Increased Levels of Adipocyte and Epidermal Fatty Acid-Binding Proteins in Acute Lymphoblastic Leukemia Survivors
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ma, H.; Sun, H.; Sun, X. Survival improvement by decade of patients aged 0–14 years with acute lymphoblastic Leukemia: A SEER analysis. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014: Cancer in children and adolescents. CA A Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.W.; Ward, K.C.; Bonaventure, A.; Siegel, D.A.; Coleman, M.P. Survival among children diagnosed with acute lymphoblastic Leukemia in the United States, by race and age, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017, 123 (Suppl. 24), 5178–5189. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Childhood cancer survivor study. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Krawczuk-Rybak, M.; Panasiuk, A.; Stachowicz-Stencel, T.; Zubowska, M.; Skalska-Sadowska, J.; Sęga-Pondel, D.; Czajńska-Deptuła, A.; Sławińska, D.; Badowska, W.; Kamieńska, E.; et al. Health status of polish children and adolescents after cancer treatment. Eur. J. Pediatr. 2018, 177, 437–447. [Google Scholar] [CrossRef]
- Suh, E.; Stratton, K.L.; Leisenring, W.M.; Nathan, P.C.; Ford, J.S.; Freyer, D.R.; McNeer, J.L.; Stock, W.; Stovall, M.; Krull, K.R.; et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: A retrospective cohort analysis from the childhood cancer survivor study. Lancet Oncol. 2020, 21, 421–435. [Google Scholar] [CrossRef]
- Ness, K.K.; Kirkland, J.L.; Gramatges, M.M.; Wang, Z.; Kundu, M.; McCastlain, K.; Li-Harms, X.; Zhang, J.; Tchkonia, T.; Pluijm, S.M.F.; et al. Premature physiologic aging as a paradigm for understanding increased risk of adverse health across the lifespan of survivors of childhood cancer. J. Clin. Oncol. 2018, 36, 2206–2215. [Google Scholar] [CrossRef]
- Fulbright, J.M.; Raman, S.; McClellan, W.S.; August, K.J. Late effects of childhood Leukemia therapy. Curr. Hematol. Malig. Rep. 2011, 6, 195–205. [Google Scholar] [CrossRef]
