Phylogenetic and Molecular Analyses of More Prevalent HCV1b Subtype in the Calabria Region, Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnostic Procedures
2.3. Amplification and Sequencing of HCV NS5B and NS5A Regions
2.4. Subtyping Tool Analysis
2.5. Datasets Construction
2.6. Likelihood Mapping
2.7. Phylogenetic Analysis
2.8. Genetic Variability Analysis
2.9. Public Availability of the Sequencing Data
3. Results
3.1. Patient Demographic Characteristics and Risk Factors
3.2. Likelihood Mapping
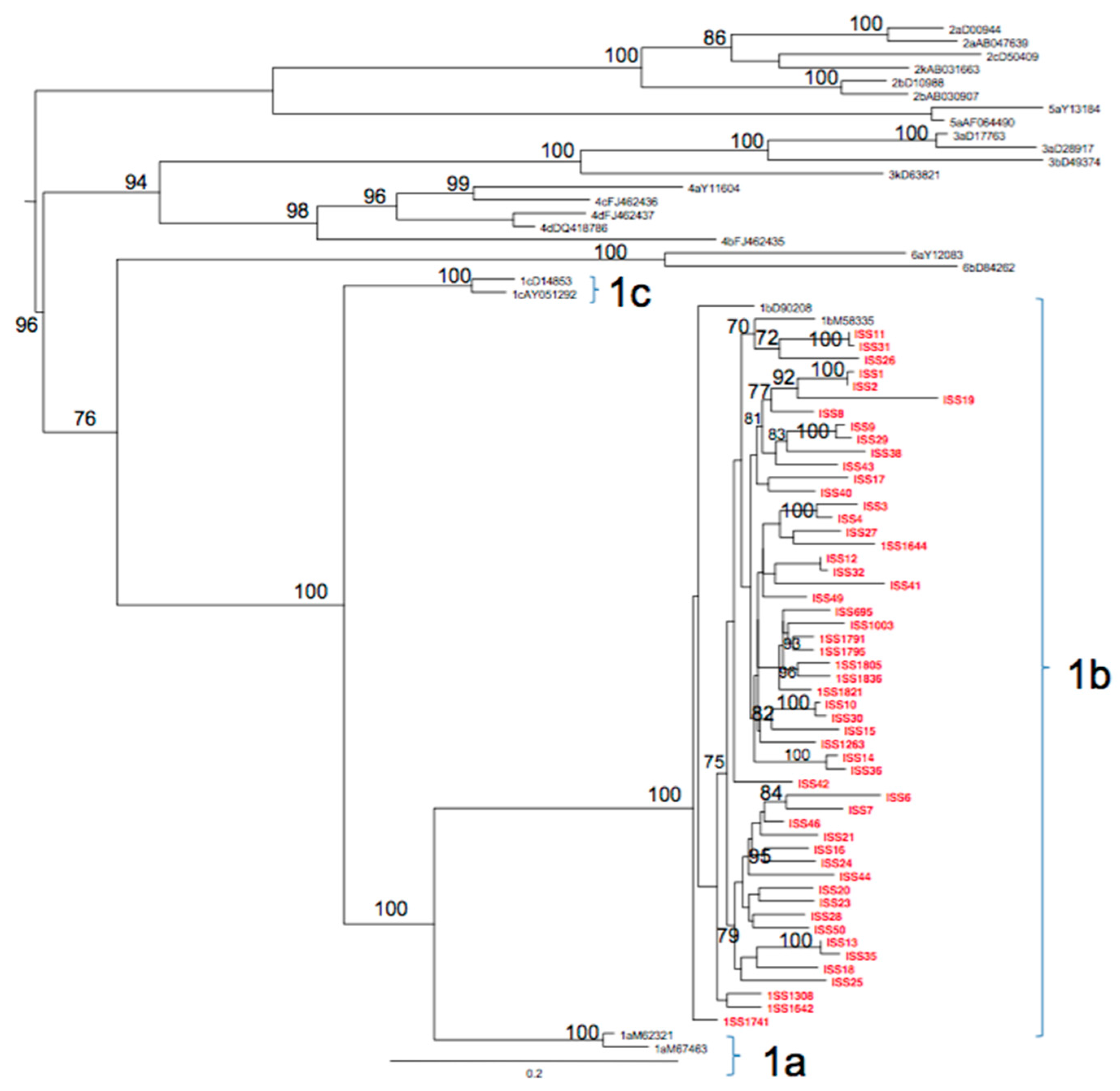
3.3. Phylogenetic Analysis
3.4. Substitutions on Target Regions in Patients Naïve to DAA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2020. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef]
- Marascio, N.; Liberto, M.C.; Barreca, G.S.; Zicca, E.; Quirino, A.; Lamberti, A.G.; Bianco, G.; Matera, G.; Surace, L.; Berardelli, G.; et al. Update on epidemiology of HCV in Italy: Focus on the Calabria Region. BMC Infect. Dis. 2014, 14, S2. [Google Scholar] [CrossRef] [PubMed]
- Kartashev, V.; Döring, M.; Nieto, L.; Coletta, E.; Kaiser, R.; Sierra, S.; Guerrero, A.; Stoiber, H.; Paar, C.; Vandamme, A.; et al. New findings in HCV genotype distribution in selected West European, Russian and Israeli regions. J. Clin. Virol. 2016, 81, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Magiorkinis, G.; Magiorkinis, E.; Paraskevis, D.; Ho, S.Y.W.; Shapiro, B.; Pybus, O.G.; Allain, J.-P.; Hatzakis, A. The Global Spread of Hepatitis C Virus 1a and 1b: A Phylodynamic and Phylogeographic Analysis. PLoS Med. 2009, 6, e1000198. [Google Scholar] [CrossRef]
- Ansaldi, F.; Bruzzone, B.; Salmaso, S.; Rota, M.C.; Durando, P.; Gasparini, R.; Icardi, G. Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J. Med. Virol. 2005, 76, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K. Hepatitis C-related hepatocellular carcinoma: Prevalence around the world, factors interacting, and role of genotypes. Epidemiol. Rev. 1999, 21, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Liberto, M.C.; Marascio, N.; Zicca, E.; Matera, G. Epidemiological features and specificities of HCV infection: A hospital-based cohort study in a university medical center of Calabria region. BMC Infect. Dis. 2012, 12, S4. [Google Scholar] [CrossRef]
- Cuypers, L.; Snoeck, J.; Vrancken, B.; Kerremans, L.; Vuagniaux, G.; Verbeeck, J.; Nevens, F.; Camacho, R.J.; Vandamme, A.M.; Van Dooren, S. A near-full length genotypic assay for HCV1b. J. Virol. Methods 2014, 209, 126–135. [Google Scholar] [CrossRef]
- Marascio, N.; Quirino, A.; Barreca, G.S.; Galati, L.; Costa, C.; Pisani, V.; Mazzitelli, M.; Matera, G.; Liberto, M.C.; Focà, A.; et al. Discussion on critical points for a tailored therapy to cure hepatitis C virus infection. Clin. Mol. Hepatol. 2019, 25, 30–36. [Google Scholar] [CrossRef]
- Torti, C.; Zazzi, M.; Abenavoli, L.; Trapasso, F.; Cesario, F.; Corigliano, D.; Cosco, L.; Costa, C.; Curia, R.L.; De Rosa, M.; et al. SINERGIE Study Group. Future research and collaboration: The “SINERGIE” project on HCV (South Italian Network for Rational Guidelines and International Epidemiology). BMC Infect. Dis. 2012, 12, S9. [Google Scholar] [CrossRef] [PubMed]
