Efficacy and Safety of Intravenous Ferric Carboxymaltose in Patients with Postoperative Anemia Following Same-Day Bilateral Total Knee Arthroplasty: A Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gombotz, H.; Rehak, P.H.; Shander, A.; Hofmann, A. Blood use in elective surgery: The Austrian benchmark study. Transfusion 2007, 47, 1468–1480. [Google Scholar] [CrossRef]
- Senthil Kumar, G.; Von Arx, O.A.; Pozo, J.L. Rate of blood loss over 48 hours following total knee replacement. Knee 2005, 12, 307–309. [Google Scholar] [CrossRef]
- Sehat, K.R.; Evans, R.; Newman, J.H. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee 2000, 7, 151–155. [Google Scholar] [CrossRef]
- Conlon, N.P.; Bale, E.P.; Herbison, G.P.; McCarroll, M. Postoperative anemia and quality of life after primary hip arthroplasty in patients over 65 years old. Anesth. Analg. 2008, 106, 1056–1061. [Google Scholar] [CrossRef]
- Clevenger, B.; Richards, T. Pre-operative anaemia. Anaesthesia 2015, 70 (Suppl. S1), 20–28. [Google Scholar] [CrossRef]
- Kotzé, A.; Harris, A.; Baker, C.; Iqbal, T.; Lavies, N.; Richards, T.; Ryan, K.; Taylor, C.; Thomas, D. British committee for standards in haematology guidelines on the identification and management of pre-operative anaemia. Br. J. Haematol. 2015, 171, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Bisbe, E.; Moltó, L.; Arroyo, R.; Muniesa, J.M.; Tejero, M. Randomized trial comparing ferric carboxymaltose vs oral ferrous glycine sulphate for postoperative anaemia after total knee arthroplasty. Br. J. Anaesth. 2014, 113, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, T.Y.; Kim, H.J.; Ro, Y.J.; Jang, H.Y.; Koh, W.U. The Effect of Intraoperative Ferric Carboxymaltose in Joint Arthroplasty Patients: A Randomized Trial. J. Clin. Med. 2019, 8, 1674. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Y.-H.; Shen, R.; Liu, C.; Sun, K.; Ye, L.; Ye, J.-J.; Yang, X.; Tian, S.-Q.; Yu, T.-B. Development and validation of a nomogram to predict perioperative blood transfusion in patients undergoing total knee arthroplasty. BMC Musculoskelet. Disord. 2020, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A.; Keating, G.M. Ferric carboxymaltose: A review of its use in iron-deficiency anaemia. Drugs 2009, 69, 739–756. [Google Scholar] [CrossRef]
