Racial Disparities in the Utilization and Outcomes of Temporary Mechanical Circulatory Support for Acute Myocardial Infarction-Cardiogenic Shock
Abstract
1. Introduction
2. Methods
Statistical Analysis
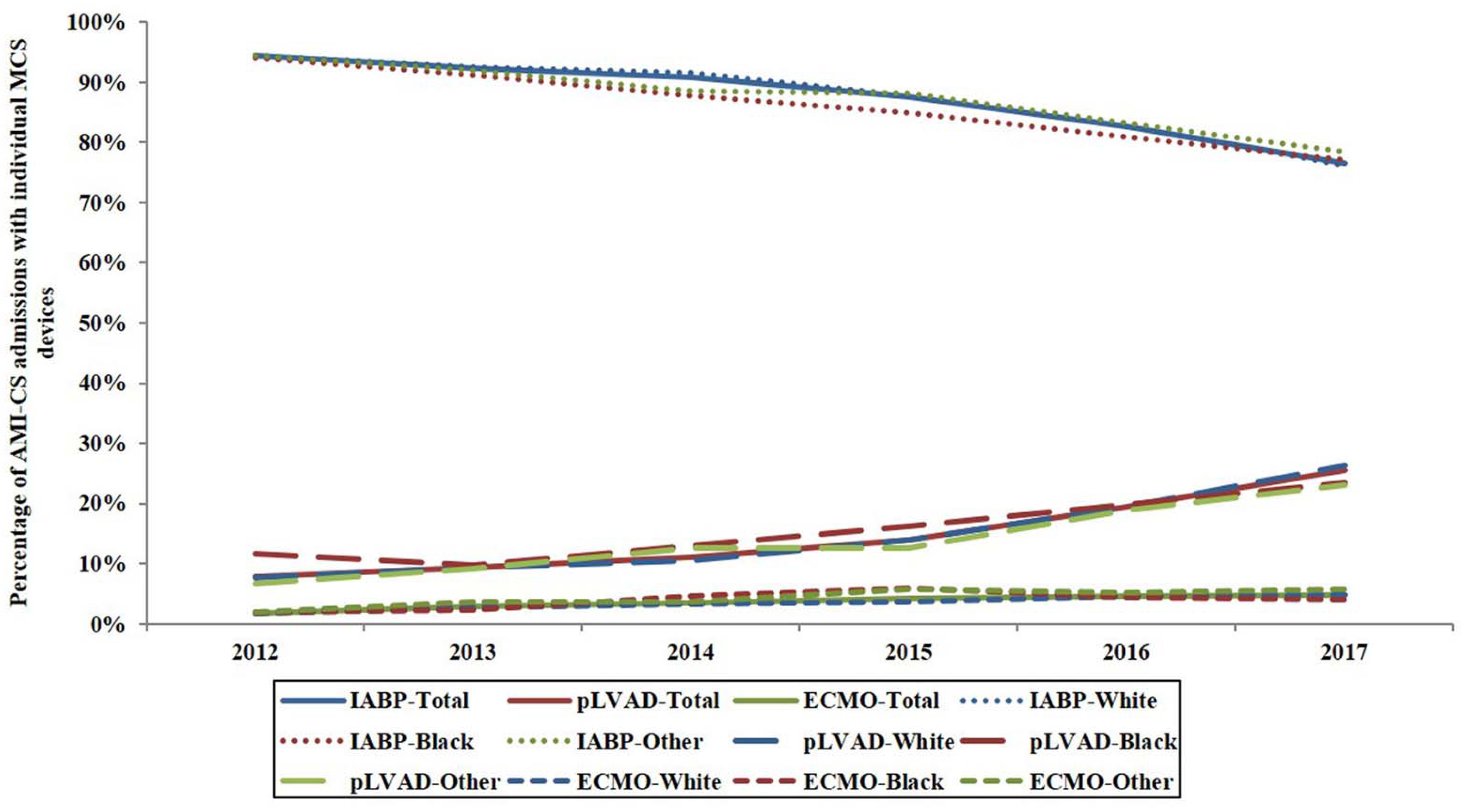
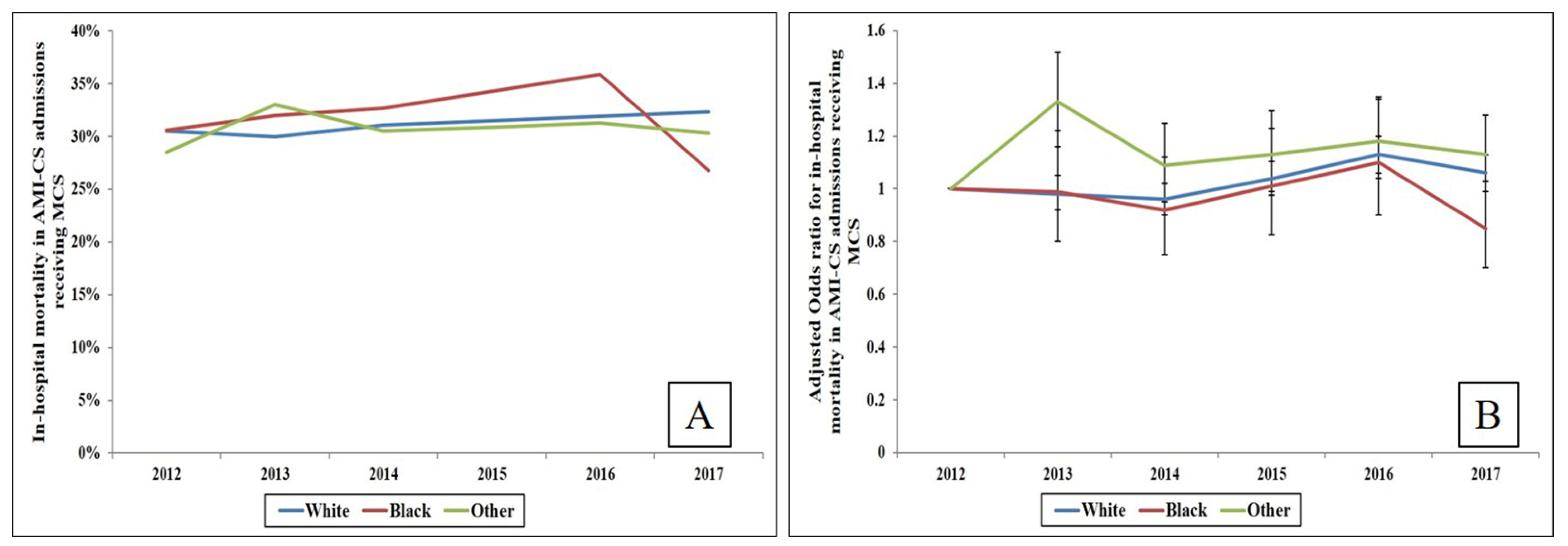
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wayangankar, S.A.; Bangalore, S.; McCoy, L.A.; Jneid, H.; Latif, F.; Karrowni, W.; Charitakis, K.; Feldman, D.N.; Dakik, H.A.; Mauri, L.; et al. Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction: A Report From the CathPCI Registry. JACC Cardiovasc. Interv. 2016, 9, 341–351. [Google Scholar] [CrossRef]
- Shah, M.; Patnaik, S.; Patel, B.; Ram, P.; Garg, L.; Agarwal, M.; Agrawal, S.; Arora, S.; Patel, N.; Wald, J.; et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin. Res. Cardiol. 2018, 107, 287–303. [Google Scholar] [CrossRef]
- White, H.D.; Assmann, S.F.; Sanborn, T.A.; Jacobs, A.K.; Webb, J.G.; Sleeper, L.A.; Wong, C.K.; Stewart, J.T.; Aylward, P.E.; Wong, S.C.; et al. Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock: Results from the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial. Circulation 2005, 112, 1992–2001. [Google Scholar] [CrossRef]
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist Debakey Cardiovasc. J. 2020, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jiritano, F.; Coco, V.L.; Matteucci, M.; Fina, D.; Willers, A.; Lorusso, R. Temporary Mechanical Circulatory Support in Acute Heart Failure. Card. Fail. Rev. 2020, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, J.; Liu, J.; Yang, N.; Smith, S.C., Jr.; Huo, Y.; Fonarow, G.C.; Ge, J.; Taubert, K.A.; Morgan, L.; et al. Sex Differences in In-Hospital Management and Outcomes of Patients With Acute Coronary Syndrome. Circulation 2019, 139, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.M.; Ungar, L.; Abnousi, F.; Asch, S.M.; Heidenreich, P.A. Racial Differences in Quality of Care and Outcomes After Acute Coronary Syndrome. Am. J. Cardiol. 2018, 121, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Rathore, S.S.; Radford, M.J.; Wang, Y.; Wang, Y.; Krumholz, H.M. Acute myocardial infarction in the elderly: Differences by age. J. Am. Coll. Cardiol. 2001, 38, 736–741. [Google Scholar] [CrossRef]
