Left Ventricular Remodeling in Hypertrophic Cardiomyopathy: An Overview of Current Knowledge
Abstract
1. Introduction
2. Natural History of HCM
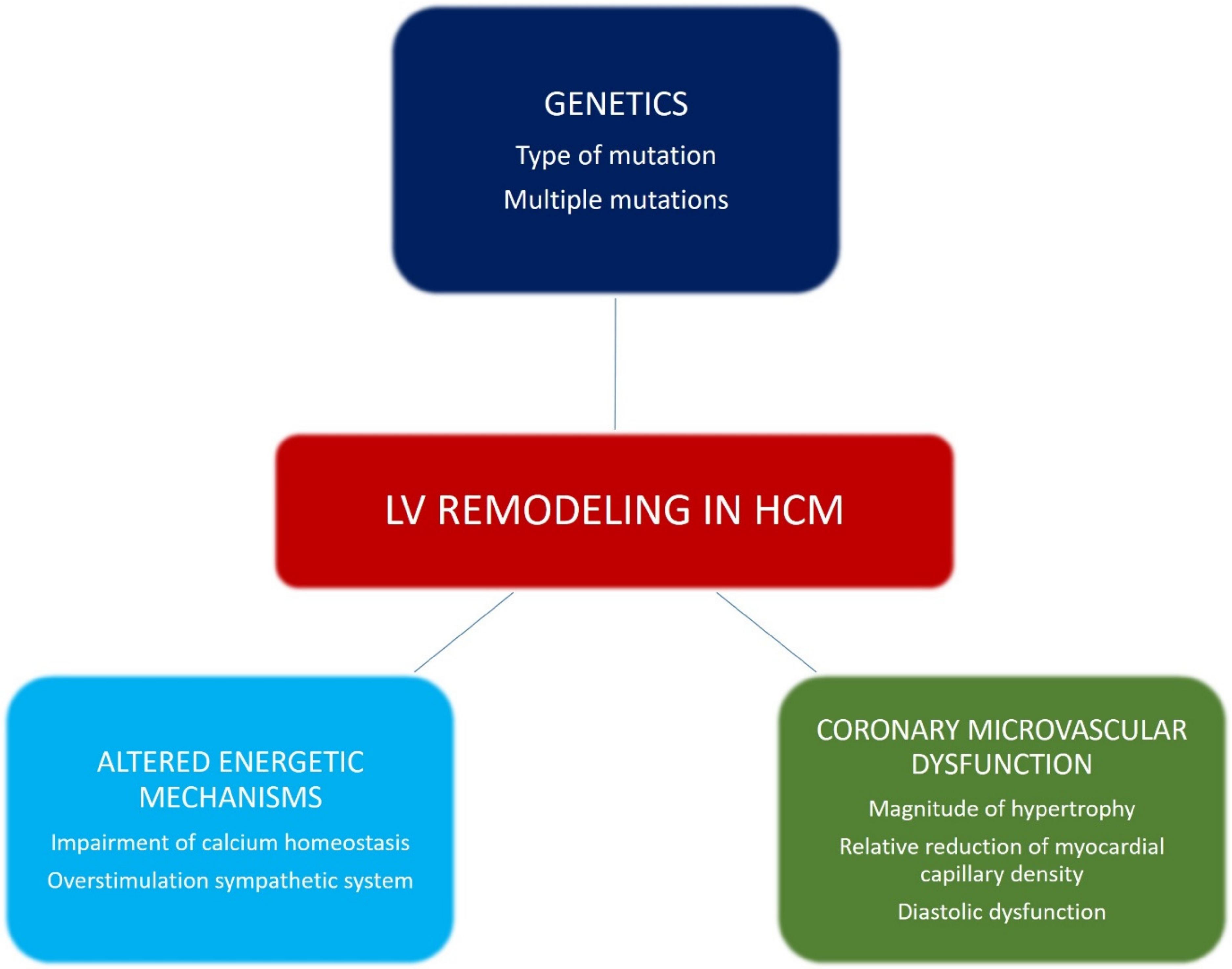
3. Pathophysiological Mechanisms of LV Remodeling in HCM
3.1. Genetics
3.2. Altered Energetic Mechanisms
3.3. Coronary Microvascular Dysfunction
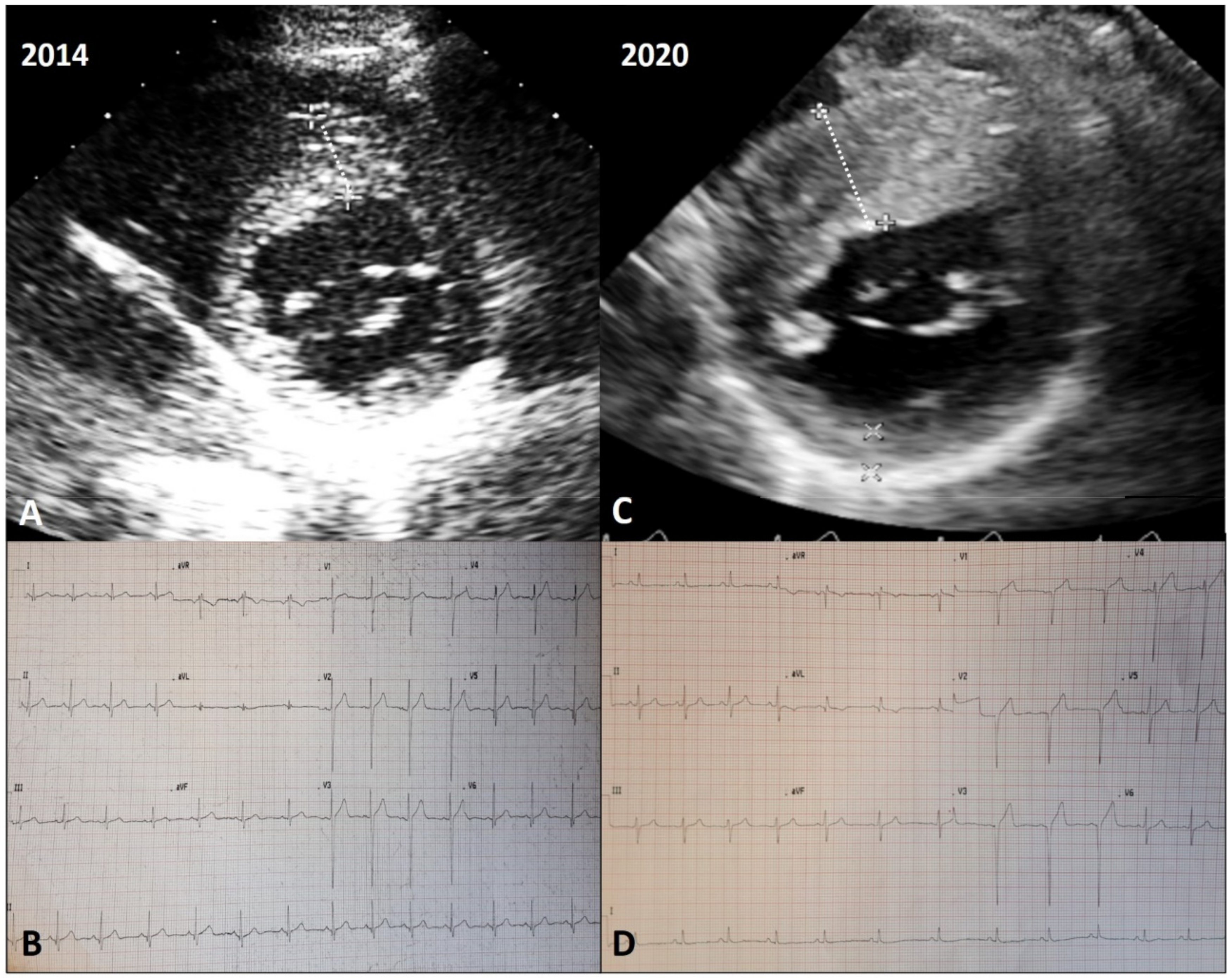
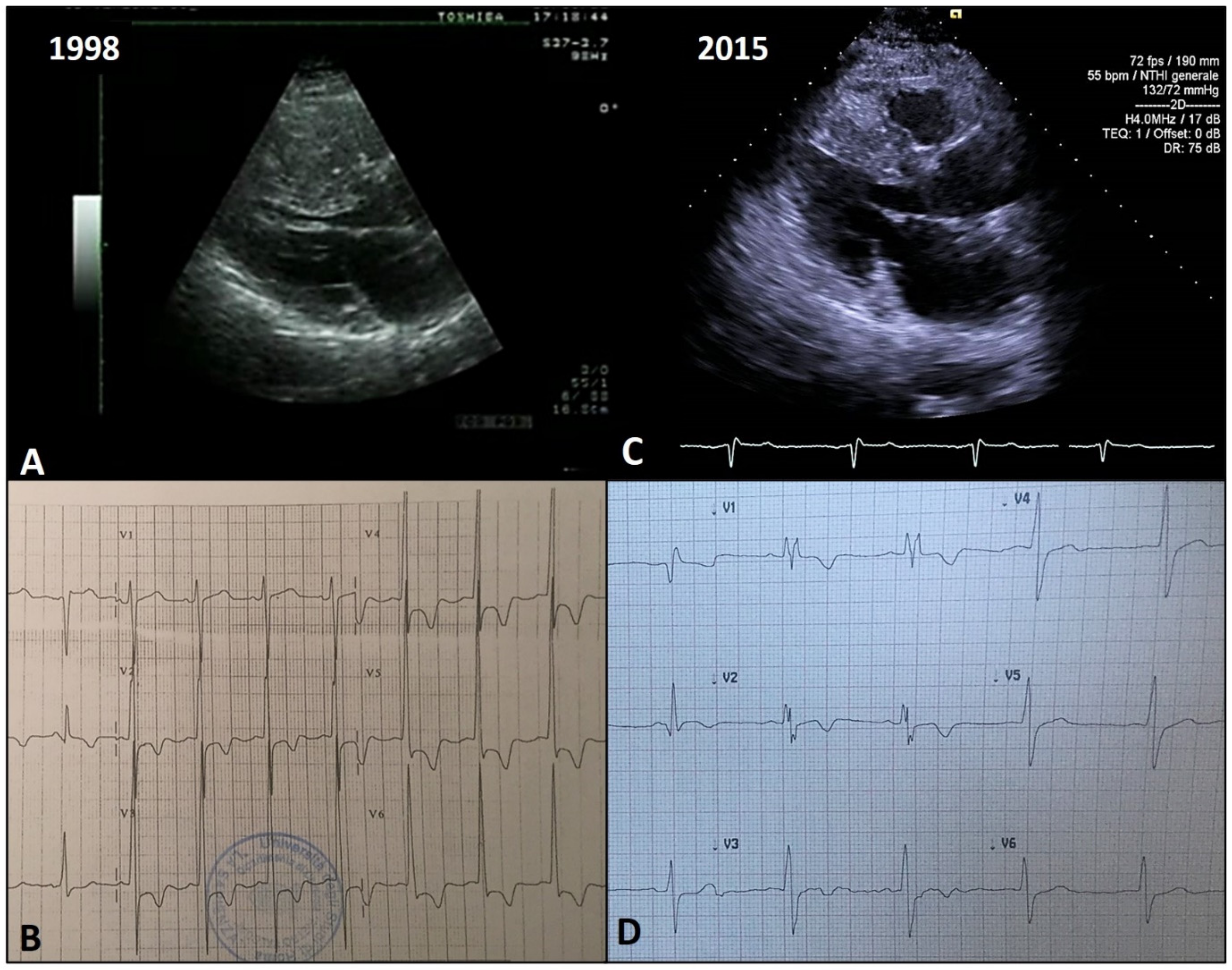
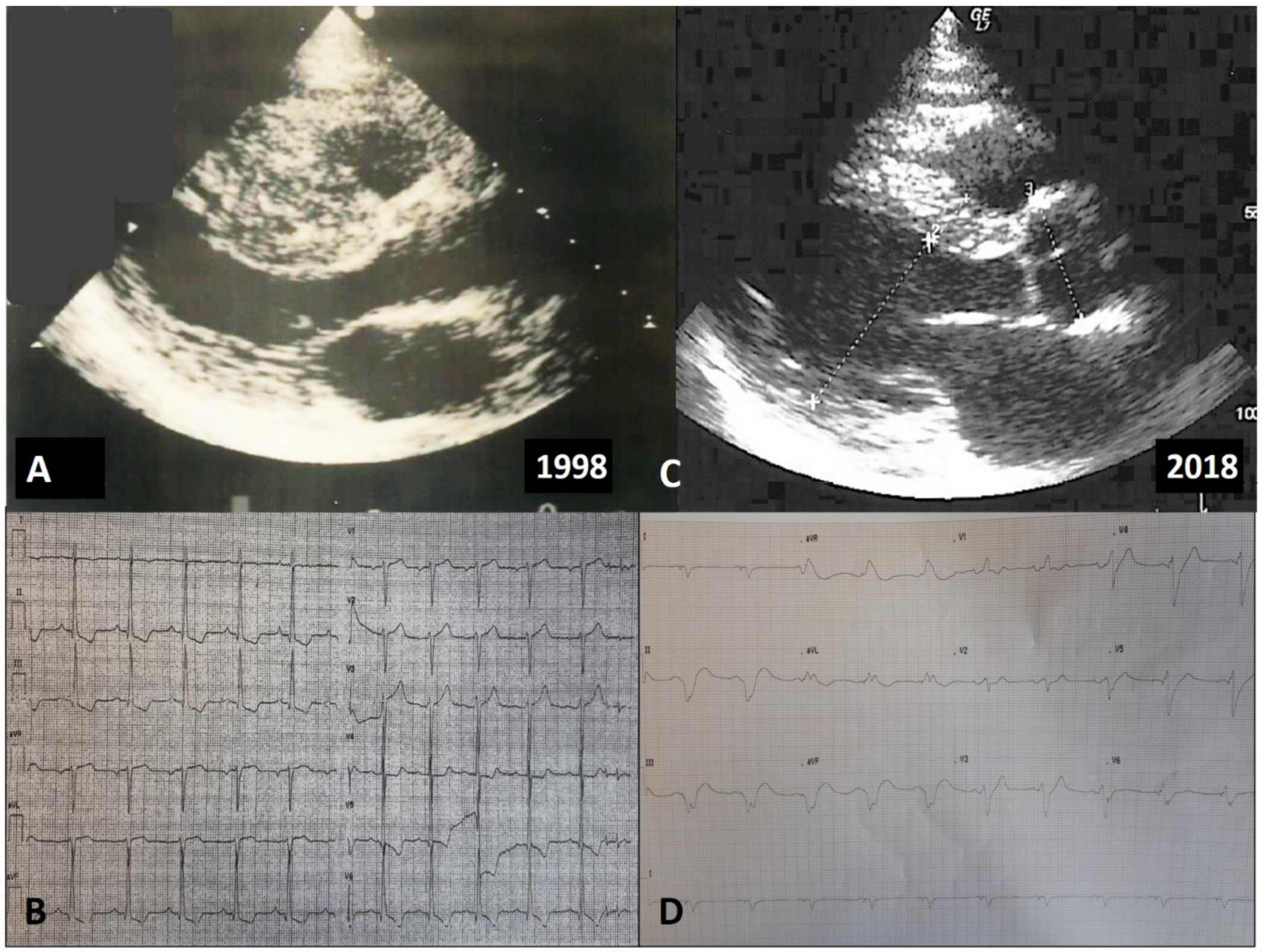
4. Types of LV Remodeling in HCM
4.1. Positive Remodeling
4.2. Benign Remodeling
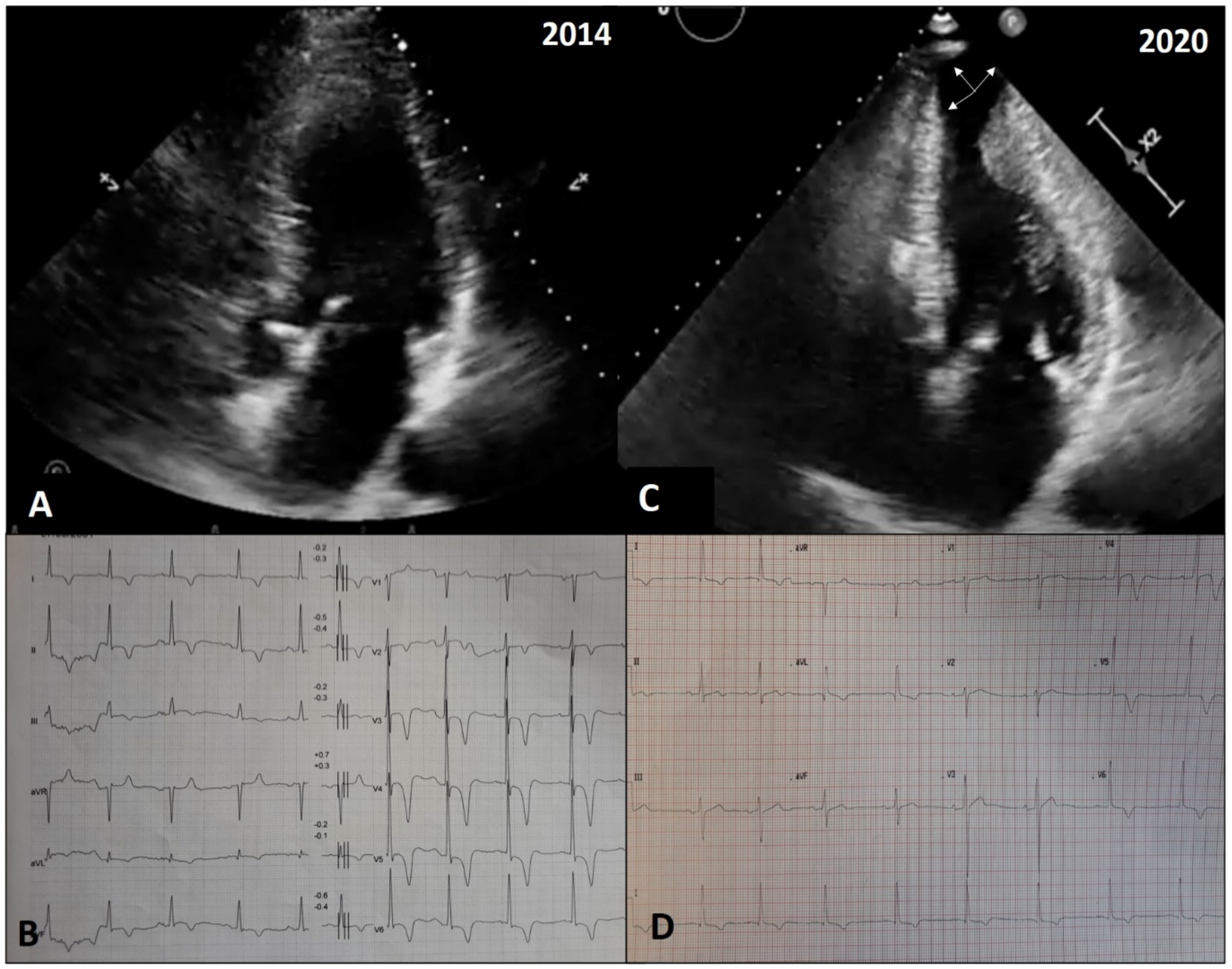
4.3. Adverse Remodeling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eaton, L.W.; Weiss, J.L.; Bulkley, B.H.; Garrison, J.B.; Weisfeldt, M.L. Regional cardiac dilatation after acute myocar- dial infarction: Recognition by two- dimensional echocardiography. N. Engl. J. Med. 1979, 300, 57–62. [Google Scholar] [CrossRef]
- McKay, R.G.; Pfeffer, M.A.; Pasternak, R.C.; Markis, J.E.; Come, P.C.; Nakao, S.; Alderman, J.D.; Ferguson, J.J.; Safian, R.D.; Grossman, W. Left ventricular remodeling after myocardial infarction: A corollary to infarct expansion. Circulation 1986, 74, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Huston, T.P.; Puffer, J.C.; Rodney, W.M. The athletic heart syndrome. N. Engl. J. Med. 1985, 313, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Spirito, P. Implications of Left Ventricular Remodeling in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 1998, 8, 1339–1344. [Google Scholar]
- Olivotto, I.; Cecchi, F.; Poggesi, C.; Yacoub, M.H. Patterns of disease progression in hypertrophic cardiomyopathy: An individualized approach to clinical staging. Circ. Heart Failure 2012, 5, 535–546. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 284, 1–55. [Google Scholar]
- Maron, B.J. Clinical course and management of hypertrophic cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef]