- Khalafallah, A.A.; Yan, C.; Al-Badri, R.; Robinson, E.; Kirkby, B.E.; Ingram, E.; Gray, Z.; Khelgi, V.; Robertson, I.K.; Kirkby, B.P. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: A prospective, open-label, randomised controlled trial. Lancet Haematol. 2016, 3, e415–e425. [Google Scholar] [CrossRef]
- Spahn, D.R. Anemia and patient blood management in hip and knee surgery: A systematic review of the literature. Anesthesiology 2010, 113, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Hadley, S.; Day, M.; Schwarzkopf, R.; Smith, A.; Slover, J.; Zuckerman, J. Is Simultaneous Bilateral Total Knee Arthroplasty (BTKA) as Safe as Staged BTKA? Am. J. Orthop. (Belle Mead N.J.) 2017, 46, E224–E229. [Google Scholar]
- Rizzo, J.D.; Brouwers, M.; Hurley, P.; Seidenfeld, J.; Somerfield, M.R.; Temin, S. American society of clinical oncology/american society of hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J. Oncol. Pract. 2010, 6, 317–320. [Google Scholar] [CrossRef]
- Liu, D.; Dan, M.; Martinez Martos, S.; Beller, E. Blood Management Strategies in Total Knee Arthroplasty. Knee Surg. Relat. Res. 2016, 28, 179–187. [Google Scholar] [CrossRef]
- Mufarrih, S.H.; Qureshi, N.Q.; Ali, A.; Malik, A.T.; Naim, H.; Noordin, S. Total knee Arthroplasty: Risk factors for allogeneic blood transfusions in the South Asian population. BMC Musculoskelet. Disord. 2017, 18, 359. [Google Scholar] [CrossRef] [PubMed]
- Viberg, B.; Gundtoft, P.H.; Schønnemann, J.; Pedersen, L.; Andersen, L.R.; Titlestad, K.; Madsen, C.F.; Lauritsen, J.; Overgaard, S. Introduction of national guidelines for restrictive blood transfusion threshold for hip fracture patients—A consecutive cohort study based on complete follow-up in national databases. J. Orthop. Surg. Res. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, e841–e842. [Google Scholar] [CrossRef]
- Eaton, C.B.; Hochberg, M.C.; Assaf, A.; Cryer, B.L.; Lu, B.; Sands, G.; Rodriguez, B.; LaCroix, A.; Lessin, L.; Limacher, M.C.; et al. The cross-sectional relationship of hemoglobin levels and functional outcomes in women with self-reported osteoarthritis: Results from the Women’s Health Initiative. Semin. Arthritis Rheum. 2011, 41, 406–414. [Google Scholar] [CrossRef][Green Version]