- Marascio, N.; Mazzitelli, M.; Scarlata, G.G.M.; Giancotti, A.; Barreca, G.S.; Lamberti, A.G.; Divenuto, F.; Costa, C.; Trecarichi, E.M.; Matera, G.; et al. HCV Antibody Prevalence and Genotype Evolution in a Teaching Hospital, Calabria Region, Southern Italy Over A Decade (2008–2018). Open Microbiol. 2020, 14, 84–90. [Google Scholar] [CrossRef]
- Guadagnino, V.; Stroffolini, T.; Caroleo, B.; Menniti Ippolito, F.; Rapicetta, M.; Ciccaglione, A.R.; Chionne, P.; Madonna, E.; Costantino, A.; De Sarro, G.; et al. Hepatitis C virus infection in an endemic area of Southern Italy 14 years later: Evidence for a vanishing infection. Dig. Liver Dis. 2013, 45, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; de Ledinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- Pybus, O.G.; Barnes, E.; Taggart, R.; Lemey, P.; Markov, P.V.; Rasachak, B.; Syhavong, B.; Phetsouvanah, R.; Sheridan, I.; Humphreys, I.S.; et al. Genetic history of hepatitis C virus in East Asia. J. Virol. 2009, 83, 1071–1082. [Google Scholar] [CrossRef]
- Lindström, I.; Kjellin, M.; Palanisamy, N.; Bondeson, K.; Wesslén, L.; Lannergard, A.; Lennerstrand, J. Prevalence of polymorphisms with significant resistance to NS5A inhibitors in treatment-naive patients with hepatitis C virus genotypes 1a and 3a in Sweden. Infect. Dis. 2015, 47, 555–562. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. Available online: https://usegalaxy.org/ (accessed on 27 March 2020). [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- de Oliveira, T.; Deforche, K.; Cassol, S.; Salminen, M.; Paraskevis, D.; Seebregts, C.; Snoeck, J.; van Rensburg, E.J.; Wensing, A.M.; van de Vijver, D.A.; et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics 2005, 21, 3797–3800. Available online: http://dbpartners.stanford.edu/RegaSubtyping/html/subtypinghcvSUB.html (accessed on 20 April 2020). [CrossRef]
- Struck, D.; Lawyer, G.; Ternes, A.M.; Schmit, J.C.; Perez Bercoff, D. COMET: Adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014, 42, e144. Available online: https://comet.lih.lu/index.php?cat=hcv (accessed on 20 April 2020). [CrossRef]
- Schmidt, H.A.; Strimmer, K.; Vingron, M.; von Haeseler, A. TREE-PUZZLE: Maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 2002, 18, 502–504. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalaghatgi, P.; Sikorski, A.M.; Knops, E.; Rupp, D.; Sierra, S.; Heger, E.; Neumann-Fraune, M.; Beggel, B.; Walker, A.; Timm, J.; et al. Geno2pheno[HCV]—A web-based interpretation system to support hepatitis C treatment decisions in the era of direct-acting antiviral agents. PLoS ONE 2016, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, V.C.; Cento, V.; Lenci, I.; Aragri, M.; Rossi, P.; Barbaliscia, S.; Melis, M.; Verucchi, G.; Magni, C.F.; Teti, E.; et al. HCV Italian Resistance Network Study Group. Multiclass HCV resistance to direct-acting antiviral failure in real-life patients advocates for tailored second-line therapies. Liver Int. 2017, 37, 514–528. [Google Scholar] [CrossRef]