- Swan, D.A.; Bell, B.; Oakley, C.M.; Goodwin, J. Analysis of symptomatic course and prognosis and treatment of hypertrophic obstructive cardiomyopathy. Br. Heart J. 1971, 33, 671–685. [Google Scholar] [CrossRef][Green Version]
- Spirito, P.; Chiarella, F.; Carratino, L.; Berisso, M.Z.; Bellotti, P.; Vecchio, C. Clinical course and prognosis of hypertrophic cardiomyopathy in an outpatient population. N. Engl. J. Med. 1989, 320, 749–755. [Google Scholar] [CrossRef]
- Maron, B.J.; Spirito, P. Impact of patient selection biases on the perception of hypertrophic cardiomyopathy and its natural history. Am. J. Cardiol. 1993, 72, 970–972. [Google Scholar] [CrossRef]
- Cecchi, F.; Olivotto, I.; Montereggi, A.; Santoro, G.; Dolara, A.; Maron, B.J. Hypertrophic cardiomyopathy in Tuscany: Clinical course and outcome in an unselected regional population. J. Am. Coll. Cardiol. 1995, 26, 1529–1536. [Google Scholar] [CrossRef]
- Spirito, P.; Autore, C.; Rapezzi, C.; Bernabò, P.; Badagliacca, R.; Maron, M.S.; Bongioanni, S.; Coccolo, F.; Estes, N.M.; Barillà, C.S.; et al. Syncope and Risk of Sudden Death in Hypertrophic Cardiomyopathy. Circulation 2009, 119, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.A.; Mohiddin, S.A.; Tripodi, D.; Winkler, J.B.; Fananapazir, L. Efficacy of implantable cardioverter-defibrillator for primary and secondary prevention of sudden cardiac death in hypertrophic cardiomyopathy. PACE 2003, 26, 1887–1896. [Google Scholar] [CrossRef]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.S.; et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy. Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Cecchi, F.; Casey, S.A.; Dolara, A.; Traverse, J.H.; Maron, B.J. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001, 104, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Autore, C.; Musumeci, M.B. The natural history of hypertrophic cardiomyopathy. Eur. Heart J. Suppl. 2020, 22, L11–L14. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How hypertrophic cardiomyopathy became a contemporary tratabile genetic disease with low mortality. Shaped by 50 years of clinical research and practice. JAMA Cardiol. 2016, 1, 98–105. [Google Scholar] [CrossRef]