- Palmeri, S.T.; Lowe, A.M.; Sleeper, L.A.; Saucedo, J.F.; Desvigne-Nickens, P.; Hochman, J.S.; Investigators, S. Racial and ethnic differences in the treatment and outcome of cardiogenic shock following acute myocardial infarction. Am. J. Cardiol. 2005, 96, 1042–1049. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Dunlay, S.M.; Barsness, G.W.; Miller, P.E.; Cheungpasitporn, W.; Stulak, J.M.; Rihal, C.S.; Holmes, D.R.; Bell, M.R.; Miller, V.M. Sex Disparities in the Use and Outcomes of Temporary Mechanical Circulatory Support for Acute Myocardial Infarction-Cardiogenic Shock. CJC Open 2020. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Patlolla, S.H.; Dunlay, S.M.; Prasad, A.; Bell, M.R.; Jaffe, A.S.; Gersh, B.J.; Rihal, C.S.; Holmes, D.R., Jr.; Barsness, G.W. Regional Variation in the Management and Outcomes of Acute Myocardial Infarction with Cardiogenic Shock in the United States. Circ. Heart Fail. 2020, 13, e006661. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, J.; Essa, M.; Lansky, A.J.; Sugeng, L. Frequency of Management of Cardiogenic Shock with Mechanical Circulatory Support Devices According to Race. Am. J. Cardiol. 2020, 125, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- HCUP. Introduction to HCUP National Inpatient Sample (NIS) 2012. Available online: https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2012.jsp (accessed on 21 March 2021).
- Vallabhajosyula, S.; Kumar, V.; Vallabhajosyula, S.; Subramaniam, A.V.; Patlolla, S.H.; Verghese, D.; Ya’Qoub, L.; Stulak, J.M.; Sandhu, G.S.; Prasad, A.; et al. Acute myocardial infarction-cardiogenic shock in patients with prior coronary artery bypass grafting: A 16-year national cohort analysis of temporal trends, management and outcomes. Int. J. Cardiol. 2020, 310, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Dunlay, S.M.; Prasad, A.; Kashani, K.; Sakhuja, A.; Gersh, B.J.; Jaffe, A.S.; Holmes, D.R., Jr.; Barsness, G.W. Acute Noncardiac Organ Failure in Acute Myocardial Infarction With Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 1781–1791. [Google Scholar] [CrossRef]
- Lauridsen, M.D.; Gammelager, H.; Schmidt, M.; Nielsen, H.; Christiansen, C.F. Positive predictive value of International Classification of Diseases, 10th revision, diagnosis codes for cardiogenic, hypovolemic, and septic shock in the Danish National Patient Registry. BMC Med. Res. Methodol. 2015, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.; Blais, C.; Hamel, D.; Brown, K.; Rinfret, S.; Cartier, R.; Giguere, M.; Carroll, C.; Beauchamp, C.; Bogaty, P. Evaluation of care and surveillance of cardiovascular disease: Can we trust medico-administrative hospital data? Can. J. Cardiol. 2012, 28, 162–168. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Arora, S.; Lahewala, S.; Kumar, V.; Shantha, G.P.S.; Jentzer, J.C.; Stulak, J.M.; Gersh, B.J.; Gulati, R.; Rihal, C.S.; et al. Temporary Mechanical Circulatory Support for Refractory Cardiogenic Shock Before Left Ventricular Assist Device Surgery. J. Am. Heart Assoc. 