; Astarcioglu, I. Acute Renal Injury Evaluation After Liver Transplantation: With RIFLE Criteria. Transpl. Proc. 2015, 47, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Park, M.H.; Kim, H.J.; Lim, H.Y.; Shim, H.S.; Sohn, J.T.; Kim, C.S.; Lee, S.M. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: A propensity score matched case-control study. Medicine 2015, 94, e273. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Vyas, G.N. Adverse effects of plasma transfusion. Transfusion 2012, 52, 65S–79S. [Google Scholar] [CrossRef]
- Sachs, U.J. Non-infectious serious hazards in plasma transfusion. Transfus. Apher. Sci. 2010, 43, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Rohde, J.M.; Dimcheff, D.E.; Blumberg, N.; Saint, S.; Langa, K.M.; Kuhn, L.; Hickner, A.; Rogers, M.A. Health care-associated infection after red blood cell transfusion: A systematic review and meta-analysis. JAMA 2014, 311, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.; Wendel, S.; Bercovitz, R.S.; Cid, J.; Cohn, C.; Dunbar, N.M.; O Apelseth, T.; Popovsky, M.; Stanworth, S.J.; Tinmouth, A.; et al. Transfusion reactions: Prevention, diagnosis, and treatment. Lancet 2016, 388, 2825–2836. [Google Scholar] [CrossRef]
| Characteristic | Baseline High Ig M Titer (≥1:128) (n = 55) | Baseline Low Ig M Titer (≤1:64) (n = 75) | p-Value |
|---|---|---|---|
| Age, year, median (range) | 53 (49–59) | 55 (50–62) | 0.128 |
| Male, n | 44 (58.7) | 37 (67.3) | 0.317 |
| Body-mass index, kg/m2 | 23.5 (22.0–26.3) | 23.1 (20.9–25.4) | 0.168 |
| MELD score | 13 (8–20) | 11 (9–19) | 0.803 |
| Child class, n (A/B/C) | 25 (45.5)/21 (38.2)/9 (16.4) | 37 (49.3)/23 (30.7)/15 (20.0) | 0.652 |
| ABO blood group, recipient, n | <0.001 | ||
| O/A/B | 43 (78.2)/5 (9.1)/7 (12.7) | 18 (24.0)/29 (38.7)/28 (37.3) | |
| ABO blood group, donor, n | 0.492 | ||
| A/B/AB | 23 (41.8)/22 (40.0)/10 (18.2) | 38 (50.7)/28 (37.3)/9 (12.0) | |
| Etiology of liver disease, n | |||
| Alcoholic liver disease, n | 8 (14.5) | 11 (14.7) | 0.985 |
| HBV hepatitis, n | 4 (7.3) | 7 (9.3) | 0.677 |
| HCV hepatitis, n | 1 (1.8) | 2 (2.7) | 0.999 |
| Cholestatic disease, n | 5 (9.1) | 10 (13.3) | 0.582 |
| Non-alcoholic steatohepatitis, n | 3 (5.5) | 1 (1.3) | 0.310 |
| Hepatocellular carcinoma, n | 34 (61.8) | 44 (58.7) | 0.717 |
| Type of graft used (right vs. left lobe) | 53 (96.4)/2 (3.6) | 70 (93.3)/5 (6.7) | 0.698 |
| Graft-to-recipient body weight ratio | 1.23 (1.12–1.48) | 1.18 (1.09–1.43) | 0.451 |
| Cold ischemic time, min | 82 (69–97) | 81 (72–96) | 0.280 |
| Warm ischemic time, min | 26 (19–31) | 27 (23–32) | 0.080 |
| Baseline estimated glomerular filtration rate, mL/min/1.73 m2 | 92.6 (67.9–126.7) | 93.1 (65.0–123.8) | 0.459 |
| Cross-matching prior to transplant (positive vs. negative) | 1 (1.8)/54 (98.2) | 2 (2.7)/73 (97.3) | 0.750 |
| Initial baseline Ig M titer | 1:1024 (1:512–1:2048) | 1:64 (1:16–1:128) | <0.001 |
| Initial baseline Ig G titter | 1:256 (1:128–1:512) | 1:32 (1:16–1:64) | 0.010 |
| Final pre-LT Ig M titer | 1:16 (1:8–1:32) | 1:4 (negative–1:8) | 0.011 |