- Essig, S.; Li, Q.; Chen, Y.; Hitzler, J.; Leisenring, W.; Greenberg, M.; Sklar, C.; Hudson, M.M.; Armstrong, G.T.; Krull, K.R.; et al. Estimating the risk for late effects of therapy in children newly diagnosed with standard risk acute lymphoblastic leukemia using an historical cohort: A report from the childhood cancer survivor study. Lancet Oncol. 2014, 15, 841–851. [Google Scholar] [CrossRef]
- Bizzarri, C.; Bottaro, G.; Pinto, R.M.; Cappa, M. Metabolic syndrome and diabetes mellitus in childhood cancer survivors. Pediatr. Endocrinol. Rev. 2014, 11, 365–373. [Google Scholar]
- Nam, G.E.; Kaul, S.; Wu, Y.P.; Nelson, R.E.; Wright, J.; Fluchel, M.N.; Hacking, C.C.; Kirchhoff, A.C. A meta-analysis of body mass index of adolescent and adult survivors of pediatric acute lymphoblastic Leukemia. J. Cancer Surviv. 2015, 9, 412–421. [Google Scholar] [CrossRef]
- Morel, S.; Leahy, J.; Fournier, M.; Lamarche, B.; Garofalo, C.; Grimard, G.; Poulain, F.; Delvin, E.; Laverdière, C.; Krajinovic, M.; et al. Lipid and lipoprotein abnormalities in acute lymphoblastic Leukemia survivors. J. Lipid Res. 2017, 58, 982–993. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte fatty acid–binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Furuhashi, M.; Ishimura, S.; Ota, H.; Miura, T. Lipid chaperones and metabolic inflammation. Int. J. Inflam. 2011, 2011. [Google Scholar] [CrossRef]
- Yeung, D.C.Y.; Wang, Y.; Xu, A.; Cheung, S.C.W.; Wat, N.M.S.; Fong, D.Y.T.; Fong, C.H.Y.; Chau, M.T.; Sham, P.C.; Lam, K.S.L. Epidermal fatty-acid-binding protein: A new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur. Heart J. 2008, 29, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, S.; Furuhashi, M.; Watanabe, Y.; Hoshina, K.; Fuseya, T.; Mita, T.; Okazaki, Y.; Koyama, M.; Tanaka, M.; Akasaka, H.; et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE 2013, 8, e81318. [Google Scholar] [CrossRef] [PubMed]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef]
- Kułaga, Z.; Grajda, A.; Gurzkowska, B.; Góźdź, M.; Wojtyło, M.; Świąder, A.; Różdżyńska-Świątkowska, A.; Litwin, M. Polish 2012 growth references for preschool children. Eur. J. Pediatr. 2013, 172, 753–761. [Google Scholar] [CrossRef]