- Benson, D.A.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2014, 42, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.J. HCV routes of transmission: What goes around comes around. Semin. Liver Dis. 2011, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, A.D.; Joy, J.B.; Montoya, V.; Luo, I.; Poon, A.F.; Jacka, B.; Lamoury, F.; Applegate, T.; Montaner, J.; Khudyakov, Y.; et al. A molecular phylogenetics-based approach for identifying recent hepatitis C virus transmission events. Infect. Genet. Evol. 2015, 33, 101–109. [Google Scholar] [CrossRef]
- Ciccozzi, M.; Equestre, M.; Costantino, A.; Marascio, N.; Quirino, A.; Lo Presti, A.; Cella, E.; Bruni, R.; Liberto, M.C.; Focà, A.; et al. Hepatitis C virus genotype 4d in Southern Italy: Reconstruction of its origin and spread by a phylodynamic analysis. J. Med. Virol. 2012, 84, 1613–1619. [Google Scholar] [CrossRef]
- Marascio, N.; Ciccozzi, M.; Equestre, M.; Lo Presti, A.; Costantino, A.; Cella, E.; Bruni, R.; Liberto, M.C.; Pisani, G.; Zicca, E.; et al. Back to the origin of HCV 2c subtype and spreading to the Calabria region (Southern Italy) over the last two centuries: A phylogenetic study. Infect. Genet. Evol. 2014, 26, 352–358. [Google Scholar] [CrossRef]
- Serraino, R.; Mazzitelli, M.; Greco, G.; Serapide, F.; Scaglione, V.; Marascio, N.; Trecarichi, E.M.; Torti, C. Risk factors for hepatitis B and C among healthy population: A community-based survey from four districts of Southern Italy. Infez. Med. 2020, 28, 223–226. [Google Scholar]
- Marascio, N.; Pavia, G.; Strazzulla, A.; Dierckx, T.; Cuypers, L.; Vrancken, B.; Barreca, G.S.; Mirante, T.; Malanga, D.; Oliveira, D.M.; et al. The SINERGIE-UMG Study Group. Detection of Natural Resistance-Associated Substitutions by Ion Semiconductor Technology in HCV1b Positive, Direct-Acting Antiviral Agents-Naïve Patients. Int. J. Mol. Sci. 2016, 17, 1416. [Google Scholar] [CrossRef]
- Calvaruso, V.; Petta, S.; Craxì, A. Is global elimination of HCV realistic? Liver Int. 2018, 38, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, L.; Li, G.; Libin, P.; Piampongsant, S.; Vandamme, A.M.; Theys, K. Genetic Diversity and Selective Pressure in Hepatitis C Virus Genotypes 1–6: Significance for Direct-Acting Antiviral Treatment and Drug Resistance. Viruses 2015, 7, 5018–5039. [Google Scholar] [CrossRef] [PubMed]
- Gaspareto, K.V.; Ribeiro, R.M.; de Mello Malta, F.; Gomes-Gouvêa, M.S.; Muto, N.H.; Romano, C.M.; Mendes-Correa, M.C.; Carrilho, F.J.; Sabino, E.C.; Rebello Pinho, J.R. Resistance-associated variants in HCV subtypes 1a and 1b detected by Ion Torrent sequencing platform. Antivir. Ther. 2016, 21, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Mizokami, M.; Pianko, S.; Mangia, A.; Han, K.H.; Martin, R.; Svarovskaia, E.; Dvory-Sobol, H.; Doehle, B.; Hedskog, C.; et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: Prevalence and effect on treatment outcome. J. Hepatol. 2017, 66, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Marascio, N.; Pavia, G.; Romeo, I.; Talarico, C.; Di Salvo, S.; Reale, M.; Marano, V.; Barreca, G.S.; Fabiani, F.; Perrotti, N.; et al. Real-life 3D therapy failure: Analysis of NS5A 93H RAS plus 108 K polymorphism in complex with ombitasvir by molecular modeling. J. Med. Virol. 2018, 90, 1257–1263. [Google Scholar] [CrossRef]