| Final pre-LT Ig G titer | 1:8 (1:4–1:32) | 1:2 (negative–1:8) | 0.006 |
| Postoperative peak Ig M titer | 1:128 (1:16 to 1:256) | 1:4 (negative–1:16) | 0.004 |
| Postoperative peak Ig G titer | 1:32 (1:8 to 1:64) | 1:4 (negative–1:16) | 0.015 |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age, recipient | 1.06 | 0.99–1.13 | 0.089 |
| Body-mass index, recipient | 1.08 | 1.01–1.26 | 0.042 |
| MELD score | 1.09 | 1.00–1.22 | 0.040 |
| Preoperative hemoglobin, g/dL | 0.86 | 0.65–1.13 | 0.181 |
| Postreperfusion syndrome | 1.16 | 0.81–1.24 | 0.075 |
| Intraoperative pRBC transfusion, per unit | 1.07 | 1.04–1.12 | <0.001 |
| Intraoperative FFP transfusion, per unit | 1.06 | 1.02–1.11 | <0.001 |
| Peak tacrolimus trough level during postoperative seven days, ng/ml | 1.08 | 0.91–1.32 | 0.420 |
| Initial baseline Ig M titer | 1.06 | 1.02–1.09 | 0.010 |
| Initial baseline Ig G titter | 1.02 | 0.99–1.05 | 0.159 |
| Final pre-LT Ig M titer | 1.00 | 0.98–1.02 | 0.859 |
| Final pre-LT Ig G titer | 1.02 | 0.97–1.04 | 0.112 |
| Postoperative peak Ig M titer | 1.08 | 1.04–1.13 | <0.001 |
| Postoperative peak Ig G titer | 1.00 | 0.99–1.01 | 0.110 |
| Characteristic | Baseline High Ig M Titer (≥1:128) (n = 55) | Baseline Low Ig M Titer (≤1:64) (n = 75) | p-Value |
|---|---|---|---|
| Number of pre-LT TPE sessions, n | 7 (6–8) | 4 (2–6) | <0.001 |
| Titer reduction rate | 1.58 (1.18–2.12) | 1.63 (1.24–2.02) | 0.812 |
| Post-LT TPE due to isoagglutinin titer rebound, n | 37 (67.3%) | 11 (14.7%) | <0.001 |
| Antibody-mediated rejection due to anti-ABO antibody, n | - | - | - |
| Acute cellular rejection, n | 3 (5.5%) | 4 (5.3%) | 0.976 |
| Intraoperative estimated blood loss, mL | 1800 (1100–3600) | 1800 (1200–3200) | 0.850 |
| Perioperative transfusion amount, n | |||
| Packed red blood cell | 4 (0–10) | 4 (0–9) | 0.486 |
| Fresh frozen plasma | 4 (2–11) | 3 (0–6) | 0.048 |
| Platelet, apheresis | 0 (0–1) | 0 (0–1) | 0.567 |
| IVIG with splenectomy, n | 12 (21.8) | 4 (5.3) | 0.005 |
| Acute kidney injury, during 1 week | 29 (52.7%) | 26 (34.7%) | 0.021 |
| Stage 1, n | 16 (29.1%) | 18 (24.0%) | |
| Stage 2 or 3, n | 13 (23.6%) | 8 (10.7%) | |
| Postoperative hemodialysis, n | 9 (16.4%) | 8 (10.7%) | 0.341 |
| Long-term hemodialysis (>6 months), n | 2 (3.6) | 1 (1.3) | 0.573 |
| Estimated glomerular filtration rate at 1 year after transplantation, mL/min/1.73 m2 | 91.4 (65.3–121.2) | 92.5 (64.5–122.7) | 0.459 |
| Early allograft dysfunction, n | 4 (7.3) | 2 (2.7) | 0.216 |
| Length of hospital stay, days | 19 (16–25) | 18 (15–24) | 0.356 |
| Length of intensive care unit stay, days | 5 (4–7) | 5 (4–7) | 0.561 |
| Postoperative infection, n | |||
| CMV/Bacterial/Fungal | 5 (6.1%)/3 (5.5%)/0 (0%) | 5 (6.7%)/4 (5.3%)/1 (1.3%) | 0.390 |
| Anastomotic biliary complication, n | 3 (5.5%) | 2 (2.7%) | 0.414 |
| Vascular complication, n | 1 (1.8%) | 2 (2.7%) | 0.750 |
| Reoperation, n | 2 (3.6%) | 1 (1.3%) | 0.388 |
| Characteristic | High Postoperative Peak Ig M Titer (≥1:32) (n = 59) | Low Postoperative Peak Ig M Titer (≤1:16) (n = 71) | p-Value |
