Diagnostic Splenectomy: Characteristics, Pre-Operative Investigations, and Identified Pathologies for 20 Patients
Abstract
1. Introduction
2. Materials and Methods
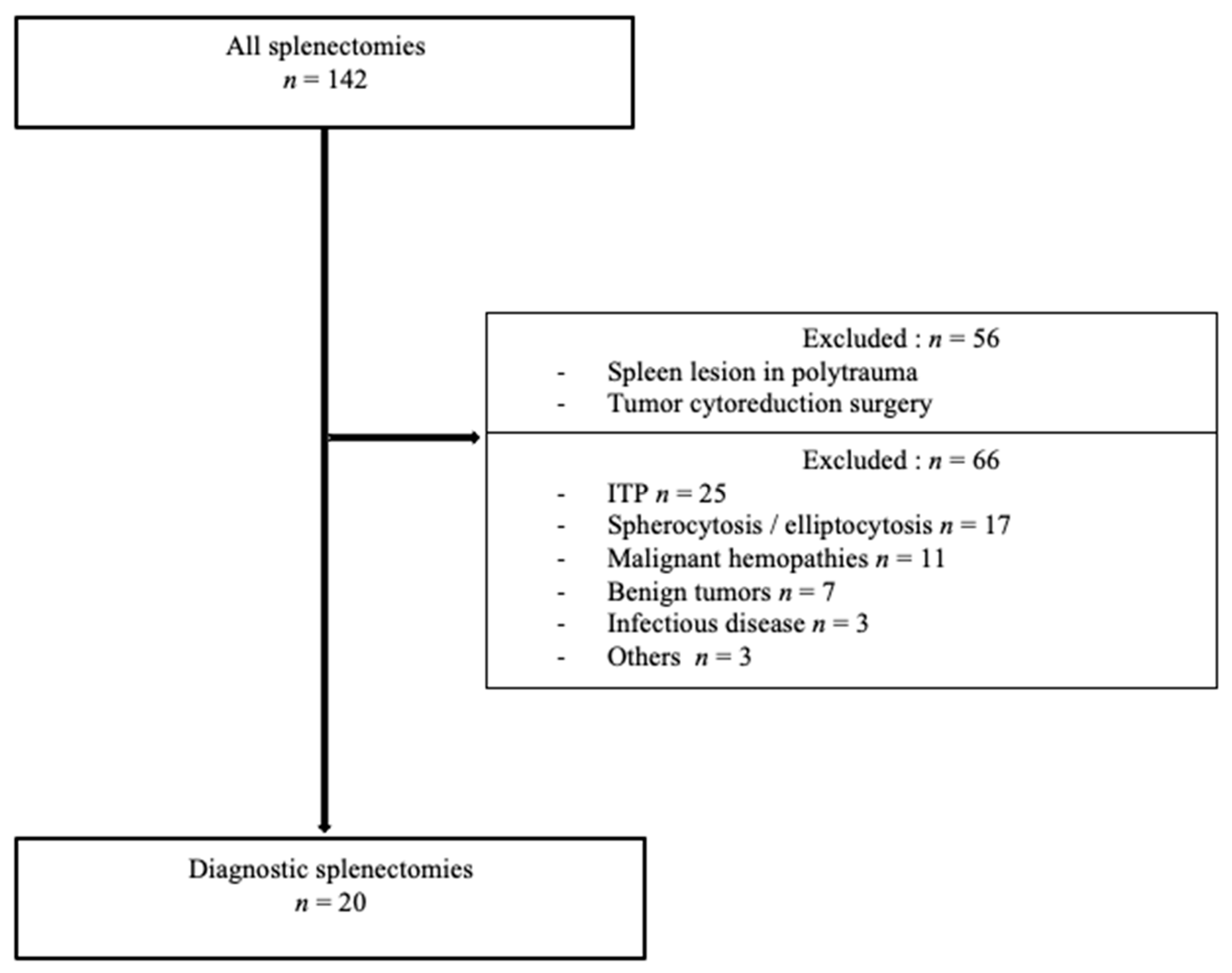
2.1. Inclusion and Exclusion Criteria
2.2. Outcomes
2.3. Variables and Analysis
2.4. Data Protection
2.5. Statistical Analysis
3. Results
3.1. Demographics and Patient Characteristics
3.2. Diagnostic Investigations Prior to Splenectomy
3.3. Identified Pathologies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Magtibay, P.M.; Adams, P.B.; Silverman, M.B.; Cha, S.S.; Podratz, K.C. Splenectomy as part of cytoreductive surgery in ovarian cancer. Gynecol. Oncol. 2006, 102, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Guédon, A.; Ribeil, J.-A.; Suarez, F.; Tamburini, J.; Gaujoux, S. Indications et résultats de la splénectomie dans les pathologies hématologiques. J. Chir. Visc. 2017, 154, 433–442. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Arnold, D.M.; McCrae, K.R. Splenectomy for immune thrombocytopenia: Down but not out. Blood 2018, 131, 1172–1182. [Google Scholar] [CrossRef]
- Ahmed, R.; Devasia, A.J.; Viswabandya, A.; Lakshmi, K.M.; Abraham, A.; Karl, S.; Mathai, J.; Jacob, P.M.; Abraham, D.; Srivastava, A.; et al. Long-term outcome following splenectomy for chronic and persistent immune thrombocytopenia (ITP) in adults and children. Ann. Hematol. 2016, 95, 1429–1434. [Google Scholar] [CrossRef]
- Philippe, P. Diagnostic et prise en charge de l’anémie hémolytique auto-immune. Presse Méd. 2007, 36, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Olszewski, A.J. Diagnostic and therapeutic splenectomy for splenic lymphomas: Analysis of the National Cancer Data Base. Hematol. Amst. Neth. 2019, 24, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Ciftciler, R.; Pasayeva, A.; Aksu, S.; Ozcebe, O.; Sayınalp, N.; Malkan, U.Y.; Buyukasık, Y.; Haznedaroglu, I.C. Indications and outcomes of splenectomy for hematological disorders. Open Med. 2019, 14, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Pastores, G.M.; Hughes, D.A. Gaucher Disease. In GeneReviews; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Stephens, K., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1269/ (accessed on 12 May 2020).
- Zimran, A.; Belmatoug, N.; Bembi, B.; Deegan, P.; Elstein, D.; Fernandez-Sasso, D.; Giraldo, P.; Goker-Alpan, O.; Lau, H.; Lukina, E.; et al. Demographics and patient characteristics of 1209 patients with Gaucher disease: Descriptive analysis from the Gaucher Outcome Survey (GOS). Am. J. Hematol. 2017, 93, 205–212. [Google Scholar] [CrossRef]