- McCarthy, H.D.; Ashwell, M. A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message—’keep your waist circumference to less than half your height’. Int. J. Obes. 2006, 30, 988–992. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Kułaga, K.; Grupa Badaczy, O.L.A.F. Distribution of blood pressure in school-aged children and adolescents reference population. Stand. Med. 2010, 7, 853–864. [Google Scholar]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatric Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Liu, S.; Chung, M.; Kelly, M.J. Growth patterns during and after treatment in patients with pediatric ALL: A meta-analysis. Pediatr. Blood Cancer 2015, 62, 1452–1460. [Google Scholar] [CrossRef]
- Möhlig, M.; Weickert, M.O.; Ghadamgadai, E.; Machlitt, A.; Pfüller, B.; Arafat, A.M.; Pfeiffer, A.F.H.; Schöfl, C. Adipocyte fatty acid-binding protein is associated with markers of obesity, but is an unlikely link between obesity, insulin resistance, and hyperandrogenism in polycystic ovary syndrome women. Eur. J. Endocrinol. 2007, 157, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Tso, A.W.K.; Cheung, B.M.Y.; Wang, Y.; Wat, N.M.S.; Fong, C.H.Y.; Yeung, D.C.Y.; Janus, E.D.; Sham, P.C.; Lam, K.S.L. Circulating adipocyte–fatty acid binding protein levels predict the development of the metabolic syndrome. Circulation 2007, 115, 1537–1543. [Google Scholar] [CrossRef]
- Yeung, D.C.Y.; Xu, A.; Cheung, C.W.S.; Wat, N.M.S.; Yau, M.H.; Fong, C.H.Y.; Chau, M.T.; Lam, K.S.L. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arter. Thromb. Vasc. Biol. 2007, 27, 1796–1802. [Google Scholar] [CrossRef]
- Hsu, B.G.; Chen, Y.C.; Lee, R.P.; Lee, C.C.; Lee, C.J.; Wang, J.H. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ. J. 2010, 74, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Furuhashi, M.; Ishimura, S.; Koyama, M.; Okazaki, Y.; Mita, T.; Fuseya, T.; Yamashita, T.; Tanaka, M.; Yoshida, H.; et al. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am. J. Hypertens. 