- Watkins, H.; Ashrafian, H.; Redwood, C. Inherited Cardiomyopathies. N. Engl. J. Med. 2011, 364, 1643–1656. [Google Scholar] [CrossRef]
- Kelly, M.; Semsarian, C. Multiple mutations in genetic cardiovascular disease: A marker of disease severity. Circ. Cardiovasc. Genet. 2009, 2, 182–190. [Google Scholar] [CrossRef]
- Van Dijk, S.J.; Dooijes, D.; dos Remedios, C.; Michels, M.; Lamers, J.M.J.; Winegrad, S.; Schlossarek, S.; Carrier, L.; ten Cate, F.J.; Stienen, G.J.M.; et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: Haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation 2009, 119, 1473–1483. [Google Scholar] [CrossRef]
- Ho, C.Y. Hypertrophic cardiomyopathy: Preclinical and early phenotype. J. Cardiovasc. Trans. Res. 2009, 2, 462–470. [Google Scholar] [CrossRef]
- Marian, A.J.; Roberts, R. The molecular genetic basis for hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 2001, 33, 655–670. [Google Scholar] [CrossRef]
- Ashrafian, H.; McKenna, W.J.; Watkins, H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ. Res. 2011, 109, 86–96. [Google Scholar] [CrossRef]
- Schäfers, M.; Dutka, D.; Rhodes, C.G.; Lammertsma, A.A.; Hermansen, F.; Schober, O.; Camici, P.G. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ. Res. 1998, 82, 57–62. [Google Scholar] [CrossRef]
- Olivotto, I.; Girolami, F.; Nistri, S.; Rossi, A.; Rega, L.; Garbini, F.; Grifoni, C.; Cecchi, F.; Yacoub, M.H. The Many Faces of Hypertrophic Cardiomyopathy: From Developmental Biology to Clinical Practice. J. Cardiovasc. Transl. Res. 2009, 2, 349–367. [Google Scholar] [CrossRef]
- Maron, B.J.; Bonow, R.O.; Cannon, R.O.; Leon, M.B.; Epstein, S.E. Hypertrophic cardiomyopathy: Interrelation of clinical manifestations, pathophysiology, and therapy. N. Engl. J. Med. 1987, 316, 780–789. [Google Scholar] [CrossRef]
