MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury
Abstract
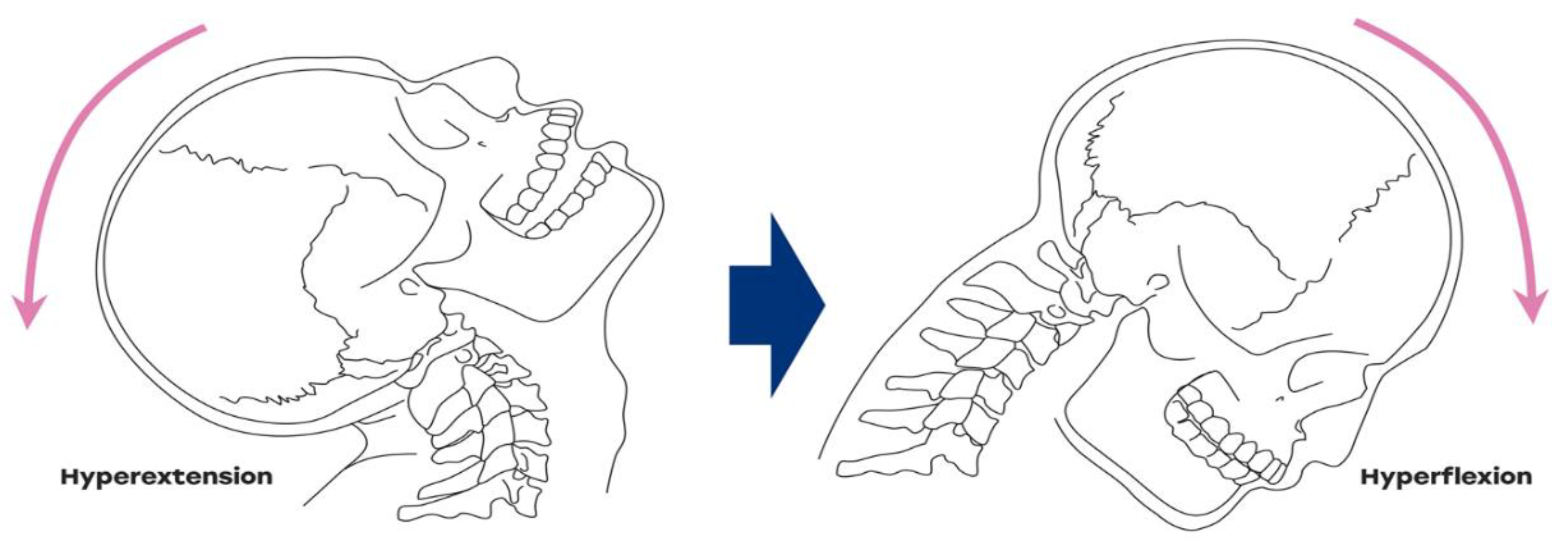
1. Introduction
2. Materials and Methods
2.1. Participants and Demographic Data
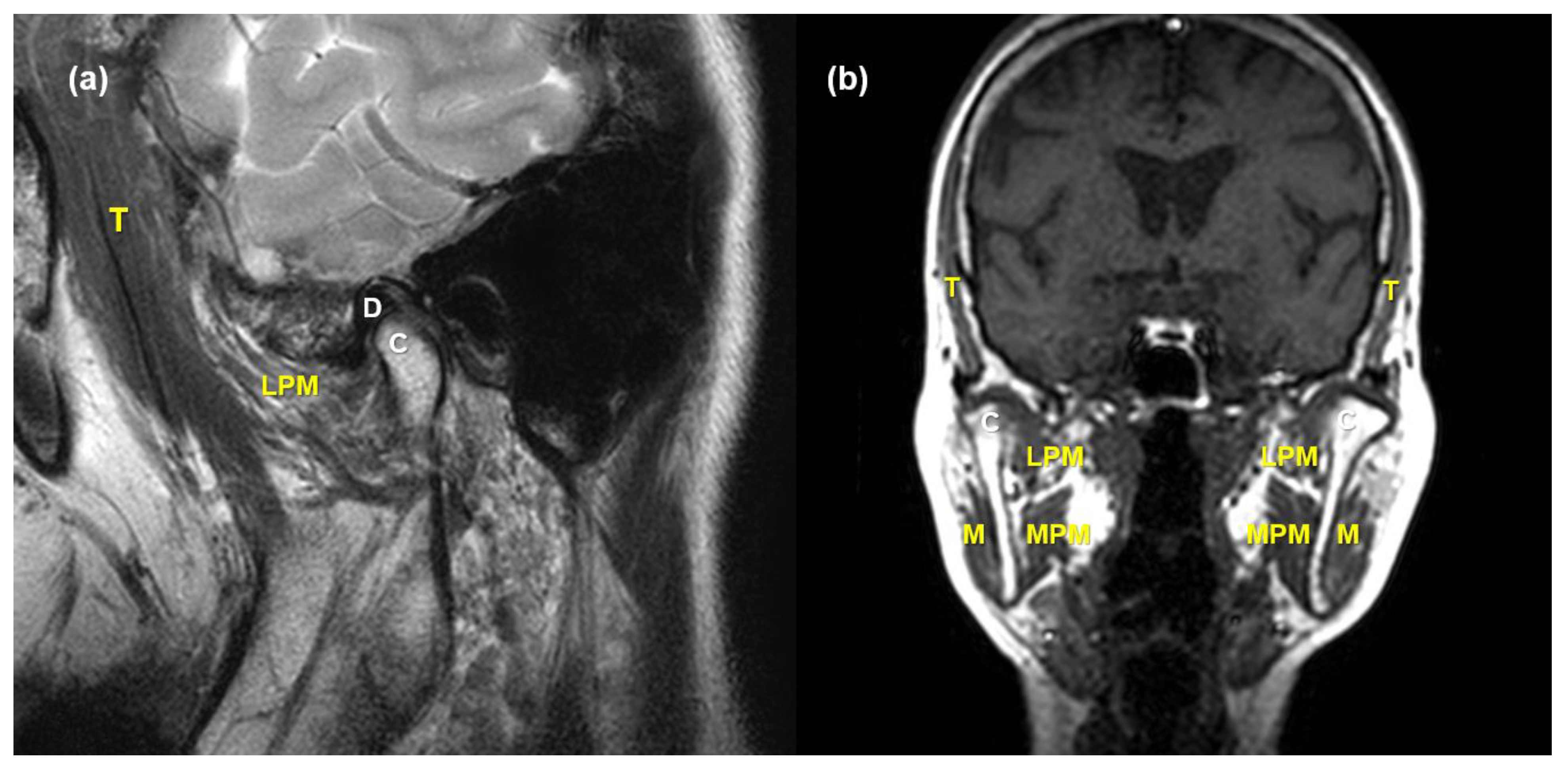
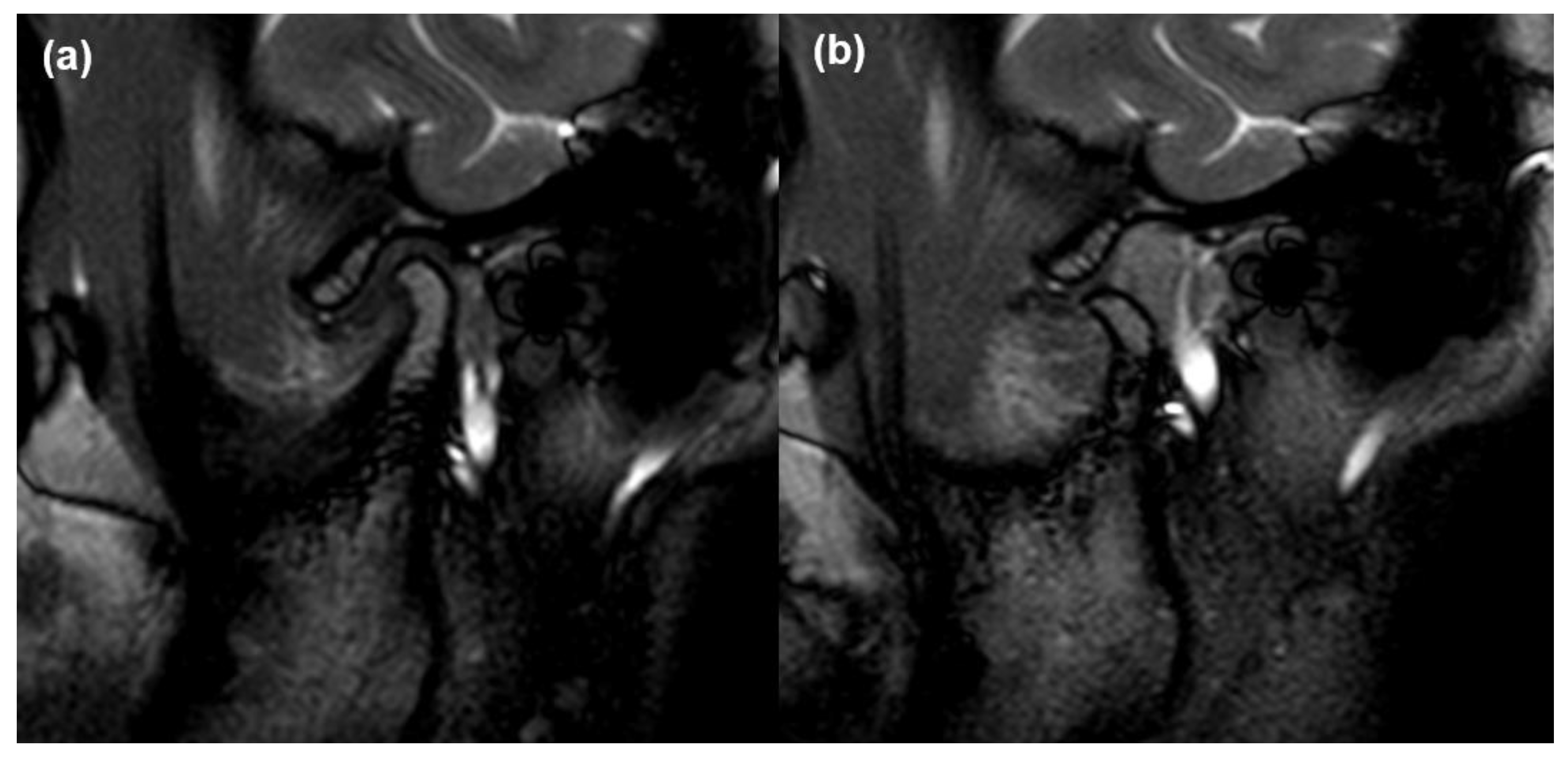
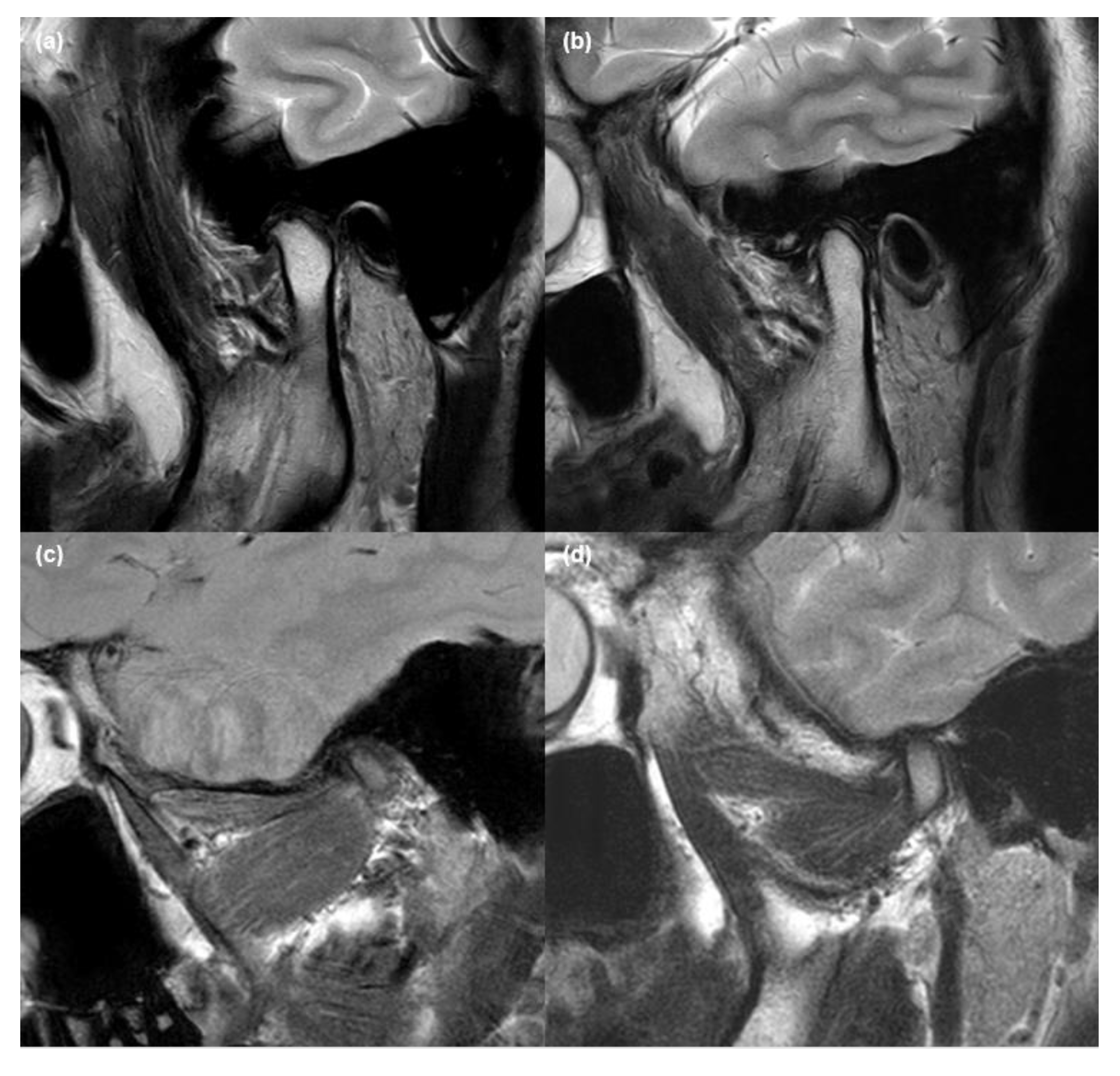
2.2. MRI Acquisition and Analysis
2.3. Validation of MRI Findings
2.4. Validation of Clinical Signs and Symptoms of TMD
2.4.1. Contributing Factors and Comorbidities
2.4.2. Characteristics of Pain
2.4.3. Palpation Index (PI) and Neck PI
2.5. Statistical Methods
3. Results
3.1. Demographic Patient Characteristics
3.2. Analysis of Pain Intensity
3.3. Clinical Characteristics in wTMD and iTMD Groups
3.4. Distribution of MRI Findings
3.5. Distribution of Muscle Tenderness
3.6. Associated MRI Findings with Increasing TMD Index
3.7. Diagnostic Classification of Pain-Related TMD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haggman-Henrikson, B.; Lampa, E.; Marklund, S.; Wanman, A. Pain and Disability in the Jaw and Neck Region following Whiplash Trauma. J. Dent. Res. 2016, 95, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, W.O.; Skovron, M.L.; Salmi, L.R.; Cassidy, J.D.; Duranceau, J.; Suissa, S.; Zeiss, E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: Redefining “whiplash” and its management. Spine 1995, 20, 1s–73s. [Google Scholar]
- Yadla, S.; Ratliff, J.K.; Harrop, J.S. Whiplash: Diagnosis, treatment, and associated injuries. Curr. Rev. Musculoskelet. Med. 2008, 1, 65–68. [Google Scholar] [CrossRef]
- Fernandez, C.E.; Amiri, A.; Jaime, J.; Delaney, P. The relationship of whiplash injury and temporomandibular disorders: A narrative literature review. J. Chiropr. Med. 2009, 8, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Haggman-Henrikson, B.; Rezvani, M.; List, T. Prevalence of whiplash trauma in TMD patients: A systematic review. J. Oral Rehabil. 