- Cianci, P.; Tartaglia, N.; Altamura, A.; Fersini, A.; Vovola, F.; Sanguedolce, F.; Ambrosi, A.; Neri, V. A recurrent epidermoid cyst of the spleen: Report of a case and literature review. World J. Surg. Oncol. 2016, 14, 1–4. [Google Scholar] [CrossRef]
- Kaiser, M.M.; Fischer, F.; Wessel, L.M. Laparoskopische organerhaltende Operationen bei nicht-parasitären Milzzysten: Möglichkeiten und Grenzen. Zent. Chir. 2008, 133, 142–147. [Google Scholar] [CrossRef]
- Browning, M.G.; Bullen, N.; Nokes, T.; Tucker, K.; Coleman, M. The evolving indications for splenectomy. Br. J. Haematol. 2016, 177, 321–324. [Google Scholar] [CrossRef]
- Han, B.; Yang, Z.; Yang, T.; Gao, W.; Sang, X.; Zhao, Y.; Shen, T. Diagnostic Splenectomy in Patients with Fever of Unknown Origin and Splenomegaly. Acta Haematol. 2008, 119, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hangge, P.T.; Sheaffer, W.W.; Neville, M.; Wasif, N.; Gray, R.J.; Pockaj, B.A.; Stucky, C.-C.H. The diagnostic value of splenectomy in patients without a definitive preoperative diagnosis. Am. J. Surg. 2019, 217, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, J.; Ralph, R.; Mathuram, A.; Prakash, V.; Nayak, S.; Zachariah, A. Role of Diagnostic Splenectomy in Patients Presenting with Pyrexia of Unknown Origin with Splenomegaly and Non-Contributory Pre-surgical Evaluation. J. Assoc. Physicians India 2019, 67, 42–45. [Google Scholar]
- Carr, J.A.; Shurafa, M.; Velanovich, V. Surgical indications in idiopathic splenomegaly. Arch. Surg. 2002, 137, 64–68. [Google Scholar] [CrossRef]
- Griffina, G.; Shenoi, S.; Hughes, G.C. Hemophagocytic lymphohistiocytosis: An update on pathogenesis, diagnosis, and therapy. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101515. [Google Scholar] [CrossRef]
- Mulders-Manders, C.; Simon, A.; Bleeker-Rovers, C. Fever of unknown origin. Clin. Med. 2015, 15, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Cai, H.; Cao, X.; Chen, M.; Li, J.; Zhu, T.; Duan, M.; Wang, S.; Han, B.; et al. Patients with Fever of Unknown Origin and Splenomegaly: Diagnostic Value of Splenectomy and Preoperative Risk Factors Suggestive of Underlying Lymphomas. Acta Haematol. 2017, 137, 240–246. [Google Scholar] [CrossRef]
- Bleeker-Rovers, C.P.; Vos, F.J.; Mudde, A.H.; Dofferhoff, A.S.M.; De Geus-Oei, L.-F.; Rijnders, A.J.; Krabbe, P.F.M.; Corstens, F.H.M.; Van Der Meer, J.W.M.; Oyen, W.J.G. A prospective multi-centre study of the value of FDG-PET as part of a structured diagnostic protocol in patients with fever of unknown origin. Eur. J. Nucl. Med. Mol. Imaging 2006, 34, 694–703. [Google Scholar] [CrossRef]
- Anderson, H.; Price, P. What does positron emission tomography offer oncology? Eur. J. Cancer 2000, 36, 2028–2035. [Google Scholar] [CrossRef]