- Cannon, R.O.; Rosing, D.R.; Maron, B.J.; Leon, M.B.; Bonow, R.O.; Watson, R.M.; Epstein, S.E. Myocardial ischemia in patients with hypertrophic cardiomyopathy: Contribution of inadequate vasodilator reserve and elevated ventricular filling pressures. Circulation 1985, 7, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Raphael, C.E.; Cooper, R.; Parker, K.H.; Collinson, J.; Vassiliou, V.; Pennell, D.J.; De Silva, R.; Hsu, L.Y.; Greve, A.M.; Nijjer, S.; et al. Mechanisms of Myocardial Ischemia in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 1651–1660. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Girolami, F.; Torricelli, F.; Camici, P.G. Relevance of Coronary Microvascular Flow Impairment to Long-Term Remodeling and Systolic Dysfunction in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 1043–1048. [Google Scholar] [CrossRef]
- Cecchi, F.; Sgalambro, A.; Baldi, M.; Sotgia, B.; Antoniucci, D.; Camici, P.G.; Sciagrà, R.; Olivotto, I. Microvascular Dysfunction, Myocardial Ischemia, and Progression to Heart Failure in Patients with Hypertrophic Cardiomyopathy. J. Cardiovasc. Transl. Res. 2009, 2, 452–461. [Google Scholar] [CrossRef]
- Maurizi, N.; Michels, M.; Rowin, E.J.; Semsarian, C.; Girolami, F.; Tomberli, B.; Cecchi, F.; Maron, M.S.; Olivotto, I.; Maron, B.J. Clinical Course and Significance of Hypertrophic Cardiomyopathy Without Left Ventricular Hypertrophy. Circulation 2019, 139, 830–833. [Google Scholar] [CrossRef]
- Christiaans, I.; Birnie, E.; Bonsel, G.J. Manifest disease, risk factors for sudden cardiac death, and cardiac events in a large nationwide cohort of predictively tested hypertrophic cardiomyopathy mutation carriers: Determining the best cardiological screening strategy. Eur. Heart J. 2011, 32, 1161–1170. [Google Scholar] [CrossRef]
- Gray, B.; Ingles, J.; Semsarian, C. Natural history of genotype positive-phenotype negative patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2011, 152, 258–259. [Google Scholar] [CrossRef]