2014, 41, 59–68. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, K.M.; Auh, Q.S.; Hong, J.P. Magnetic Resonance Imaging-Based Prediction of the Relationship between Whiplash Injury and Temporomandibular Disorders. Front. Neurol. 2017, 8, 725. [Google Scholar] [CrossRef]
- Myrtveit, S.M.; Skogen, J.C.; Sivertsen, B.; Steingrímsdóttir, Ó.A.; Stubhaug, A.; Nielsen, C.S. Pain and pain tolerance in whiplash-associated disorders: A population-based study. Eur. J. Pain 2016, 20, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, L.; Lord, S.; Bogduk, N. Whiplash injury. Pain 1994, 58, 283–307. [Google Scholar] [CrossRef]
- Von Korff, M.; Le Resche, L.; Dworkin, S.F. First onset of common pain symptoms: A prospective study of depression as a risk factor. Pain 1993, 55, 251–258. [Google Scholar] [CrossRef]
- Lim, P.F.; Smith, S.; Bhalang, K.; Slade, G.D.; Maixner, W. Development of temporomandibular disorders is associated with greater bodily pain experience. Clin. J. Pain 2010, 26, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Skármeta, N.P.; Pesce, M.C.; Saldivia, J.; Espinoza-Mellado, P.; Montini, F.; Sotomayor, C. Changes in understanding of painful temporomandibular disorders: The history of a transformation. Quintessence Int. 2019, 50, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, K.M.; Auh, Q.S.; Hong, J.P. Sex-related differences in symptoms of temporomandibular disorders and structural changes in the lateral pterygoid muscle after whiplash injury. J. Oral Rehabil. 2019, 46, 1107–1120. [Google Scholar] [CrossRef]
- Curatolo, M.; Bogduk, N.; Ivancic, P.C.; McLean, S.A.; Siegmund, G.P.; Winkelstein, B.A. The role of tissue damage in whiplash-associated disorders: Discussion paper 1. Spine 2011, 36, S309–S315. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef]