2012, 25, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Fuseya, T.; Furuhashi, M.; Yuda, S.; Muranaka, A.; Kawamukai, M.; Mita, T.; Ishimura, S.; Watanabe, Y.; Hoshina, K.; Tanaka, M.; et al. Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc. Diabetol. 2014, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Okura, T.; Fujioka, Y.; Sumi, K.; Matsuzawa, K.; Izawa, S.; Ueta, E.; Kato, M.; Taniguchi, S.; Yamamoto, K. Serum fatty acid-binding protein 4 (FABP4) concentration is associated with insulin resistance in peripheral tissues, a clinical study. PLoS ONE 2017, 12, e0179737. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calvo, R.; Girona, J.; Alegret, J.M.; Bosquet, A.; Ibarretxe, D.; Masana, L. Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J. Endocrinol. 2017, 233, R173–R184. [Google Scholar] [CrossRef]
- Choi, K.M.; Yannakoulia, M.; Park, M.S.; Cho, G.J.; Kim, J.H.; Lee, S.H.; Hwang, T.G.; Yang, S.J.; Kim, T.N.; Yoo, H.J.; et al. Serum adipocyte fatty acid–binding protein, retinol-binding protein 4, and adiponectin concentrations in relation to the development of the metabolic syndrome in Korean boys: A 3-y prospective cohort study12345. Am. J. Clin. Nutr 2011, 93, 19–26. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Patryn, E.; Bednarz-Misa, I.; Mierzchala, M.; Hotowy, K.; Czapinska, E.; Kustrzeba-Wojcicka, I.; Gamian, A.; Noczynska, A. Circulating adipocyte fatty acid-binding protein, juvenile obesity, and metabolic syndrome. J. Pediatr. Endocrinol. Metab. 2011, 24, 921–928. [Google Scholar] [CrossRef]
- Hu, X.; Ma, X.; Pan, X.; Luo, Y.; Xu, Y.; Xiong, Q.; Bao, Y.; Jia, W. Association of androgen with gender difference in serum adipocyte fatty acid binding protein levels. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Gurney, J.G.; Ness, K.K.; Sibley, S.D.; O’Leary, M.; Dengel, D.R.; Lee, J.M.; Youngren, N.M.; Glasser, S.P.; Baker, K.S. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic Leukemia. Cancer 2006, 107, 1303–1312. [Google Scholar] [CrossRef]
