Home Management of Heart Failure and Arrhythmias in Patients with Cardiac Devices during Pandemic
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehra, M.R.; Desai, S.S.; Kuy, S.; Henry, T.D.; Patel, A.N. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N. Engl. J. Med. 2020, 382, e102. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- HRS COVID-19 Task Force Update: April 15, 2020. Management of Cardiac Implantable Electronic Devices (CIED). Available online: https://www.hrsonline.org/COVID19-Challenges-Solutions/hrs-covid-19-task-force-updateapril-15-2020 (accessed on 5 May 2020).
- Varma, N.; Epstein, A.E.; Irimpen, A.; Schweikert, R.; Love, C. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up. Circulation 2010, 122, 325–332. [Google Scholar] [CrossRef] [PubMed]
- de Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during Covid-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Metzler, B.; Siostrzonek, P.; Binder, R.K.; Bauer, A.; Reinstadler, S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: The pandemic response causes cardiac collateral damage. Eur. Heart J. 2020, 41, 1852–1853. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, S.; Fileti, L.; Reggi, A.; Moschini, C.; Lorenzetti, S.; Rubboli, A. Impact of the COVID-19 pandemic on admissions for acute coronary syndrome: Review of the literature and single-center experience. G. Ital. Cardiol. 2020, 21, 502–508. [Google Scholar] [CrossRef]
- Piccolo, R.; Bruzzese, D.; Mauro, C.; Aloia, A.; Baldi, C.; Boccalatte, M.; Bottiglieri, G.; Briguori, C.; Caiazzo, G.; Calabrò, P.; et al. Population Trends in Rates of Percutaneous Coronary Revascularization for Acute Coronary Syndromes Associated With the COVID-19 Outbreak. Circulation 2020, 141, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Klersy, C.; De Silvestri, A.; Gabutti, G.; Regoli, F.; Auricchio, A. A Meta-Analysis of Remote Monitoring of Heart Failure Patients. J. Am. Coll. Cardiol. 2009, 54, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Landolina, M.; Perego, G.B.; Lunati, M.; Curnis, A.; Guenzati, G.; Vicentini, A.; Parati, G.; Borghi, G.; Zanaboni, P.; Valsecchi, S.; et al. Remote Monitoring Reduces Healthcare Use and Improves Quality of Care in Heart Failure Patients with Implantable Defibrillators. Circulation 2012, 125, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients with Implanted Devices. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Sharma, V.; Johnson, J.W.; Warman, E.N.; Baicu, C.F.; Bennett, T.D. Prediction of All-Cause Mortality Based on the Direct Measurement of Intrathoracic Impedance. Circ. Heart Fail. 2016, 9, e002543. [Google Scholar] [CrossRef] [PubMed]