|---|---|---|---|
| Number of pre-LT TPE sessions, n | 8 (6–9) | 5 (3–5) | <0.001 |
| Titer reduction rate | 1.58 (1.16–1.94) | 1.71 (1.26–2.04) | 0.378 |
| Post-LT TPE due to isoagglutinin titer rebound, n | 59 (100%) | - | |
| Antibody-mediated rejection due to anti-ABO antibody, n | - | - | - |
| Acute cellular rejection, n | 4 (6.8%) | 3 (4.2%) | 0.701 |
| Intraoperative estimated blood loss, ml | 1600 (1100–3100) | 1800 (1200–2900) | 0.646 |
| Perioperative transfusion amount, n | |||
| Packed red blood cell | 4 (0–10) | 4 (0–9) | 0.375 |
| Fresh frozen plasma | 4 (3–11) | 3 (1–6) | 0.040 |
| Platelet, apheresis | 0 (0–1) | 0 (0–1) | 0.657 |
| IVIG with splenectomy, n | 11 (18.6) | 5 (7.0) | 0.061 |
| Acute kidney injury, during 1 week | 40 (67.8%) | 15 (21.1%) | <0.001 |
| Stage 1, n | 20 (33.9%) | 14 (19.7%) | |
| Stage 2 or 3, n | 20 (33.9%) | 1 (1.4%) | |
| Postoperative hemodialysis, n | 14 (23.7%) | 3 (4.2%) | 0.001 |
| Long-term hemodialysis (>6 months), n | 3 (5.1) | - | 0.055 |
| Estimated glomerular filtration rate at 1 year after transplantation, mL/min/1.73 m2 | 91.3 (67.2–123.6) | 91.1 (67.7–124.4) | 0.830 |
| Early allograft dysfunction, n | 5 (8.5) | 1 (1.4) | 0.056 |
| Length of hospital stay, days | 20 (14–27) | 18 (15–22) | 0.344 |
| Length of intensive care unit stay, days | 6 (5–8) | 5 (4–7) | 0.055 |
| Postoperative infection, n | |||
| CMV/Bacterial/Fungal | 7 (11.9%)/5 (8.5%)/0 (0%) | 3 (4.gh2%)/2 (2.8%)/1 (1.4%) | 0.104 |
| Anastomotic biliary complication, n | 3 (5.1%) | 2 (2.8%) | 0.441 |
| Vascular complication, n | 2 (3.4%) | 1 (1.4%) | 0.590 |
| Reoperation, n | 2 (3.4%) | 1 (1.4%) | 0.590 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Bae, J.; Yoon, H.-K.; Lee, H.-J.; Yang, S.-M.; Choe, S.H.; Jung, C.-W.; Suh, K.-S.; Kim, W.H. Perioperative ABO Blood Group Isoagglutinin Titer and the Risk of Acute Kidney Injury after ABO-Incompatible Living Donor Liver Transplantation. J. Clin. Med. 2021, 10, 1679. https://doi.org/10.3390/jcm10081679
Cho H, Bae J, Yoon H-K, Lee H-J, Yang S-M, Choe SH, Jung C-W, Suh K-S, Kim WH. Perioperative ABO Blood Group Isoagglutinin Titer and the Risk of Acute Kidney Injury after ABO-Incompatible Living Donor Liver Transplantation. Journal of Clinical Medicine. 2021; 10(8):1679. https://doi.org/10.3390/jcm10081679
Chicago/Turabian StyleCho, Hyeyeon, Jinyoung Bae, Hyun-Kyu Yoon, Ho-Jin Lee, Seong-Mi Yang, Suk Hyung Choe, Chul-Woo Jung, Kyung-Suk Suh, and Won Ho Kim. 2021. "Perioperative ABO Blood Group Isoagglutinin Titer and the Risk of Acute Kidney Injury after ABO-Incompatible Living Donor Liver Transplantation" Journal of Clinical Medicine 10, no. 8: 1679. https://doi.org/10.3390/jcm10081679
APA StyleCho, H., Bae, J., Yoon, H.-K., Lee, H.-J., Yang, S.-M., Choe, S. H., Jung, C.-W., Suh, K.-S., & Kim, W. H. (2021). Perioperative ABO Blood Group Isoagglutinin Titer and the Risk of Acute Kidney Injury after ABO-Incompatible Living Donor Liver Transplantation. Journal of Clinical Medicine, 10(8), 1679. https://doi.org/10.3390/jcm10081679