- Besson, F.L.; Chaumet-Riffaud, P.; Playe, M.; Noel, N.; Lambotte, O.; Goujard, C.; Prigent, A.; Durand, E. Contribution of 18F-FDG PET in the diagnostic assessment of fever of unknown origin (FUO): A stratification-based meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1887–1895. [Google Scholar] [CrossRef]
- Schönau, V.; Vogel, K.; Englbrecht, M.; Wacker, J.; Schmidt, D.; Manger, B.; Kuwert, T.; Schett, G. The value of 18F-FDG-PET/CT in identifying the cause of fever of unknown origin (FUO) and inflammation of unknown origin (IUO): Data from a prospective study. Ann. Rheum. Dis. 2018, 77, 70–77. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.-M.; Li, Y.; Hua, F.-C.; Wang, Q.-S.; Zhang, X.-L.; Cheng, C.; Wu, H.; Yao, Z.M.; Zhang, W.F.; et al. 18F-FDGPET/CT in fever of unknown origin and inflammation of unknown origin: A Chinese multi-center study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 159–165. [Google Scholar] [CrossRef]
- Sreedharanunni, S.; Sachdeva, M.U.S.; Malhotra, P.; Ahluwalia, J.; Naseem, S.; Prakash, G.; Khadwal, A.; Sharma, P.; Kumar, N.; Varma, N.; et al. Role of blood and bone marrow examination in the diagnosis of mature lymphoid neoplasms in patients presenting with isolated splenomegaly. Hematology 2015, 20, 530–537. [Google Scholar] [CrossRef]
- Hong, F.S.; Fox, L.C.; Chai, K.L.; Htun, K.; Clucas, D.; Morgan, S.; Cole-Sinclair, M.F.; Juneja, S. Role of bone marrow biopsy for fever of unknown origin in the contemporary Australian context. Intern. Med. J. 2019, 49, 850–854. [Google Scholar] [CrossRef]
- Olson, M.C.; Atwell, T.D.; Harmsen, W.S.; Konrad, A.; King, R.L.; Lin, Y.; Wall, D.J. Safety and Accuracy of Percutaneous Image-Guided Core Biopsy of the Spleen. Am. J. Roentgenol. 2016, 206, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S.; Libson, E.; Sella, T.; Lebensart, P.; Sosna, J. Percutaneous image-guided splenic procedures: Update on indications, technique, complications, and outcomes. Semin. Ultrasound CT MRI 2007, 28, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Kleinstern, G.; Spectre, G.; Da’As, N.; Ziv, E.; Kalish, Y. Thromboembolic Events Following Splenectomy: Risk Factors, Prevention, Management and Outcomes. World J. Surg. 2017, 42, 675–681. [Google Scholar] [CrossRef] [PubMed]


| Male/Female, n (%) | 13/7 (65/35) |
| Laparoscopy, n (%) | 8 (40) |
| Laparotomy, n (%) | 12 (60) |
| Median age at splenectomy (years) Median (interquartile range (IQR)) | 65 (59.5–76.5) |
| Splenomegaly, n (%) | 13 (65) |
| Hepatomegaly, n (%) | 6 (30) |
| Enlarged lymph nodes, n (%) | 7 (35) |
| Fever/B symptoms, n (%) | 10 (50) |
| Other symptoms, n (%) * | 4 (20) |