- Strand, V.; Luo, X.; Bushmakin, A.; Cappelleri, J.; Assaf, A.; Cuffel, B. Effect of blood loss on physical functioning: Pooled analysis of patients with osteoarthritis or rheumatoid arthritis. Arthritis Rheum. 2009, 60, S318. [Google Scholar]
- Kim, Y.W.; Bae, J.M.; Park, Y.K.; Yang, H.K.; Yu, W.; Yook, J.H.; Noh, S.H.; Han, M.; Ryu, K.W.; Sohn, T.S.; et al. Effect of Intravenous Ferric Carboxymaltose on Hemoglobin Response Among Patients with Acute Isovolemic Anemia Following Gastrectomy: The FAIRY Randomized Clinical Trial. JAMA 2017, 317, 2097–2104. [Google Scholar] [CrossRef]
- Williams, V.S.; Smith, M.Y.; Fehnel, S.E. The validity and utility of the BPI interference measures for evaluating the impact of osteoarthritic pain. J. Pain Symptom Manag. 2006, 31, 48–57. [Google Scholar] [CrossRef]
- Mease, P.J.; Spaeth, M.; Clauw, D.J.; Arnold, L.M.; Bradley, L.A.; Russell, I.J.; Kajdasz, D.K.; Walker, D.J.; Chappell, A.S. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res. 2011, 63, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, A.; Martín-Fernández, J.; Arenaza, J.; García, I.; Tomás-García, N.; Trujillo-Martín, E.; García-Perez, L. Validation of the EQ-5D-5L in patients with hip or knee osteoarthritis. Value Health 2017, 20, A760. [Google Scholar] [CrossRef]
- Cuenca, J.; García-Erce, J.A.; Martínez, F.; Cardona, R.; Pérez-Serrano, L.; Muñoz, M. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int. J. Surg. 2007, 5, 89–94. [Google Scholar] [CrossRef]
- Gonzalez-Porras, J.R.; Colado, E.; Conde, M.P.; Lopez, T.; Nieto, M.J.; Corral, M. An individualized pre-operative blood saving protocol can increase pre-operative haemoglobin levels and reduce the need for transfusion in elective total hip or knee arthroplasty. Transfus. Med. 2009, 19, 35–42. [Google Scholar] [CrossRef]