- Scaglione, V.; Mazzitelli, M.; Costa, C.; Pisani, V.; Greco, G.; Serapide, F.; Lionello, R.; La Gamba, V.; Marascio, N.; Trecarichi, E.M.; et al. Virological and Clinical Outcome of DAA Containing Regimens in a Cohort of Patients in Calabria Region (Southern Italy). Medicina 2020, 56, 101. [Google Scholar] [CrossRef]

| Absolute Number (%) | |||
|---|---|---|---|
| Characteristics | Overall | Patients from University Hospital | Subjects from Sersale Village |
| Gender | |||
| M | 22 (41.5) | 21 (48.7) | 1 (8.3) |
| F | 31 (58.5) | 20 (51.3) | 11 (91.7) |
| Risk factors | |||
| Surgical intervention | 7 (13.2) | 7 (17.1) | - |
| Blood transfusion | 2 (3.8) | 2 (4.8) | - |
| Dental treatment therapy | 9 (16.9) | 9 (21.9) | - |
| Cohabitation | 1 (1.9) | 1 (2.4) | - |
| Multiple * | 31 (58.5) | 22 (53.6) | 9 (75) |
| Not available | 3 (5.7) | - | 3 (25) |
| Clinical parameters | |||
| cirrhotic status | - | 14 (34.1) | not available |
| HCV RNA median level | 3,792,576 IU/mL | 2,280,000 IU/mL | 3,918,625 IU/mL |
| Median (range) | |||
| Age (years) | 70 (31–90) | 68 (31–85) | 71 (65–90) |
| Total | 53 | 41 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marascio, N.; Costantino, A.; Taffon, S.; Lo Presti, A.; Equestre, M.; Bruni, R.; Pisani, G.; Barreca, G.S.; Quirino, A.; Trecarichi, E.M.; et al. Phylogenetic and Molecular Analyses of More Prevalent HCV1b Subtype in the Calabria Region, Southern Italy. J. Clin. Med. 2021, 10, 1655. https://doi.org/10.3390/jcm10081655
Marascio N, Costantino A, Taffon S, Lo Presti A, Equestre M, Bruni R, Pisani G, Barreca GS, Quirino A, Trecarichi EM, et al. Phylogenetic and Molecular Analyses of More Prevalent HCV1b Subtype in the Calabria Region, Southern Italy. Journal of Clinical Medicine. 2021; 10(8):1655. https://doi.org/10.3390/jcm10081655
Chicago/Turabian StyleMarascio, Nadia, Angela Costantino, Stefania Taffon, Alessandra Lo Presti, Michele Equestre, Roberto Bruni, Giulio Pisani, Giorgio Settimo Barreca, Angela Quirino, Enrico Maria Trecarichi, and et al. 2021. "Phylogenetic and Molecular Analyses of More Prevalent HCV1b Subtype in the Calabria Region, Southern Italy" Journal of Clinical Medicine 10, no. 8: 1655. https://doi.org/10.3390/jcm10081655
APA StyleMarascio, N., Costantino, A., Taffon, S., Lo Presti, A., Equestre, M., Bruni, R., Pisani, G., Barreca, G. S., Quirino, A., Trecarichi, E. M., Costa, C., Mazzitelli, M., Serapide, F., Matera, G., Torti, C., Liberto, M. C., & Ciccaglione, A. R. (2021). Phylogenetic and Molecular Analyses of More Prevalent HCV1b Subtype in the Calabria Region, Southern Italy. Journal of Clinical Medicine, 10(8), 1655. https://doi.org/10.3390/jcm10081655