| Leukopenia (leucocytes < 4 × 109/L), n (%) | 5 (26) |
| Lymphopenia (lymphocytes < 1.5 × 109/L), n (%) | 4 (24) |
| Anemia (hemoglobin < 12 g/dL), n (%) | 16 (84) |
| Thrombopenia (<150 × 109/L), n (%) | 10 (53) |
| Abnormal plasma protein electrophoresis | 11 (79) |
| Elevated level of LDH (>430 IU/L), n (%) | 7 (41) |
| Diagnostic Procedure for Pathology Prior to Splenectomy | N. of Performed Procedures | N. of Informative Procedures | Suspected Diagnosis after Procedure or Contributive Information | Final Diagnosis after Splenectomy |
|---|---|---|---|---|
| Bone-marrow biopsy | 13 | 4 | 3 suggestive of LG NHL | 2 DLBCL complicating LG NHL, 1 LG NHL |
| 1 suggestive of T-cell lymphoma | 1 pseudo-inflammatory tumor | |||
| Liver biopsy | 2 | 1 | 1 suggestive of T-cell lymphoproliferation | 1 T gamma-delta splenic lymphoma |
| Superficial lymph-node biopsies | 3 | 1 | 1 suggestive of marginal zone lymphoma | 1 marginal zone lymphoma |
| Lymphocyte phenotyping | 10 | 6 | 4 suggestive of LG NHL, | 3 LG NHL, 1 DBCL complicating LG NHL |
| 1 suggestive of secondary LGL | 1 histiocytic sarcoma | |||
| 1 suggestive of T-cell lymphoproliferation | 1 T gamma-delta splenic lymphoma | |||
| Microbial investigations | 16 | 3 | 1 HIV-1 | |
| 1 replicating hepatitis B | No infectious disease identified | |||
| 1 active hepatitis C | ||||
| FDG PET/CT | 18 | 16 | 16 hypermetabolic spleens, 8 patients with other hypermetabolic loci (tonsils, deep or superficial lymphadenopathy, vertebral disco fixation, muscular tissue fixation) | 6 DLBCL 4 LG NHL 1 Hodgkin lymphoma 1 T gamma delta splenic lymphoma 1 pseudo-inflammatory tumor 1 hemangioma 1 granulomatosis 1 without diagnosis |
| Echocardiography | 7 | 0 | None | Not applicable |
| Bone-marrow aspiration | 10 | 0 | None | Not applicable |
| Karyotype | 5 | 0 | None | Not applicable |
| Other sites biopsies (Bones, gastric and duodenal, spleen, temporal artery) | 10 | 0 | None | Not applicable |
| Patients with Splenomegaly n = 13 | Patients without Splenomegaly n = 7 | p | |
|---|---|---|---|
| Clinical presentation | |||
| Fever/B symptoms, n (%) | 8 (62) | 2 (29) | 0.35 |
| Hepatomegaly, n (%) | 6 (46) | 0 (0) | 0.052 |
| Lymph nodes, n (%) | 9 (69) | 2 (29) | 0.16 |
| Other organ involvement, n (%) | 3 (23) | 1 (14) | 1 |
| Isolated abdominal symptoms, n (%) | 0 (0) | 3 (43) | 0.03 |
| Biological presentation | |||
| Anemia, n (%) | 12/12 (100) | 4/7 (57) | 0.04 |
| Thrombopenia, n (%) | 9/12 (75) | 1/7 (14) | 0.02 |
| Leukopenia, n (%) | 5/12 (42) | 0/7 (0) | 0.11 |
| Lymphopenia, n (%) | 4/10 (40) | 0/7 (0) | 0.10 |
| Pancytopenia, n (%) | 4/12 (33) | 0/7 (0) | 0.25 |