2018, 7, e010193. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Arora, S.; Sakhuja, A.; Lahewala, S.; Kumar, V.; Shantha, G.P.S.; Egbe, A.C.; Stulak, J.M.; Gersh, B.J.; Gulati, R.; et al. Trends, Predictors, and Outcomes of Temporary Mechanical Circulatory Support for Postcardiac Surgery Cardiogenic Shock. Am. J. Cardiol. 2019, 123, 489–497. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Prasad, A.; Sandhu, G.S.; Bell, M.R.; Gulati, R.; Eleid, M.F.; Best, P.J.M.; Gersh, B.J.; Singh, M.; Lerman, A.; et al. Mechanical Circulatory Support-Assisted Early Percutaneous Coronary Intervention in Acute Myocardial Infarction with Cardiogenic Shock: 10-Year National Temporal Trends, Predictors and Outcomes. EuroIntervention 2019. [Google Scholar] [CrossRef]
- Ziaeian, B.; Kominski, G.F.; Ong, M.K.; Mays, V.M.; Brook, R.H.; Fonarow, G.C. National Differences in Trends for Heart Failure Hospitalizations by Sex and Race/Ethnicity. Circ. Cardiovasc. Qual. Outcomes 2017, 10. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Angraal, S.; Couch, T.; Welsh, J.W.; Nallamothu, B.K.; Girotra, S.; Chan, P.S.; Krumholz, H.M. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA 2017, 318, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Fengler, K.; Fuernau, G.; Desch, S.; Eitel, I.; Neumann, F.J.; Olbrich, H.G.; de Waha, A.; de Waha, S.; Richardt, G.; Hennersdorf, M.; et al. Gender differences in patients with cardiogenic shock complicating myocardial infarction: A substudy of the IABP-SHOCK II-trial. Clin. Res. Cardiol. 2015, 104, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Dunlay, S.M.; Barsness, G.W.; Rihal, C.S.; Holmes, D.R., Jr.; Prasad, A. Hospital-Level Disparities in the Outcomes of Acute Myocardial Infarction with Cardiogenic Shock. Am. J. Cardiol. 2019, 124, 491–498. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef]
- Dzavik, V.; Steeper, L.A.; Cocke, T.P.; Moscucci, M.; Saucedo, J.; Hosat, S.; Jiang, X.; Slater, J.; LeJemtel, T.; Hochman, J.S.; et al. Early revascularization is associated with improved survival in elderly patients with acute myocardial infarction complicated by cardiogenic shock: A report from the SHOCK Trial Registry. Eur. Heart J. 2003, 24, 828–837. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.; Dixon, S.; Alaswad, K.; Patel, K.; Almany, S.; Khandelwal, A.; Hanson, I.; George, A.; Ashbrook, M.; et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter. Cardiovasc. Interv. 2018, 91, 454–461. [Google Scholar] [CrossRef]