- Maron, B.J.; Spirito, P.; Wesley, Y.; Arce, J. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N. Engl. J. Med. 1986, 315, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Maron, B.J. Absence of progression of left ventricular hypertrophy in adult patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1987, 9, 1013–1017. [Google Scholar] [CrossRef]
- Domenicucci, S.; Lazzeroni, E.; Roelandt, J.; Cate, F.J.T.; Vletter, W.B.; Arntzenius, A.C.; Das, S.K. Progression of hypertrophic cardiomyopathy. A cross sectional echocardiographic study. Heart 1985, 53, 405–411. [Google Scholar] [CrossRef]
- Doolan, G.; Nguyen, L.; Chung, J.; Ingles, J.; Semsarian, C. Progression of left ventricular hypertrophy and the angiotensin-converting enzyme gene polymorphism in hypertrophic cardiomyopathy. Int. J. Cardiol. 2004, 96, 157–163. [Google Scholar] [CrossRef]
- Semsarian, C.; French, J.; Trent, R.J.; Richmond, D.R.; Jeremy, R.W. The natural history of left ventricular wall thickening in hypertrophic cardiomyopathy. Aust. N. Z. J. Med. 1997, 27, 51–58. [Google Scholar] [CrossRef]
- Canepa, M.; Fumagalli, C.; Tini, G.; Vincent-Tompkins, J.; Day, S.M.; Ashley, E.A.; Mazzarotto, F.; Ware, J.S.; Michels, M.; Jacoby, D.; et al. Temporal Trend of Age at Diagnosis in Hypertrophic Cardiomyopathy: An Analysis of the International Sarcomeric Human Cardiomyopathy Registry. Circ. Heart Fail. 2020, 13, e007230. [Google Scholar] [CrossRef]
- Spirito, P.; Maron, B.J. Relation between extent of left ventricular hypertrophy and age in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1988, 13, 820–823. [Google Scholar] [CrossRef]
- Louie, E.K.; Maron, B.J. Hypertrophic cardiomyopathy with extreme increase in left ventricular wall thickness: Functional and morphologic features and clinical significance. J. Am. Coll. Cardiol. 1986, 8, 57. [Google Scholar] [CrossRef]