- Yamokoski, L.M.; Haas, G.J.; Gans, B.; Abraham, W.T. OptiVol® fluid status monitoring with an implantable cardiac device: A heart failure management system. Expert Rev. Med. Devices 2007, 4, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.J.-B.; Pereda, D.C.; Vega, D.S.; Díaz, S.D.P.; Ruiz, J.M.M.; Gómez, J.L.Z.; Salinas, G.L.A. Heart Failure in the Time of COVID-19. Cardiology 2020, 145, 481–484. [Google Scholar] [CrossRef]
- Hall, M.E.; Vaduganathan, M.; Khan, M.S.; Papadimitriou, L.; Long, R.C.; Hernandez, G.A.; Moore, C.K.; Lennep, B.W.; Mcmullan, M.R.; Butler, J. Reductions in Heart Failure Hospitalizations during the COVID-19 Pandemic. J. Card. Fail. 2020, 26, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Bromage, D.I.; Cannatà, A.; Rind, I.A.; Gregorio, C.; Piper, S.; Shah, A.M.; McDonagh, T.A. The impact of COVID-19 on heart failure hospitalization and management: Report from a Heart Failure Unit in London during the peak of the pandemic. Eur. J. Heart Fail. 2020, 22, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Barutçu, A.; Temiz, A.; Bekler, A.; Altun, B.; Kırılmaz, B.; Aksu, F.U.; Kucuk, U.; Gazi, E.; Kirilmaz, B. Arrhythmia Risk Assessment Using Heart Rate Variability Parameters in Patients with Frequent Ventricular Ectopic Beats without Structural Heart Disease. Pacing Clin. Electrophysiol. 2014, 37, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Perkiömäki, J.S.; Huikuri, H.V.; Koistinen, J.M.; Mäkikallio, T.; Castellanos, A.; Myerburg, R.J. Heart rate variability and dispersion of QT interval in patients with vulnerability to ventricular tachycardia and ventricular fibrillation after previous myocardial infarction. J. Am. Coll. Cardiol. 1997, 30, 1331–1338. [Google Scholar] [CrossRef]
- Crea, F. Focus on atrial fibrillation, syncope, and arrhythmias during COVID-19 pandemic. Eur. Heart J. 2021, 42, 361–364. [Google Scholar] [CrossRef] [PubMed]
| n or Mean ± SD | % | |
|---|---|---|
| Population | 312 | 100 |
| Female | 151 | 48.4 |
| Male | 161 | 51.6 |
| Age | 71 ± 7.2 | |
| Hypertension | 194 | 62.1 |
| Diabetes | 87 | 27.9 |
| COPD | 56 | 18 |
| CRF | 63 | 20 |
| AF/AFL | 104 | 33.3 |
| Echocardiographic Data | ||
| EF | 43.5 ± 9.8 | |
| Diastolic Dysfunction (cumulative) | 192 | 61.5 |
| Grade I Grade II Grade III | 118 63 11 | 37.8 20.2 3.5 |
| Mitral Valve Disease | 51 | 16.3 |
| Aortic Valve Disease | 28 | 8.9 |
| Tricuspid Valve Disease | 44 | 14.1 |
| Medical Therapy | ||
| ACEi/ARB/ARNI | 262 | 84 |
| Anticoagulant therapy | 104 | 33.3 |
| Statin therapy | 123 | 39.4 |