- Nottage, K.A.; Ness, K.K.; Li, C.; Srivastava, D.; Robison, L.L.; Hudson, M.M. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic Leukaemia—From the St. Jude lifetime cohort. Br. J. Haematol. 2014, 165, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Rodday, A.M.; Kelly, M.J.; Must, A.; MacPherson, C.; Roberts, S.B.; Saltzman, E.; Parsons, S.K. Predictors of being overweight or obese in survivors of pediatric acute lymphoblastic Leukemia (ALL). Pediatr. Blood Cancer 2014, 61, 1263–1269. [Google Scholar] [CrossRef]
- Smith, W.A.; Li, C.; Nottage, K.; Mulrooney, D.A.; Armstrong, G.T.; Lanctot, J.Q.; Chemaitilly, W.; Laver, J.H.; Srivastava, D.K.; Robison, L.L.; et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: A report from the St. Jude lifetime cohort study. Cancer 2014, 120, 2742–2750. [Google Scholar] [CrossRef]
- Saultier, P.; Auquier, P.; Bertrand, Y.; Vercasson, C.; Oudin, C.; Contet, A.; Plantaz, D.; Poirée, M.; Ducassou, S.; Kanold, J.; et al. Metabolic syndrome in long-term survivors of childhood acute Leukemia treated without hematopoietic stem cell transplantation: An, L.E.A. study. Haematologica 2016, 101, 1603–1610. [Google Scholar] [CrossRef]
- Özdemir, Z.C.; Düzenli Kar, Y.; Demiral, M.; Sırmagül, B.; Bör, Ö.; Kırel, B. The Frequency of metabolic syndrome and serum osteopontin levels in survivors of childhood acute lymphoblastic Leukemia. J. Adolesc. Young Adult Oncol. 2018, 7, 480–487. [Google Scholar] [CrossRef]
- Levy, E.; Samoilenko, M.; Morel, S.; England, J.; Amre, D.; Bertout, L.; Drouin, S.; Laverdière, C.; Krajinovic, M.; Sinnett, D.; et al. Cardiometabolic risk factors in childhood, adolescent and young adult survivors of acute lymphoblastic Leukemia—A petale cohort. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Adams-Huet, B.; Victor, R.G.; Church, T.S.; Snell, P.G.; Dunn, A.L.; Eshelman-Kent, D.A.; Ross, R.; Janiszewski, P.M.; Turoff, A.J.; et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic Leukemia. J. Clin. Oncol. 2009, 27, 3698–3704. [Google Scholar] [CrossRef] [PubMed]