- Sareen, J. Posttraumatic stress disorder in adults: Impact, comorbidity, risk factors, and treatment. Can. J. Psychiatry 2014, 59, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Vedana, L.; Landulpho, A.B.; Andrade e Silva, F.; Buarque e Silva, W.A. Electromyographic evaluation during masticatory function, in patients with temporomandibular disorders following interocclusal appliance treatment. Electromyogr. Clin. Neurophysiol. 2010, 50, 33–38. [Google Scholar]
- Chen, H.; Whittle, T.; Gal, J.A.; Murray, G.M.; Klineberg, I.J. The medial pterygoid muscle: A stabiliser of horizontal jaw movement. J. Oral Rehabil. 2017, 44, 779–790. [Google Scholar] [CrossRef]
- Schellhas, K.P. MR imaging of muscles of mastication. Am. J. Roentgenol. 1989, 153, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Zanoteli, E.; Yamashita, H.K.; Suzuki, H.; Oliveira, A.S.; Gabbai, A.A. Temporomandibular joint and masticatory muscle involvement in myotonic dystrophy: A study by magnetic resonance imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 94, 262–271. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Pedrinelli, A.; Hernandez, A.J. Muscle injury–physiopathology, diagnosis, treatment and clinical presentation. Rev. Bras. Ortop. 2011, 46, 247–255. [Google Scholar] [CrossRef]
- Taskaya-Yilmaz, N.; Ogutcen-Toller, M. Magnetic resonance imaging evaluation of temporomandibular joint disc deformities in relation to type of disc displacement. J. Oral Maxillofac. Surg. 2001, 59, 860–865, discussion 865–866. [Google Scholar] [CrossRef]
- Ariji, Y.; Ariji, E. Magnetic resonance and sonographic imagings of masticatory muscle myalgia in temporomandibular disorder patients. Jpn. Dent. Sci. Rev. 2017, 53, 11–17. [Google Scholar] [CrossRef]