| Beta blocker | 158 | 50.6 |
| Antiplatelet therapy | 94 | 30.1 |
| Aldosterone antagonist | 106 | 33.6 |
| Diuretics | 141 | 45.2 |
| Digoxin | 12 | 3.8 |
| Antiarrhythmic Drugs | 59 | 18.9 |
| CIEDs | ||
| ICD/CRT-D | 127 | 40.7 |
| PM | 185 | 59.3 |
| 2019 | 2020 | ||||
|---|---|---|---|---|---|
| Event | N | % | n | % | p-Value |
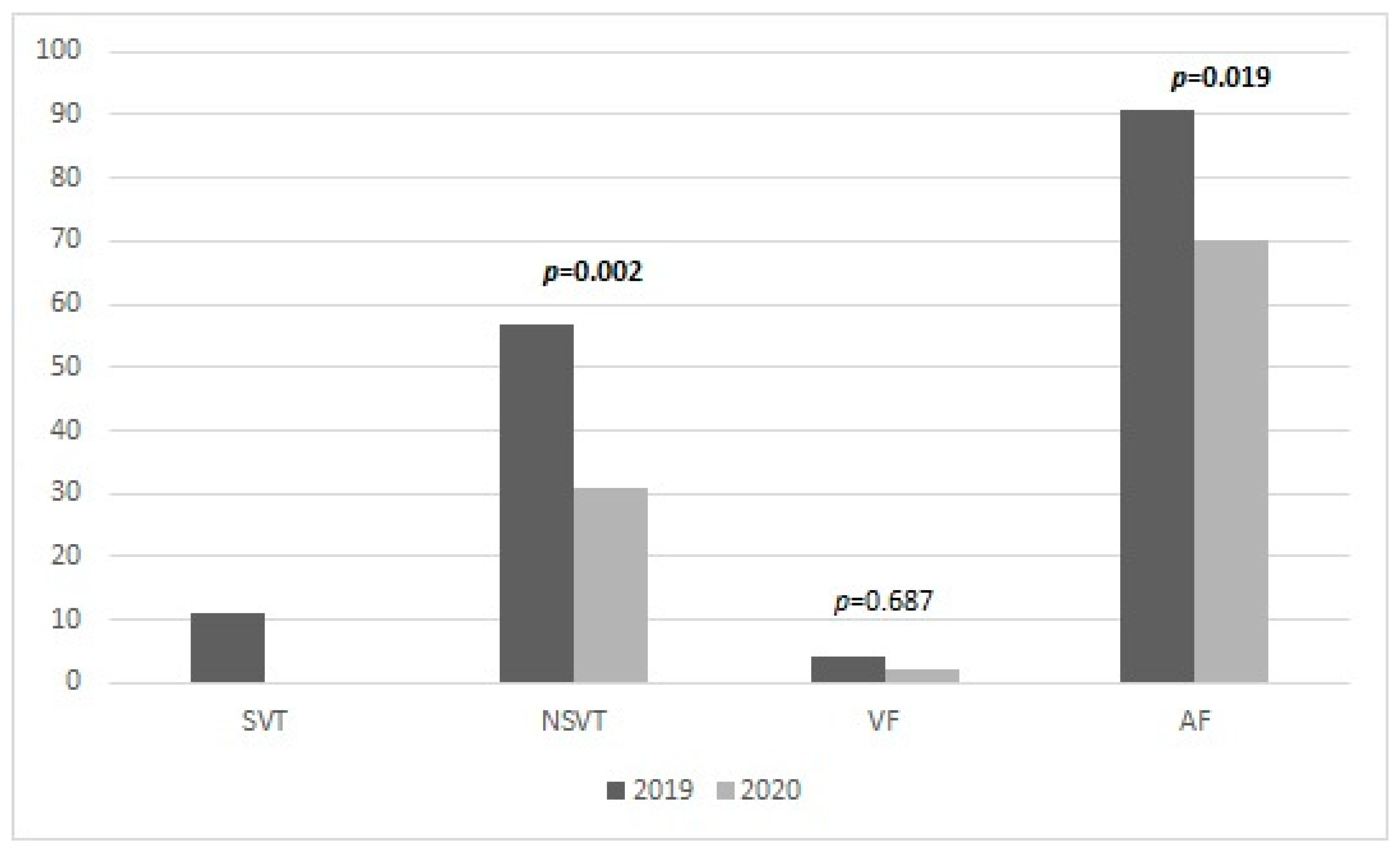
| VF | 4 | 1.3 | 2 | 0.6 | 0.687 |
| NSVT | 57 | 18.3 | 31 | 9.9 | 0.002 |
| SVT | 11 | 3.5 | 0 | 0 | |
| ATP | 5 | 1.6 | 8 | 2.6 | 0.581 |
| DC Shock | 1 | 0.3 | 0 | 0 | |
| A-Shock | 3 | 1 | 0 | 0 | |
| I-Shock | 0 | 0 | 0 | 0 | |
| AF | 91 | 29.2 | 70 | 22.4 | 0.019 |
| HF Alarm | 55 | 176 | 79 | 25.3 | 0.012 |
| VLNA | 6 | 1.9 | 14 | 4.5 | 0.115 |
| ALNA | 23 | 7.4 | 22 | 7.1 | 1.000 |
| ERI | 6 | 1.9 | 1 | 0.3 | 0.125 |
| PMT | 16 | 5.1 | 18 | 5.8 | 0.791 |
| Hospitalization | 20 | 6.4 | 2 | 0.6 | <0.001 |
| Event | n (%) | S.E. | p-Value | OR | CI (95%) |
|---|---|---|---|---|---|
| VF | 4 (1.3) | 1.606 | 0.001 | 262.444 | 11.26–6114.34 |
| NSVT | 57 (18.3) | 0.738 | 0.017 | 5.788 | 1.36–24.60 |
| SVT | 11 (3.5) | 1.101 | 0.001 | 39.264 | 4.53–339.94 |
| AF | 91 (29.2) | 0.791 | 0.004 | 9.667 | 2.05–45.55 |
| HF Alarm | 55 (17.6) | 0.785 | 0.004 | 9.651 | 2.07–44.97 |
| VLNA | 6 (1.9) | 1.161 | <0.001 | 66.909 | 6.88–650.66 |
| ALNA | 23 (7.4) | 1.173 | 0.028 | 13.138 | 1.31–130,942 |
| ERI | 6 (1.9) | 1.466 | <0.001 | 827.547 | 46.72–14654.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteucci, A.; Bonanni, M.; Centioni, M.; Zanin, F.; Geuna, F.; Massaro, G.; Sangiorgi, G. Home Management of Heart Failure and Arrhythmias in Patients with Cardiac Devices during Pandemic. J. Clin. Med. 2021, 10, 1618. https://doi.org/10.3390/jcm10081618
Matteucci A, Bonanni M, Centioni M, Zanin F, Geuna F, Massaro G, Sangiorgi G. Home Management of Heart Failure and Arrhythmias in Patients with Cardiac Devices during Pandemic. Journal of Clinical Medicine. 2021; 10(8):1618. https://doi.org/10.3390/jcm10081618
Chicago/Turabian StyleMatteucci, Andrea, Michela Bonanni, Marco Centioni, Federico Zanin, Francesco Geuna, Gianluca Massaro, and Giuseppe Sangiorgi. 2021. "Home Management of Heart Failure and Arrhythmias in Patients with Cardiac Devices during Pandemic" Journal of Clinical Medicine 10, no. 8: 1618. https://doi.org/10.3390/jcm10081618
APA StyleMatteucci, A., Bonanni, M., Centioni, M., Zanin, F., Geuna, F., Massaro, G., & Sangiorgi, G. (2021). Home Management of Heart Failure and Arrhythmias in Patients with Cardiac Devices during Pandemic. Journal of Clinical Medicine, 10(8), 1618. https://doi.org/10.3390/jcm10081618