- Kim, S.K.; Seo, W.Y.; Kim, H.J.; Yoo, J.J. Postoperative Intravenous Ferric Carboxymaltose Reduces Transfusion Amounts after Orthopedic Hip Surgery. Clin. Orthop. Surg. 2018, 10, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Gaskell, H.; Rose, P.; Allan, J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011, 11, 4. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, X.; Li, X.; Zhang, H.; Wu, M.; Liu, J.; Palmen, M.; Roubert, B.; Li, C. Pharmacokinetic, Pharmacodynamic, and Safety Profiles of Ferric Carboxymaltose in Chinese Patients with Iron-deficiency Anemia. Clin. Ther. 2020, 42, 276–285. [Google Scholar] [CrossRef]
- Zager, R.A.; Johnson, A.C.; Hanson, S.Y.; Wasse, H. Parenteral iron formulations: A comparative toxicologic analysis and mechanisms of cell injury. Am. J. Kidney Dis. 2002, 40, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S. Review of issues relating to iron and infection. Am. J. Kidney Dis. 1999, 34, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E.; Frawley, W.H.; Griffith, K.E.; Forestner, J.E.; Minei, J.P. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: A meta-analysis. J. Trauma 2003, 54, 908–914. [Google Scholar] [CrossRef]
- Walters, B.A.; Van Wyck, D.B. Benchmarking iron dextran sensitivity: Reactions requiring resuscitative medication in incident and prevalent patients. Nephrol. Dial. Transplant. 2005, 20, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The safety of intravenous iron preparations: Systematic review and meta-analysis. Mayo Clin. Proc. 2015, 90, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, S.; Longrois, D.; Montravers, P.; Beaumont, C. Hepcidin and anemia of the critically ill patient: Bench to bedside. Anesthesiology 2011, 114, 688–694. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.P.; Wells, A.W.; Whitehead, S.; Brewster, N. Recovery from post-operative anaemia. Transfus. Med. 2005, 15, 413–418. [Google Scholar] [CrossRef]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT®) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef]
- Na, H.S.; Shin, S.Y.; Hwang, J.Y.; Jeon, Y.T.; Kim, C.S.; Do, S.H. Effects of intravenous iron combined with low-dose recombinant human erythropoietin on transfusion requirements in iron-deficient patients undergoing bilateral total knee replacement arthroplasty. Transfusion 2011, 51, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, C.R. Effects of Femoral Lateral Bowing on Coronal Alignment and Component Position after Total Knee Arthroplasty: A Comparison of Conventional and Navigation-Assisted Surgery. Knee Surg. Relat. Res. 2018, 30, 64–73. [Google Scholar] [CrossRef]