- Dworkin, S.F. Research Diagnostic criteria for Temporomandibular Disorders: Current status & future relevance. J. Oral Rehabil. 2010, 37, 734–743. [Google Scholar] [CrossRef]
- Truelove, E.; Pan, W.; Look, J.O.; Mancl, L.A.; Ohrbach, R.K.; Velly, A.M.; Huggins, K.H.; Lenton, P.; Shiffman, E.L. The Research Diagnostic Criteria for Temporomandibular Disorders. III: Validity of Axis I diagnoses. J. Orofac. Pain 2010, 24, 35–47. [Google Scholar]
- Markiewicz, M.R.; Ohrbach, R.; McCall, W.D., Jr. Oral behaviors checklist: Reliability of performance in targeted waking-state behaviors. J. Orofac. Pain 2006, 20, 306–316. [Google Scholar] [PubMed]
- Goulet, J.P.; Clark, G.T.; Flack, V.F. Reproducibility of examiner performance for muscle and joint palpation in the temporomandibular system following training and calibration. Community Dent. Oral Epidemiol. 1993, 21, 72–77. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, S.C.; Nogueira, R.V.; Duarte, A.P.; Vasconcelos, B.C.; Almeida Rde, A. Analysis of helkimo and craniomandibular indexes for temporomandibular disorder diagnosis on rheumatoid arthritis patients. Braz. J. Otorhinolaryngol. 2007, 73, 19–26. [Google Scholar] [CrossRef]
- Cote, P.; Cassidy, J.D.; Carroll, L.; Frank, J.W.; Bombardier, C. A systematic review of the prognosis of acute whiplash and a new conceptual framework to synthesize the literature. Spine 2001, 26, E445–E458. [Google Scholar] [CrossRef]
- Haggman-Henrikson, B.; List, T.; Westergren, H.T.; Axelsson, S.H. Temporomandibular disorder pain after whiplash trauma: A systematic review. J. Orofac. Pain 2013, 27, 217–226. [Google Scholar] [CrossRef]
- Carroll, L.J.; Ferrari, R.; Cassidy, J.D. Reduced or painful jaw movement after collision-related injuries: A population-based study. J. Am. Dent. Assoc. 2007, 138, 86–93. [Google Scholar] [CrossRef]