- Basir, M.B.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Prasad, A.; Bell, M.R.; Sandhu, G.S.; Eleid, M.F.; Dunlay, S.M.; Schears, G.J.; Stulak, J.M.; Singh, M.; Gersh, B.J.; et al. Extracorporeal Membrane Oxygenation Use in Acute Myocardial Infarction in the United States, 2000 to 2014. Circ. Heart Fail. 2019, 12, e005929. [Google Scholar] [CrossRef]
- Khera, R.; Cram, P.; Lu, X.; Vyas, A.; Gerke, A.; Rosenthal, G.E.; Horwitz, P.A.; Girotra, S. Trends in the use of percutaneous ventricular assist devices: Analysis of national inpatient sample data, 2007 through 2012. JAMA Intern. Med. 2015, 175, 941–950. [Google Scholar] [CrossRef]
- Subramaniam, A.V.; Barsness, G.W.; Vallabhajosyula, S.; Vallabhajosyula, S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiol. Ther. 2019, 8, 211–228. [Google Scholar] [CrossRef]
- Chi, G.C.; Kanter, M.H.; Li, B.H.; Qian, L.; Reading, S.R.; Harrison, T.N.; Jacobsen, S.J.; Scott, R.D.; Cavendish, J.J.; Lawrence, J.M.; et al. Trends in Acute Myocardial Infarction by Race and Ethnicity. J. Am. Heart Assoc. 2020, 9, e013542. [Google Scholar] [CrossRef] [PubMed]
- Crow, S.; Fischer, A.C.; Schears, R.M. Extracorporeal life support: Utilization, cost, controversy, and ethics of trying to save lives. Semin. Cardiothorac. Vasc. Anesth. 2009, 13, 183–191. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Prasad, A.; Dunlay, S.M.; Murphree, D.H., Jr.; Ingram, C.; Mueller, P.S.; Gersh, B.J.; Holmes, D.R., Jr.; Barsness, G.W. Utilization of Palliative Care for Cardiogenic Shock Complicating Acute Myocardial Infarction: A 15-Year National Perspective on Trends, Disparities, Predictors, and Outcomes. J. Am. Heart Assoc. 2019, 8, e011954. [Google Scholar] [CrossRef]
- Bosson, N.; Fang, A.; Kaji, A.H.; Gausche-Hill, M.; French, W.J.; Shavelle, D.; Thomas, J.L.; Niemann, J.T. Racial and ethnic differences in outcomes after out-of-hospital cardiac arrest: Hispanics and Blacks may fare worse than non-Hispanic Whites. Resuscitation 2019, 137, 29–34. [Google Scholar] [CrossRef]
- Chen, L.M.; Nallamothu, B.K.; Spertus, J.A.; Tang, Y.; Chan, P.S. Racial Differences in Long-Term Outcomes among Older Survivors of In-Hospital Cardiac Arrest. Circulation 2018, 138, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Nichol, G.; Krumholz, H.M.; Spertus, J.A.; Jones, P.G.; Peterson, E.D.; Rathore, S.S.; Nallamothu, B.K. American Heart Association National Registry of Cardiopulmonary Resuscitation, I. Racial differences in survival after in-hospital cardiac arrest. JAMA 2009, 302, 1195–1201. [Google Scholar] [CrossRef]