- Kim, M.S.; Koh, I.J.; Choi, Y.J.; Kim, Y.D.; In, Y. Correcting Severe Varus Deformity Using Trial Components During Total Knee Arthroplasty. J. Arthroplast. 2017, 32, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, Y.B.; Song, M.K.; Lim, J.W.; Lee, H.J. Reliability and Validity of the Femorotibial Mechanical Axis Angle in Primary Total Knee Arthroplasty: Navigation versus Weight Bearing or Supine Whole Leg Radiographs. Knee Surg. Relat. Res. 2018, 30, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Oh, H.C.; Park, S.H.; Kim, J.K.; Kim, S.H. Does Obesity Affect Clinical and Radiological Outcomes in Minimally Invasive Total Knee Arthroplasty? Minimum 5-Year Follow-up of Minimally Invasive TKA in Obese Patients. Clin. Orthop. Surg. 2018, 10, 315–321. [Google Scholar] [CrossRef]
- Basora, M.; Pereira, A.; Coca, M.; Tió, M.; Lozano, L. Cost-effectiveness analysis of ferric carboxymaltose in pre-operative haemoglobin optimisation in patients undergoing primary knee arthroplasty. Blood Transfus. 2018, 16, 438–442. [Google Scholar] [PubMed]
- Rognoni, C.; Ortalda, V.; Biasi, C.; Gambaro, G. Economic Evaluation of Ferric Carboxymaltose for the Management of Hemodialysis Patients with Iron Deficiency Anemia in Italy. Adv. Ther. 2019, 36, 3253–3264. [Google Scholar] [CrossRef]
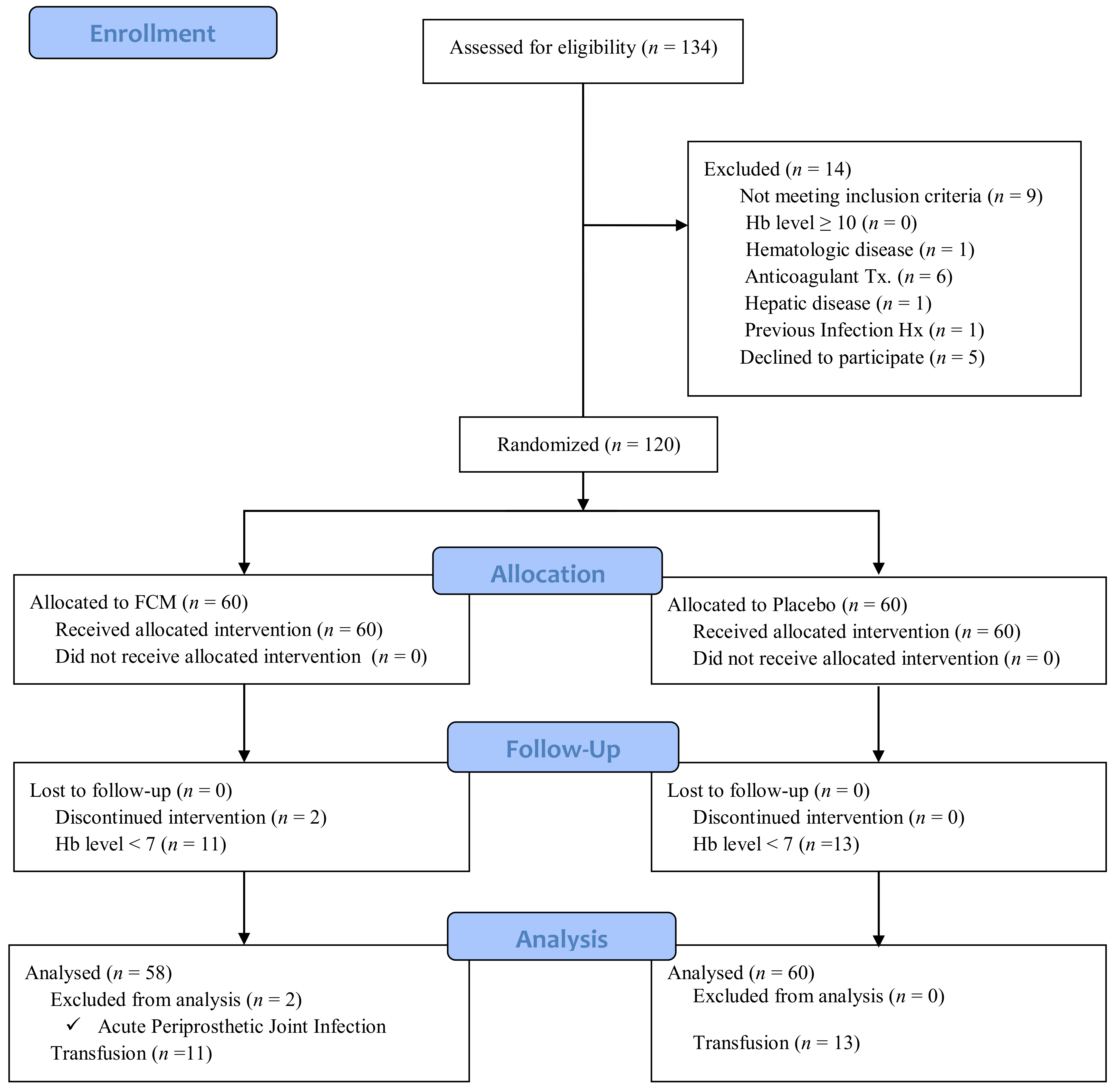
| Characteristics | Ferric Carboxymaltose (n = 58) | Placebo (n = 60) | p-Value |
|---|---|---|---|
| Age, mean (SD), y | 69.2 (4.7) | 70.3 (4.1) | 0.169 |
| Sex | 0.819 | ||
| Male | 6 (10.3%) | 7 (11.7%) | |
| Female | 52 (89.7%) | 53 (88.3%) | |
| Body Mass Index, mean (SD) | 26.5 (3.8) | 27.2 (3.2) | 0.253 |
| Comorbidities | |||
| Hypertension | 34 (58.6%) | 31 (51.7%) | 0.448 |
| Diabetes | 12 (20.7%) | 8 (13.8%) | 0.287 |
| Cardiovascular | 4 (6.9%) | 5 (8.3%) | 0.769 |
| Brain | 0 (0%) | 3 (5.0%) | 0.085 |
| Thyroid | 3 (5.2%) | 1 (1.7%) | 0.293 |
| Kidney | 1 (1.7%) | 1 (1.7%) | 0.981 |
| Lung | 4 (6.9%) | 2 (3.3%) | 0.378 |
| Liver | 0 (0%) | 1 (1.7%) | 0.323 |