- Johansen, M.K.; Graven-Nielsen, T.; Olesen, A.S.; Arendt-Nielsen, L. Generalised muscular hyperalgesia in chronic whiplash syndrome. Pain 1999, 83, 229–234. [Google Scholar] [CrossRef]
- Freynhagen, R.; Parada, H.A.; Calderon-Ospina, C.A.; Chen, J.; Rakhmawati Emril, D.; Fernández-Villacorta, F.J.; Franco, H.; Ho, K.Y.; Lara-Solares, A.; Li, C.C.; et al. Current understanding of the mixed pain concept: A brief narrative review. Curr. Med. Res. Opin. 2019, 35, 1011–1018. [Google Scholar] [CrossRef]
- Pereira-Silva, R.; Costa-Pereira, J.T.; Alonso, R.; Serrão, P.; Martins, I.; Neto, F.L. Attenuation of the Diffuse Noxious Inhibitory Controls in Chronic Joint Inflammatory Pain Is Accompanied by Anxiodepressive-Like Behaviors and Impairment of the Descending Noradrenergic Modulation. Int. J. Mol. Sci. 2020, 21, 2973. [Google Scholar] [CrossRef]
- Edwards, R.R.; Grace, E.; Peterson, S.; Klick, B.; Haythornthwaite, J.A.; Smith, M.T. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur. J. Pain 2009, 13, 1043–1047. [Google Scholar] [CrossRef]
- Generaal, E.; Vogelzangs, N.; Macfarlane, G.J.; Geenen, R.; Smit, J.H.; Penninx, B.W.; Dekker, J. Reduced hypothalamic-pituitary-adrenal axis activity in chronic multi-site musculoskeletal pain: Partly masked by depressive and anxiety disorders. BMC Musculoskelet. Disord. 2014, 15, 227. [Google Scholar] [CrossRef]
- Burstein, R.; Jakubowski, M.; Garcia-Nicas, E.; Kainz, V.; Bajwa, Z.; Hargreaves, R.; Becerra, L.; Borsook, D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann. Neurol. 2010, 68, 81–91. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Vicenzino, B.; Kenardy, J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain 2003, 104, 509–517. [Google Scholar] [CrossRef]
- Herrero, J.F.; Laird, J.M.; López-García, J.A. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog. Neurobiol. 2000, 61, 169–203. [Google Scholar] [CrossRef]