| Abnormal plasma protein electrophoresis, n (%) | 8/10 (80) | 3/4 (75) | 1 |
| Elevated level of LDH, n (%) | 6/12 (50) | 1/5 (20) | 0.34 |
| Positive pre-surgery investigations | |||
| Median (IQR) duration of investigations (months) | 5 (2.25–29.5) | 5 (1–14) | 0.55 |
| Lymphocyte phenotyping, n (%) | 5/8 (63) | 1/2 (50) | 1 |
| PET/CT with hypermetabolic spleen, n (%) | 10/12 (83) | 6/6 (100) | 0.53 |
| Bone-marrow biopsy, n (%) | 3/10 (30) | 1/3 (33) | 1 |
| Identified diseases | |||
| Final diagnosis, n (%) | 12 (93) | 7 (100) | 1 |
| Lymphoma, n (%) | 10 (77) | 4 (57) | 0.61 |
| DLBCL, n (%) | 6 (46) | 2 (29) | 0.64 |
| Low-grade NHL, n (%) | 3 (23) | 1 (14) | 0.60 |
| Patients with DLBCL (n = 8) | Patients with Low-Grade NHL (n = 4) | p | |
|---|---|---|---|
| Median (IQR) duration between first medical contact and surgery (months) | 2.25 (1–12.75) | 3.5 (2.5–5.25) | 0.93 |
| B signs/fever, n (%) | 3 (38) | 2 (50) | 1 |
| Splenomegaly, n (%) | 6 (75) | 3 (75) | 1 |
| Leukopenia, n (%) | 3/7 (43) | 1 (25) | 1 |
| Anemia, n (%) | 6/7 (86) | 3 (75) | 1 |
| Thrombocytopenia, n (%) | 5/7 (71) | 2 (50) | 0.58 |
| Elevated LDH levels, n (%) | 6 (75) | 0 (0) | 0.06 |
| Elevated C-reactive protein, n (%) | 3/4 (75) | 1 (25) | 0.49 |
| Splenic PET-hyperfixation, n (%) | 6/7 (86) | 2 (50) | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maillot, J.; Malfuson, J.-V.; Lazure, T.; Benoist, S.; Cremades, A.; Hornez, E.; Besson, F.L.; Noël, N.; Lambotte, O. Diagnostic Splenectomy: Characteristics, Pre-Operative Investigations, and Identified Pathologies for 20 Patients. J. Clin. Med. 2021, 10, 1519. https://doi.org/10.3390/jcm10071519
Maillot J, Malfuson J-V, Lazure T, Benoist S, Cremades A, Hornez E, Besson FL, Noël N, Lambotte O. Diagnostic Splenectomy: Characteristics, Pre-Operative Investigations, and Identified Pathologies for 20 Patients. Journal of Clinical Medicine. 2021; 10(7):1519. https://doi.org/10.3390/jcm10071519
Chicago/Turabian StyleMaillot, Jean, Jean-Valère Malfuson, Thierry Lazure, Stéphane Benoist, Anne Cremades, Emmanuel Hornez, Florent L. Besson, Nicolas Noël, and Olivier Lambotte. 2021. "Diagnostic Splenectomy: Characteristics, Pre-Operative Investigations, and Identified Pathologies for 20 Patients" Journal of Clinical Medicine 10, no. 7: 1519. https://doi.org/10.3390/jcm10071519
APA StyleMaillot, J., Malfuson, J.-V., Lazure, T., Benoist, S., Cremades, A., Hornez, E., Besson, F. L., Noël, N., & Lambotte, O. (2021). Diagnostic Splenectomy: Characteristics, Pre-Operative Investigations, and Identified Pathologies for 20 Patients. Journal of Clinical Medicine, 10(7), 1519. https://doi.org/10.3390/jcm10071519