| Smoking | 0 (0%) | 1 (1.7%) | 0.323 |
| Alcohol | 1 (1.7%) | 0 (0%) | 0.307 |
| ASA | 0.082 | ||
| 1 | 18 (31.0%) | 28 (46.7%) | |
| 2 | 40 (69.0%) | 32 (53.3%) | |
| Torniquet time Right, mean (SD), min | 41.2 (5.4) | 41.1 (6.3) | 0.926 |
| Torniquet time Left, mean (SD), min | 42.0 (6.5) | 41.7 (6.4) | 0.822 |
| Total Hemovac amount, mean (SD), mL | 811.5 (435.4) | 823.0 (348.8) | 0.878 |
| Total blood loss during operation, mean (SD), mL | 369.7 (81.6) | 391.0 (85.2) | 0.168 |
| Hematologic Markers | Ferric Carboxymaltose (n = 58) | Placebo (n = 60) | p-Value |
|---|---|---|---|
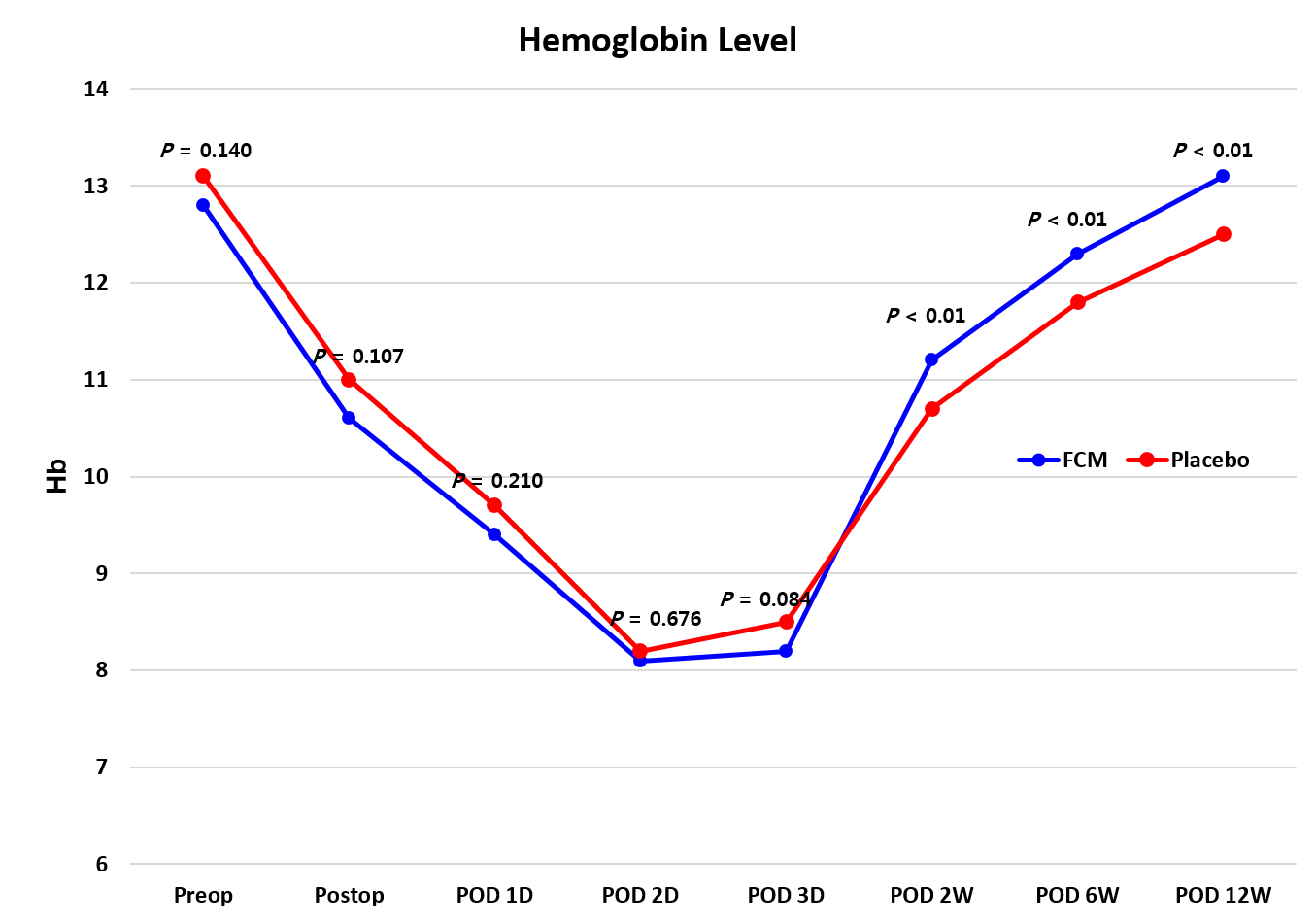
| Hemoglobin, g/dL | |||
| Preoperative | 12.8 (1.1) | 13.1 (1.3) | 0.140 |
| Postoperative 2 weeks | 11.2 (0.9) | 10.7 (1.0) | 0.007 |
| Postoperative 6 weeks | 12.3 (0.8) | 11.8 (0.9) | 0.004 |
| Postoperative 12 weeks | 13.1 (0.8) | 12.5 (1.1) | 0.007 |
| Serum Ferritin, ng/mL | |||
| Preoperative | 108.6 (72.1) | 115.0 (73.5) | 0.641 |
| Postoperative 2 weeks | 1191.8 (491.1) | 271.1 (226.6) | <0.001 |
| Postoperative 6 weeks | 753.4 (308.3) | 169.9 (145.9) | <0.001 |
| Postoperative 12 weeks | 475.0 (231.1) | 101.8 (98.2) | <0.001 |
| Iron, mcg/dL | |||
| Preoperative | 80.0 (32.5) | 82.6 (32.0) | 0.667 |
| Postoperative 2 weeks | 86.8 (32.2) | 56.5 (22.7) | <0.001 |
| Postoperative 6 weeks | 80.0 (24.0) | 59.0 (23.3) | <0.001 |
| Postoperative 12 weeks | 83.4 (25.3) | 60.7 (21.7) | <0.001 |
| Total Iron-Binding Capacity, mcg/dL | |||
| Preoperative | 318.1 (46.9) | 317.6 (60.2) | 0.961 |
| Postoperative 2 weeks | 269.2 (39.2) | 292.6 (44.8) | 0.004 |
| Postoperative 6 weeks | 250.8 (34.6) | 306.0 (54.7) | <0.001 |
| Postoperative 12 weeks | 255.4 (33.5) | 320.3 (57.6) | <0.001 |
| Transferrin Saturation, % | |||
| Preoperative | 25.6 (11.2) | 27.1 (10.8) | 0.497 |
| Postoperative 2 weeks | 32.7 (12.8) | 19.9 (8.6) | <0.001 |
| Postoperative 6 weeks | 32.0 (9.6) | 19.9 (8.4) | <0.001 |
| Postoperative 12 weeks | 32.4 (9.0) | 19.9 (8.8) | <0.001 |
| EQ-5D | Ferric Carboxymaltose (n = 58) | Placebo (n = 60) | p-Value |
|---|---|---|---|
| EQ 5D-1 Mobility | |||
| Preoperative | 1.9 (0.4) | 1.9 (0.4) | 0.834 |
| Postoperative 2 weeks | 2.0 (0.5) | 1.9 (0.4) | 0.680 |