- Sterling, M. Whiplash-associated disorder: Musculoskeletal pain and related clinical findings. J. Man. Manip. Ther. 2011, 19, 194–200. [Google Scholar] [CrossRef]
- Suvinen, T.I.; Kemppainen, P. Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J. Oral Rehabil. 2007, 34, 631–644. [Google Scholar] [CrossRef]
- Boniver, R. Temporomandibular joint dysfunction in whiplash injuries: Association with tinnitus and vertigo. Int. Tinnitus J. 2002, 8, 129–131. [Google Scholar] [PubMed]
- Pressman, B.D.; Shellock, F.G.; Schames, J.; Schames, M. MR imaging of temporomandibular joint abnormalities associated with cervical hyperextension/hyperflexion (whiplash) injuries. J. Magn. Reson. Imaging 1992, 2, 569–574. [Google Scholar] [CrossRef]
- Okeson, J.P. Joint intracapsular disorders: Diagnostic and nonsurgical management considerations. Dent. Clin. N. Am. 2007, 51, 85–103. [Google Scholar] [CrossRef]
- Honda, K.; Natsumi, Y.; Urade, M. Correlation between MRI evidence of degenerative condylar surface changes, induction of articular disc displacement and pathological joint sounds in the temporomandibular joint. Gerodontology 2008, 25, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.O.; Eriksson, A.; Ringqvist, M.; Thornell, L.E. Special histochemical muscle-fibre characteristics of the human lateral pterygoid muscle. Arch. Oral Biol. 1981, 26, 495–507. [Google Scholar] [CrossRef]
- Finden, S.G.; Enochs, W.S.; Rao, V.M. Pathologic changes of the lateral pterygoid muscle in patients with derangement of the temporomandibular joint disk: Objective measures at MR imaging. AJNR Am. J. Neuroradiol. 2007, 28, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Imanimoghaddam, M.; Madani, A.S.; Hashemi, E.M. The evaluation of lateral pterygoid muscle pathologic changes and insertion patterns in temporomandibular joints with or without disc displacement using magnetic resonance imaging. Int. J. Oral Maxillofac. Surg. 2013, 42, 1116–1120. [Google Scholar] [CrossRef]