- Meacham, L.R.; Chow, E.J.; Ness, K.K.; Kamdar, K.Y.; Chen, Y.; Yasui, Y.; Oeffinger, K.C.; Sklar, C.A.; Robison, L.L.; Mertens, A.C. Cardiovascular risk factors in adult survivors of pediatric cancer—A report from the childhood cancer survivor study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Latoch, E.; Muszynska-Roslan, K.; Panas, A.; Panasiuk, A.; Sawicka-Zukowska, M.; Zelazowska-Rutkowska, B.; Zabrocka, E.; Krawczuk-Rybak, M. Adipokines and insulin resistance in young adult survivors of childhood cancer. Int. J. Endocrinol. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Yan, X.; Yan, F.; Sun, Y.; Zeng, J.; Waigel, S.; Yin, Y.; Fraig, M.M.; Egilmez, N.K.; et al. Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metab. 2018, 28, 689–705.e5. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Shen, N.; Pang, J.; Zhang, Y.; Rao, E.; Bode, A.; Al-Kali, A.; Zhang, D.; Litzow, M.; Li, B.; et al. Fatty acid binding protein FABP4 mechanistically links obesity with aggressive AML by enhancing aberrant DNA methylation in AML cells. Leukemia 2017, 31, 1434–1442. [Google Scholar] [CrossRef]


| Number (%) | Median (IQR) a | |
|---|---|---|
| Patients | 62 (100%) | |
| Male | 28 (45.2%) | |
| Female | 34 (54.8%) | |
| Age at diagnosis (years) | 4.13 (3.03–6.45) | |
| Age at study (years) | 12.39 (8.17–16.08) | |
| Follow-up after treatment (years) | 7.05 (2.24–9.07) | |
| Overweight | 18 (29%) | |
| Obese | 10 (16%) | |
| Glucocorticoids: | ||
| Cumulative corticosteroid dose (mg/m2) c | 62 (100%) | 3087 (3087–3087) b |
| Prednisone (cumulative dose in mg/m2) | 62 (100%) | 1680 (1680–1680) b |
| Dexamethasone (cumulative dose in mg/m2) | 62 (100%) | 210 (210–210) b |
| Radiotherapy | 9 (14.5%) | |
| Cranial radiotherapy (CRT) (cumulative dose in Grey) | 8 (12.9%) | 12 (12–12) b |
| Total body irradiation (TBI) | 2 (3.23%) | 12 (12–12) b |
| No | 53 (85.5%) | |
| HSCT | 6 (9.7%) | |
| Metabolic derangements | ||
| 1 Metabolic risk factor | 23 (37.1%) | |
| 2 Metabolic risk factors | 5 (8.1%) | |
| 3 Metabolic risk factors | 4 (6.5%) | |
| 4 Metabolic risk factors | 1 (1.6%) |