- Groeneveld, P.W.; Heidenreich, P.A.; Garber, A.M. Racial disparity in cardiac procedures and mortality among long-term survivors of cardiac arrest. Circulation 2003, 108, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, X.; Glance, L.G. Disparities in 30-Day Rehospitalization Rates Among Medicare Skilled Nursing Facility Residents by Race and Site of Care. Med. Care 2015, 53, 1058–1065. [Google Scholar] [CrossRef]
- Smith, D.B.; Feng, Z.; Fennell, M.L.; Zinn, J.S.; Mor, V. Separate and unequal: Racial segregation and disparities in quality across U.S. nursing homes. Health Aff. 2007, 26, 1448–1458. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | White (N = 66,314) | Black (N = 7440) | Others a (N = 16,317) | p | |
|---|---|---|---|---|---|
| Age (years) | 66.6 ± 11.9 | 63.5 ± 12.6 | 64.4 ± 12.3 | <0.001 | |
| Female sex | 30.5 | 39.8 | 26.7 | <0.001 | |
| Primary payer | Medicare | 56.4 | 51.4 | 44.2 | <0.001 |
| Medicaid | 7.4 | 13.0 | 15.5 | ||
| Private | 27.6 | 24.4 | 26.4 | ||
| Others b | 8.6 | 11.2 | 13.8 | ||
| Quartile of median household income for zip code | 0–25th | 25.8 | 55.1 | 30.5 | <0.001 |
| 26th–50th | 28.2 | 18.9 | 22.2 | ||
| 51st–75th | 24.9 | 14.9 | 22.9 | ||
| 75th–100th | 21.1 | 11.1 | 24.4 | ||
| Charlson Comorbidity Index | 0–3 | 37.7 | 39.6 | 39.5 | <0.001 |
| 4–6 | 43.3 | 39.3 | 41.0 | ||
| ≥7 | 19.0 | 21.2 | 19.5 | ||
| Hospital teaching status and location | Rural | 4.9 | 2.1 | 1.2 | <0.001 |
| Urban non-teaching | 28.0 | 18.7 | 29.9 | ||
| Urban teaching | 67.0 | 79.1 | 68.9 | ||
| Hospital bed-size | Small | 9.9 | 10.3 | 10.2 | 0.03 |
| Medium | 25.8 | 27.0 | 26.2 | ||
| Large | 64.3 | 62.6 | 63.5 | ||
| Hospital region | Northeast | 18.0 | 15.6 | 16.1 | <0.001 |
| Midwest | 24.0 | 20.5 | 10.5 | ||
| South | 39.8 | 56.2 | 35.7 | ||
| West | 18.2 | 7.7 | 37.8 | ||
| AMI type | STEMI | 67.1 | 62.3 | 64.3 | <0.001 |
| NSTEMI | 32.9 | 37.7 | 35.7 | <0.001 | |
| Acute organ failure | Respiratory | 59.8 | 57.9 | 61.2 | <0.001 |
| Renal | 46.5 | 52.8 | 48.6 | <0.001 | |
| Hepatic | 15.3 | 16.1 | 16.8 | <0.001 | |
| Hematologic | 19.1 | 20.3 | 23.2 | <0.001 | |
| Neurologic | 19.6 | 21.6 | 21.2 | <0.001 | |
| Cardiac arrest | 31.3 | 31.1 | 29.9 | 0.003 | |
| Coronary angiography | 90.0 | 88.4 | 91.4 | <0.001 | |
| Percutaneous coronary intervention | 66.9 | 65.6 | 63.7 | <0.001 | |
| Pulmonary artery catheterization | 7.6 | 7.9 | 6.9 | 0.01 | |
| Invasive mechanical ventilation | 48.8 | 49.9 | 54.6 | <0.001 | |