| Postoperative 6 weeks | 1.8 (0.4) | 1.8 (0.4) | 0.451 |
| Postoperative 12 weeks | 1.7 (0.5) | 1.6 (0.5) | 0.482 |
| EQ 5D-2 Self-Care | |||
| Preoperative | 1.5 (0.6) | 1.4 (0.6) | 0.830 |
| Postoperative 2 weeks | 1.9 (0.6) | 1.8 (0.5) | 0.884 |
| Postoperative 6 weeks | 1.8 (0.4) | 1.8 (0.5) | 0.775 |
| Postoperative 12 weeks | 1.5 (0.6) | 1.6 (0.6) | 0.449 |
| EQ 5D-3 Usual Activities | |||
| Preoperative | 1.7 (0.5) | 1.7 (0.6) | 0.903 |
| Postoperative 2 weeks | 2.1 (0.4) | 2.1 (0.5) | 0.982 |
| Postoperative 6 weeks | 1.8 (0.4) | 1.8 (0.5) | 0.528 |
| Postoperative 12 weeks | 1.6 (0.6) | 1.7 (0.7) | 0.564 |
| EQ 5D-4 Pain/Discomfort | |||
| Preoperative | 2.2 (0.6) | 2.2 (0.0) | 0.418 |
| Postoperative 2 weeks | 2.0 (0.3) | 1.9 (0.3) | 0.750 |
| Postoperative 6 weeks | 1.9 (0.5) | 2.0 (0.6) | 0.309 |
| Postoperative 12 weeks | 1.6 (0.6) | 1.4 (0.5) | 0.076 |
| EQ 5D-5 Anxiety/Depression | |||
| Preoperative | 1.5 (0.6) | 1.4 (0.5) | 0.453 |
| Postoperative 2 weeks | 1.5 (0.5) | 1.5 (0.5) | 0.778 |
| Postoperative 6 weeks | 1.5 (0.5) | 1.5 (0.5) | 0.628 |
| Postoperative 12 weeks | 1.7 (0.5) | 1.7 (0.5) | 0.616 |
| BPI Interference | Ferric Carboxymaltose (n = 58) | Placebo (n = 60) | p-Value |
|---|---|---|---|
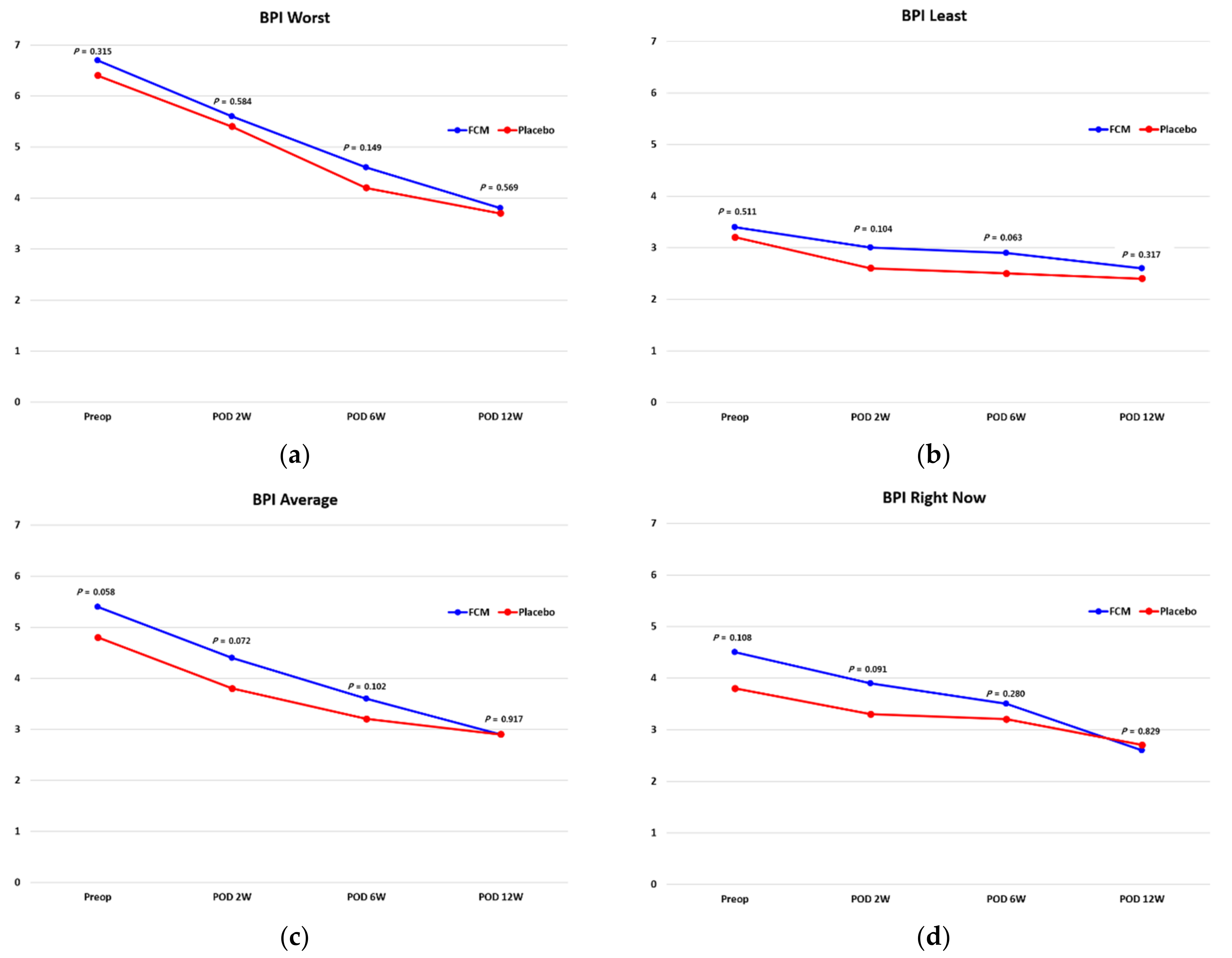
| BPI Interference General Activity | |||
| Preoperative | 6.4 (2.6) | 6.2 (1.8) | 0.639 |
| Postoperative 2 weeks | 6.5 (1.8) | 5.9 (1.8) | 0.150 |
| Postoperative 6 weeks | 5.1 (1.5) | 4.7 (1.1) | 0.157 |
| Postoperative 12 weeks | 4.2 (1.3) | 4.1 (1.1) | 0.689 |
| BPI Interference Walking | |||
| Preoperative | 6.0 (2.6) | 6.0 (1.9) | 0.868 |
| Postoperative 2 weeks | 6.1 (1.9) | 5.9 (1.9) | 0.645 |
| Postoperative 6 weeks | 5.3 (1.5) | 4.8 (1.3) | 0.125 |
| Postoperative 12 weeks | 4.5 (1.5) | 4.2 (1.4) | 0.456 |
| BPI Interference Work | |||
| Preoperative | 6.3 (2.1) | 6.1 (1.9) | 0.695 |
| Postoperative 2 weeks | 6.2 (2.1) | 6.0 (1.8) | 0.522 |
| Postoperative 6 weeks | 5.3 (1.5) | 4.8 (1.4) | 0.108 |
| Postoperative 12 weeks | 4.4 (1.4) | 4.3 (1.3) | 0.735 |
| BPI Interference Sleep | |||
| Preoperative | 5.5 (2.7) | 5.2 (2.3) | 0.498 |
| Postoperative 2 weeks | 5.5 (2.2) | 5.1 (2.1) | 0.340 |