| Total | Females | Males | p Value | |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| n = 62 | n = 34 | n = 28 | ||
| Age at diagnosis (years) | 4.13 (3.03; 6.45) | 5.23 (2.91; 7.04) | 3.74 (3.30; 5.59) | 0.784 |
| Age at study (years) | 12.36 (8.17; 16.08) | 13.55 (10.13; 16.40) | 10.89 (6.51; 14.49) | 0.164 |
| Follow-up (years) | 7.05 (2.24; 9.07) | 7.69 (3.45; 9.36) | 5.71 (1.71; 8.68) | 0.178 |
| Weight (kg) | 47.50 (31.30; 65.00) | 49.25 (38.00; 62.70) | 44.05 (24.80; 71.35) | 0.598 |
| Height (cm) | 151.25 (133.50; 162.00) | 152.50 (140.00; 160.00) | 145.75 (118.75; 166.75) | 0.648 |
| BMI (kg/m2) | 21.17 (17.93; 24.96) | 21.41 (18.82; 24.96) | 20.99 (17.53; 24.51) | 0.817 |
| WC (cm) | 72.00 (63.00; 81.00) | 72.50 (65.00; 80.00) | 71.50 (57.50; 83.00) | 1.00 |
| WHtR | 0.50 (0.45; 0.54) | 0.48 (0.45; 0.53) | 0.50 (0.45; 0.55) | 0.425 |
| ALT (U/L) | 15.00 (12.00; 23.00) | 14.00 (12.00; 22.00) | 17.00 (13.00; 23.00) | 0.513 |
| TG (mg/dL) | 91.00 (62.00; 118.00) | 84.00 (62.00; 100.00) | 98.00 (63.00; 132.00) | 0.443 |
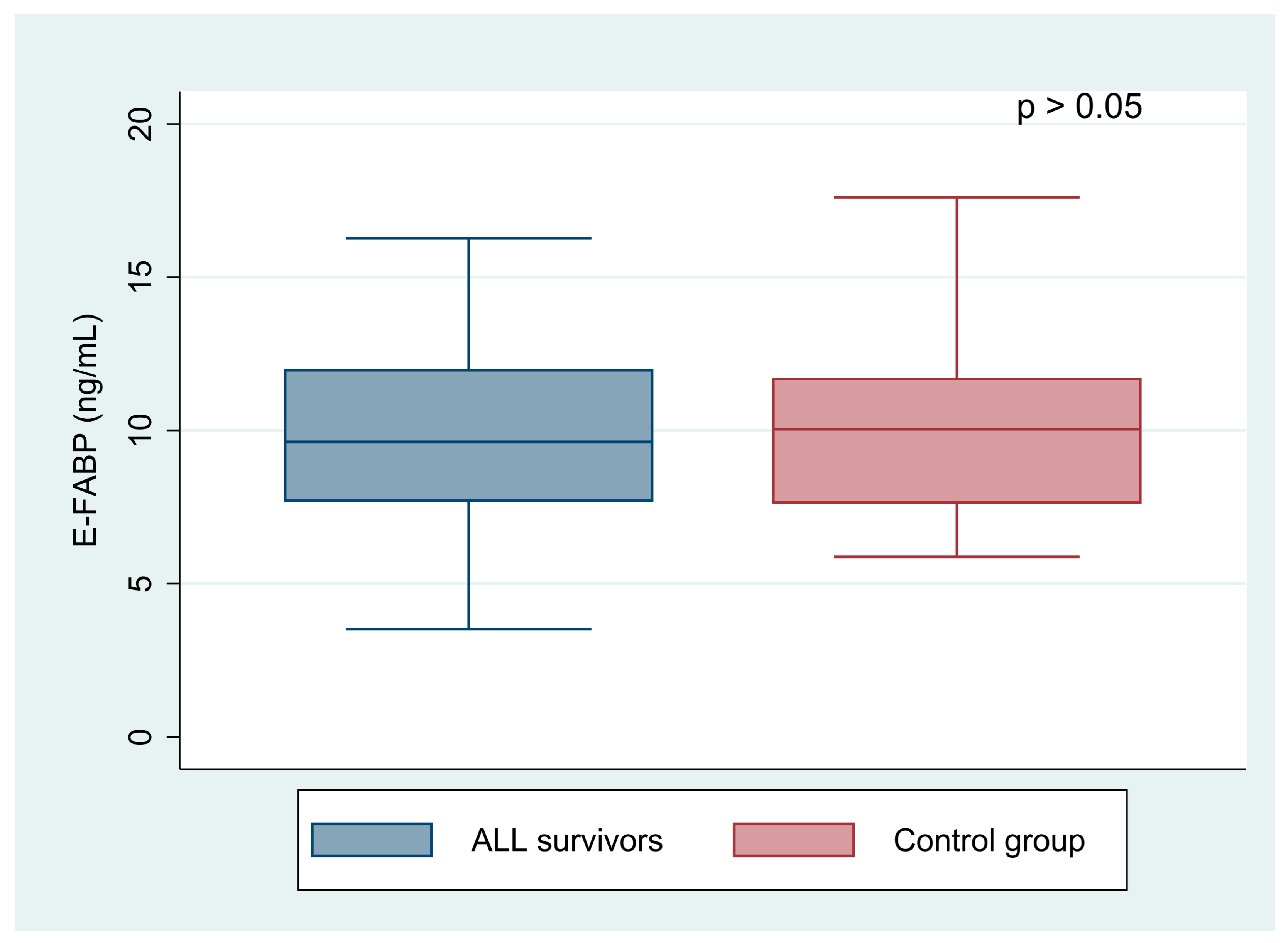
| E-FABP (ng/mL) | 10.32 (8.26; 14.08) | 11.07 (9.43; 15.08) | 9.04 (7.12; 12.00) | 0.023 |
| A-FABP (ng/mL) | 23.69 (14.62; 30.82) | 24.71 (16.21; 31.43) | 23.09 (11.66; 30.12) | 0.349 |
| Overweight/Obese | Normal Weight | p Value | |
|---|---|---|---|
| n = 28 | n = 34 | ||
| Median (IQR) | Median (IQR) | ||
| BMI (kg/m2) | 25.43 (22.36; 28.47) | 18.85 (16.18; 21.30) | <0.001 |
| Age at study (years) | 12.21 (7.43; 17.34) | 12.68 (9.21; 15.42) | 0.905 |
| Follow-up (years) | 5.58 (2.25; 8.72) | 7.58 (2.01; 9.84) | 0.427 |
| ALT (U/L) | 17.50 (13.50; 33.00) | 13.00 (11.00; 19.00) | 0.053 |
| TG (mg/dL) | 100.00 (66.00; 139.00) | 72.00 (56.00; 98.00) | 0.078 |
| E-FABP (ng/mL) | 10.86 (9.16; 17.52) | 9.78 (7.79; 12.04) | 0.055 |
| A-FABP (ng/mL) | 27.76 (20.84; 38.74) | 20.09 (12.32; 26.21) | 0.006 |
| Independent Variable | Coeff. | t | p | 95% Conf. Interval | ||
|---|---|---|---|---|---|---|
| A-FABP (ng/mL) | BMI (kg/m2) | 1.02 | 2.87 | 0.006 | 0.31 | 1.73 |