| Non-invasive ventilation | 4.1 | 4.0 | 4.5 | 0.04 | |
| Acute hemodialysis | 2.1 | 2.6 | 3.0 | <0.001 | |
| Characteristic | White (N = 66,314) | Black (N = 7440) | Others a (N = 16,317) | p | |
|---|---|---|---|---|---|
| In-hospital mortality | 31.3 | 31.4 | 30.2 | 0.04 | |
| Length of stay (days) | 10.3 ± 11.2 | 12.1 ± 14.2 | 10.9 ± 11.2 | <0.001 | |
| Palliative care | 9.5 | 8.5 | 8.2 | <0.001 | |
| Do-not-resuscitate status | 13.2 | 11.7 | 12.7 | 0.002 | |
| Total costs (x1000 USD) | 242.9 ± 262.9 | 255.1 ± 262.9 | 307.2 ± 308.3 | <0.001 | |
| Durable left ventricular assist device | 0.7 | 0.6 | 0.7 | 0.68 | |
| Cardiac transplantation | 0.1 | 0.1 | 0.0 | 0.30 | |
| Discharge disposition | Home | 37.0 | 38.5 | 42.5 | <0.001 |
| Transfer | 14.2 | 12.6 | 13.9 | ||
| Skilled nursing facility | 30.8 | 32.4 | 25.1 | ||
| Home with HHC | 17.5 | 16.0 | 17.9 | ||
| Against medical advice | 0.5 | 0.5 | 0.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojjini, R.; Patlolla, S.H.; Cheungpasitporn, W.; Kumar, A.; Sundaragiri, P.R.; Doshi, R.P.; Jaffe, A.S.; Barsness, G.W.; Holmes, D.R.; Rab, S.T.; et al. Racial Disparities in the Utilization and Outcomes of Temporary Mechanical Circulatory Support for Acute Myocardial Infarction-Cardiogenic Shock. J. Clin. Med. 2021, 10, 1459. https://doi.org/10.3390/jcm10071459
Vojjini R, Patlolla SH, Cheungpasitporn W, Kumar A, Sundaragiri PR, Doshi RP, Jaffe AS, Barsness GW, Holmes DR, Rab ST, et al. Racial Disparities in the Utilization and Outcomes of Temporary Mechanical Circulatory Support for Acute Myocardial Infarction-Cardiogenic Shock. Journal of Clinical Medicine. 2021; 10(7):1459. https://doi.org/10.3390/jcm10071459
Chicago/Turabian StyleVojjini, Rahul, Sri Harsha Patlolla, Wisit Cheungpasitporn, Arnav Kumar, Pranathi R. Sundaragiri, Rajkumar P. Doshi, Allan S. Jaffe, Gregory W. Barsness, David R. Holmes, S. Tanveer Rab, and et al. 2021. "Racial Disparities in the Utilization and Outcomes of Temporary Mechanical Circulatory Support for Acute Myocardial Infarction-Cardiogenic Shock" Journal of Clinical Medicine 10, no. 7: 1459. https://doi.org/10.3390/jcm10071459
APA StyleVojjini, R., Patlolla, S. H., Cheungpasitporn, W., Kumar, A., Sundaragiri, P. R., Doshi, R. P., Jaffe, A. S., Barsness, G. W., Holmes, D. R., Rab, S. T., & Vallabhajosyula, S. (2021). Racial Disparities in the Utilization and Outcomes of Temporary Mechanical Circulatory Support for Acute Myocardial Infarction-Cardiogenic Shock. Journal of Clinical Medicine, 10(7), 1459. https://doi.org/10.3390/jcm10071459