| Postoperative 6 weeks | 4.6 (1.6) | 4.1 (1.4) | 0.098 |
| Postoperative 12 weeks | 3.9 (1.4) | 3.8 (1.4) | 0.991 |
| BPI Interference Relation | |||
| Preoperative | 5.3 (2.8) | 5.0 (2.5) | 0.557 |
| Postoperative 2 weeks | 5.1 (2.6) | 4.9 (2.2) | 0.659 |
| Postoperative 6 weeks | 4.3 (1.9) | 4.1 (1.7) | 0.593 |
| Postoperative 12 weeks | 3.5 (1.5) | 3.8 (1.6) | 0.439 |
| BPI Interference Enjoyment | |||
| Preoperative | 5.7 (2.5) | 5.6 (2.1) | 0.810 |
| Postoperative 2 weeks | 5.7 (2.1) | 5.5 (1.9) | 0.697 |
| Postoperative 6 weeks | 4.9 (1.3) | 4.7 (1.5) | 0.517 |
| Postoperative 12 weeks | 4.0 (1.3) | 4.2 (1.4) | 0.591 |
| BPI Interference Mood | |||
| Preoperative | 5.8 (2.8) | 5.4 (2.2) | 0.488 |
| Postoperative 2 weeks | 6.0 (2.3) | 5.5 (2.1) | 0.284 |
| Postoperative 6 weeks | 5.0 (2.0) | 4.4 (1.4) | 0.084 |
| Postoperative 12 weeks | 4.3 (1.7) | 4.0 (1.4) | 0.366 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Koh, I.J.; Choi, K.Y.; Yang, S.C.; In, Y. Efficacy and Safety of Intravenous Ferric Carboxymaltose in Patients with Postoperative Anemia Following Same-Day Bilateral Total Knee Arthroplasty: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 1457. https://doi.org/10.3390/jcm10071457
Kim MS, Koh IJ, Choi KY, Yang SC, In Y. Efficacy and Safety of Intravenous Ferric Carboxymaltose in Patients with Postoperative Anemia Following Same-Day Bilateral Total Knee Arthroplasty: A Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(7):1457. https://doi.org/10.3390/jcm10071457
Chicago/Turabian StyleKim, Man Soo, In Jun Koh, Keun Young Choi, Sung Cheol Yang, and Yong In. 2021. "Efficacy and Safety of Intravenous Ferric Carboxymaltose in Patients with Postoperative Anemia Following Same-Day Bilateral Total Knee Arthroplasty: A Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 7: 1457. https://doi.org/10.3390/jcm10071457
APA StyleKim, M. S., Koh, I. J., Choi, K. Y., Yang, S. C., & In, Y. (2021). Efficacy and Safety of Intravenous Ferric Carboxymaltose in Patients with Postoperative Anemia Following Same-Day Bilateral Total Knee Arthroplasty: A Randomized Controlled Trial. Journal of Clinical Medicine, 10(7), 1457. https://doi.org/10.3390/jcm10071457