| SBP (normal vs. high) | 13.74 | 2.04 | 0.046 | 0.23 | 27.3 | |
| DBP (normal vs. high) | −0.64 | −0.12 | 0.907 | −11.6 | 10.3 | |
| E-FABP (ng/mL) | BMI (kg/m2) | 0.48 | 3.43 | 0.005 | 0.17 | 0.78 |
| Cholesterol (mg/dL) | 0.04 | 1.40 | 0.186 | −0.02 | 0.11 | |
| ≥1 Metabolic Risk Factor | Control Group | p Value | |
|---|---|---|---|
| n = 33 | n = 25 | ||
| Median (IQR) | Median (IQR) | ||
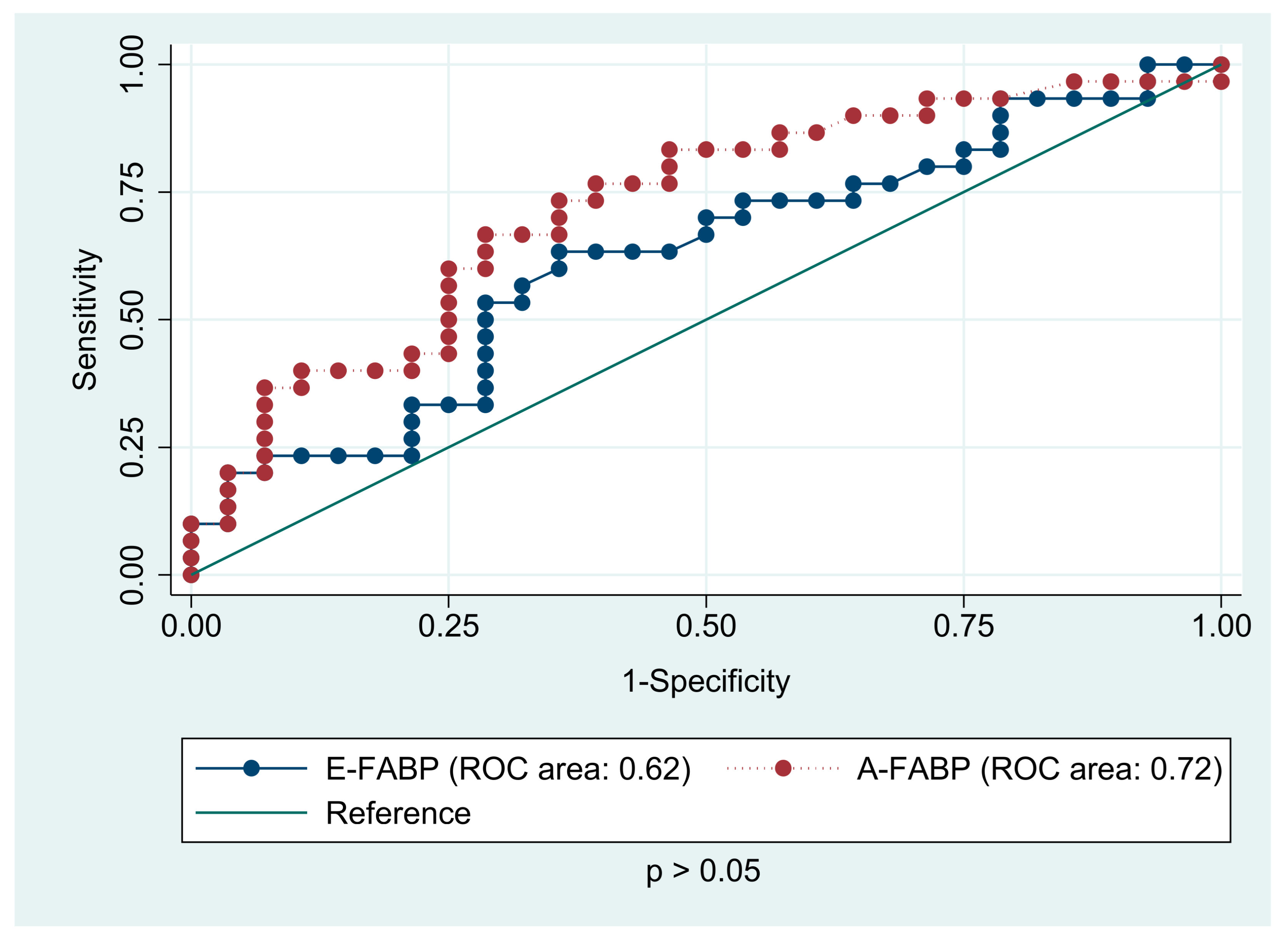
| E-FABP (ng/mL) | 11.28 (8.44; 14.60) | 10.04 (7.64; 11.68) | 0.090 |
| A-FABP (ng/mL) | 25.81 (21.32; 37.84) | 13.44 (10.48; 21.15) | <0.001 |
| ≥2 Metabolic Risk Factors | |||
| n = 10 | n = 25 | ||
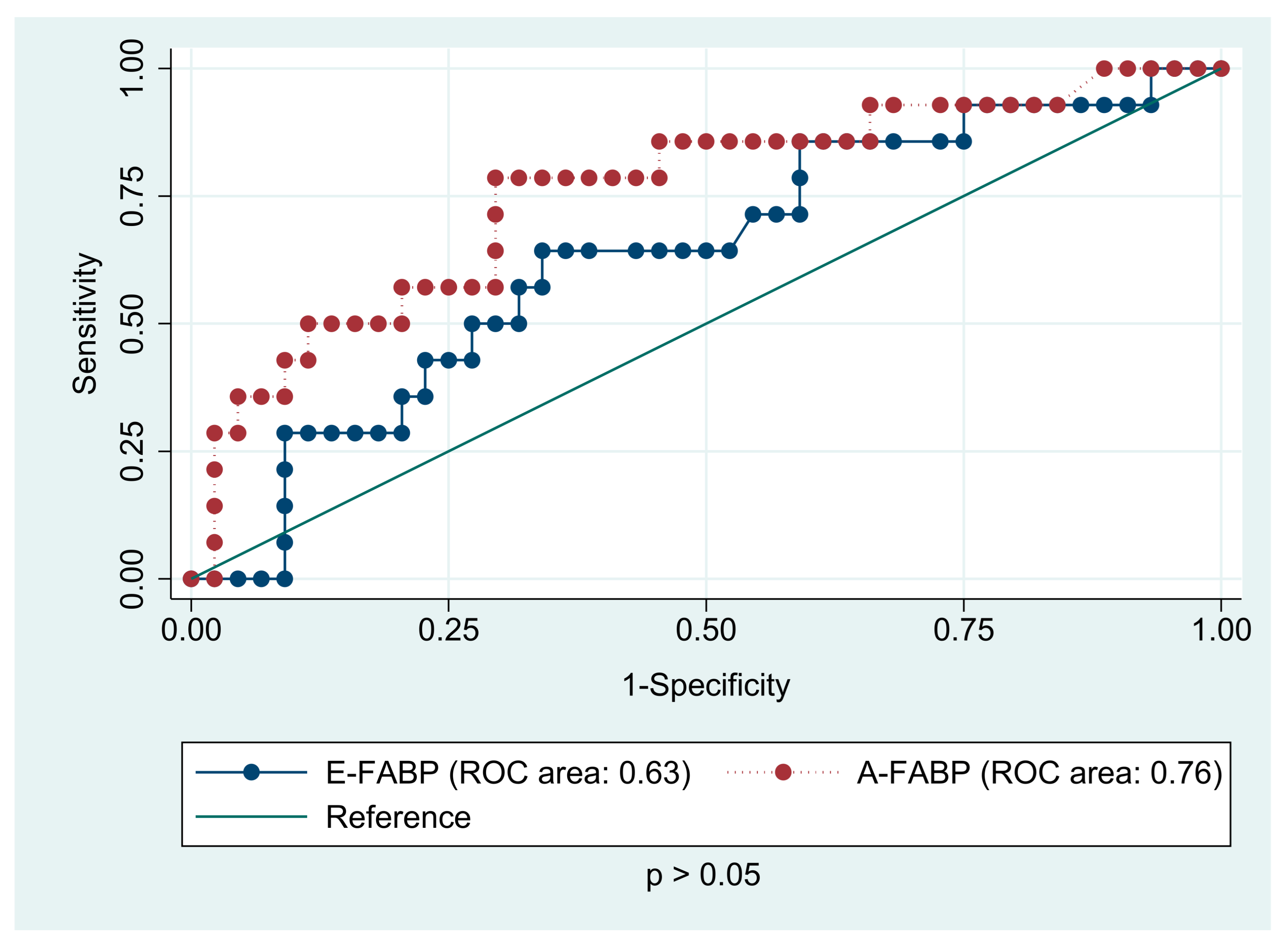
| E-FABP (ng/mL) | 13.74 (9.88; 16.27) | 10.04 (7.64; 11.68) | 0.021 |
| A-FABP (ng/mL) | 25.51 (24.32; 37.84) | 13.44 (10.48; 21.15) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konończuk, K.; Latoch, E.; Żelazowska-Rutkowska, B.; Krawczuk-Rybak, M.; Muszyńska-Rosłan, K. Increased Levels of Adipocyte and Epidermal Fatty Acid-Binding Proteins in Acute Lymphoblastic Leukemia Survivors. J. Clin. Med. 2021, 10, 1567. https://doi.org/10.3390/jcm10081567
Konończuk K, Latoch E, Żelazowska-Rutkowska B, Krawczuk-Rybak M, Muszyńska-Rosłan K. Increased Levels of Adipocyte and Epidermal Fatty Acid-Binding Proteins in Acute Lymphoblastic Leukemia Survivors. Journal of Clinical Medicine. 2021; 10(8):1567. https://doi.org/10.3390/jcm10081567
Chicago/Turabian StyleKonończuk, Katarzyna, Eryk Latoch, Beata Żelazowska-Rutkowska, Maryna Krawczuk-Rybak, and Katarzyna Muszyńska-Rosłan. 2021. "Increased Levels of Adipocyte and Epidermal Fatty Acid-Binding Proteins in Acute Lymphoblastic Leukemia Survivors" Journal of Clinical Medicine 10, no. 8: 1567. https://doi.org/10.3390/jcm10081567
APA StyleKonończuk, K., Latoch, E., Żelazowska-Rutkowska, B., Krawczuk-Rybak, M., & Muszyńska-Rosłan, K. (2021). Increased Levels of Adipocyte and Epidermal Fatty Acid-Binding Proteins in Acute Lymphoblastic Leukemia Survivors. Journal of Clinical Medicine, 10(8), 1567. https://doi.org/10.3390/jcm10081567