- Thaman, R.; Gimeno, J.R.; Reith, S.; Esteban MT, T.; Limongelli, G.; Murphy, R.T.; Mist, B.; McKenna, W.J.; Elliott, P.M. Progressive left ventricular remodeling in patients with hypertrophic cardiomyopathy and severe left ventricular hypertrophy. J. Am. Coll. Cardiol. 2004, 44, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Maron, B.J.; Bonow, R.O.; Epstein, S.E. Occurrence and significance of progressive left ventricular wall thinning and relative cavity dilatation in hypertrophic cardiomyopathy. Am. J. Cardiol. 1987, 60, 123–129. [Google Scholar] [CrossRef]
- Olivotto, I.; Maron, B.J.; Appelbaum, E.; Harrigan, C.J.; Salton, C.; Gibson, C.M.; Udelson, J.E.; O’Donnell, C.; Lesser, J.R.; Manning, W.J.; et al. Spectrum and Clinical Significance of Systolic Function and Myocardial Fibrosis Assessed by Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2010, 106, 261–267. [Google Scholar] [CrossRef]
- Melacini, P.; Basso, C.; Angelini, A.; Calore, C.; Bobbo, F.; Tokajuk, B.; Bellini, N.; Smaniotto, G.; Zucchetto, M.; Iliceto, S.; et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur. Heart J. 2010, 31, 2111–2123. [Google Scholar] [CrossRef]
- Ciró, E.; Maron, B.J.; Bonow, R.O.; Cannon, R.O.; Epstein, S.E. Relation between marked changes in left ventricular outflow tract gradient and disease progression in hypertrophic cardiomyopathy. Am. J. Cardiol. 1984, 53, 1103–1109. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Maron, B.J.; Prasad, S.K.; Cecchi, F.; Udelson, J.E.; Camici, P.G. The Case for Myocardial Ischemia in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.B.; Russo, D.; Limite, L.R.; Canepa, M.; Tini, G.; Casenghi, M.; Francia, P.; Adduci, C.; Pagannone, E.; Magri, D.; et al. Long-Term Left Ventricular Remodeling of Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 122, 1924–1931. [Google Scholar] [CrossRef]
- Harris, K.M.; Spirito, P.; Maron, M.S.; Zenovich, A.G.; Formisano, F.; Lesser, J.R.; Mackey-Bojack, S.; Manning, W.J.; Udelson, J.E.; Maron, B.J. Prevalence, Clinical Profile, and Significance of Left Ventricular Remodeling in the End-Stage Phase of Hypertrophic Cardiomyopathy. Circulation 2006, 114, 216–225. [Google Scholar] [CrossRef]
- Biagini, E.; Coccolo, F.; Ferlito, M.; Perugini, E.; Rocchi, G.; Bacchi-Reggiani, L.; Lofiego, C.; Boriani, G.; Prandstraller, D.; Picchio, F.M.; et al. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: Prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J. Am. Coll. Cardiol. 2005, 46, 1543–1550. [Google Scholar] [CrossRef]
- Kubo, T.; Gimeno, J.R.; Bahl, A.; Steffensen, U.; Steffensen, M.; Osman, E.; Thaman, R.; Mogensen, J.; Elliot, P.M.; Doi, Y.; et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J. Am. Coll. Cardiol. 2007, 49, 2419–2426. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Haas, T.S.; Garberich, R.F.; Wang, W.; Link, M.S.; Maron, M.S. Hypertrophi cardiomyopathy with left ventricular apical aneurysm. J. Am. Coll. Cardiol. 2017, 69, 1652. [Google Scholar] [CrossRef]
- Maron, M.S.; Rowin, E.J.; Maron, B.J. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: The newest high-risk phenotype. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1351–1352. [Google Scholar] [CrossRef]
- Kubo, T.; Kitaoka, H.; Okawa, M.; Yamanaka, S.; Hirota, T.; Baba, Y.; Hayato, K.; Yamasaki, N.; Matsumura, Y.; Yasuda, N.; et al. Combined Measurements of Cardiac Troponin I and Brain Natriuretic Peptide Are Useful for Predicting Adverse Outcomes in Hypertrophic Cardiomyopathy. Circ. J. 2011, 75, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Pennacchini, E.; Musumeci, M.B.; Conte, M.R.; Stöllberger, C.; Formisano, F.; Bongioanni, S.; Francia, P.; Volpe, M.; Autore, C. Electrocardiographic evolution in patients with hypertrophic cardiomyopathy who develop a left ventricular apical aneurysm. J. Electrocardiol. 2015, 48, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Adler, A.; Fardfini, K.; Hoss, S.; Hanneman, K.; Rowin, E.J.; Maron, M.S.; Maron, B.J.; Rakowski, H.; Chan, R.H. Progression of myocardial fibrosis in HCM. JACC Cardiovasc. Imaging 2020. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Carrick, R.T.; Patel, P.P.; Koethe, B.; Wells, S.; Maron, M.S. Outcomes in Patients with Hypertrophic Cardiomyopathy and Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.B.; Mastromarino, V.; Casenghi, M.; Tini, G.; Francia, P.; Maruotti, A.; Romaniello, A.; Magri, D.; Lillo, R.; Adduci, C.; et al. Pulmonary hypertension and clinical correlates in hypertrophic cardiomyopathy. Int. J. Cardiol. 2017, 248, 326–332. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musumeci, B.; Tini, G.; Russo, D.; Sclafani, M.; Cava, F.; Tropea, A.; Adduci, C.; Palano, F.; Francia, P.; Autore, C. Left Ventricular Remodeling in Hypertrophic Cardiomyopathy: An Overview of Current Knowledge. J. Clin. Med. 2021, 10, 1547. https://doi.org/10.3390/jcm10081547
Musumeci B, Tini G, Russo D, Sclafani M, Cava F, Tropea A, Adduci C, Palano F, Francia P, Autore C. Left Ventricular Remodeling in Hypertrophic Cardiomyopathy: An Overview of Current Knowledge. Journal of Clinical Medicine. 2021; 10(8):1547. https://doi.org/10.3390/jcm10081547
Chicago/Turabian StyleMusumeci, Beatrice, Giacomo Tini, Domitilla Russo, Matteo Sclafani, Francesco Cava, Alessandro Tropea, Carmen Adduci, Francesca Palano, Pietro Francia, and Camillo Autore. 2021. "Left Ventricular Remodeling in Hypertrophic Cardiomyopathy: An Overview of Current Knowledge" Journal of Clinical Medicine 10, no. 8: 1547. https://doi.org/10.3390/jcm10081547
APA StyleMusumeci, B., Tini, G., Russo, D., Sclafani, M., Cava, F., Tropea, A., Adduci, C., Palano, F., Francia, P., & Autore, C. (2021). Left Ventricular Remodeling in Hypertrophic Cardiomyopathy: An Overview of Current Knowledge. Journal of Clinical Medicine, 10(8), 1547. https://doi.org/10.3390/jcm10081547