- Elliott, J.M.; O’Leary, S.; Sterling, M.; Hendrikz, J.; Pedler, A.; Jull, G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine 2010, 35, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Uthaikhup, S.; Assapun, J.; Kothan, S.; Watcharasaksilp, K.; Elliott, J.M. Structural changes of the cervical muscles in elder women with cervicogenic headache. Musculoskelet. Sci. Pract. 2017, 29, 1–6. [Google Scholar] [CrossRef]
- Woźniak, K.; Lipski, M.; Lichota, D.; Szyszka-Sommerfeld, L. Muscle Fatigue in the Temporal and Masseter Muscles in Patients with Temporomandibular Dysfunction. Biomed Res. Int. 2015, 2015, 269734. [Google Scholar] [CrossRef]
- Garip, H.; Tufekcioglu, S.; Kaya, E. Changes in the temporomandibular joint disc and temporal and masseter muscles secondary to bruxism in Turkish patients. Saudi Med. J. 2018, 39, 81–85. [Google Scholar] [CrossRef]
- Park, K.M.; Choi, E.; Kwak, E.J.; Kim, S.; Park, W.; Jeong, J.S.; Kim, K.D. The relationship between masseter muscle thickness measured by ultrasonography and facial profile in young Korean adults. Imaging Sci. Dent. 2018, 48, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, C.H. Correlation between mandibular morphology and masticatory muscle thickness in normal occlusion and mandibular prognathism. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Korfage, J.A.; Brugman, P.; Van Eijden, T.M. Intermuscular and intramuscular differences in myosin heavy chain composition of the human masticatory muscles. J. Neurol. Sci. 2000, 178, 95–106. [Google Scholar] [CrossRef]
- Bottinelli, R.; Canepari, M.; Pellegrino, M.A.; Reggiani, C. Force-velocity properties of human skeletal muscle fibres: Myosin heavy chain isoform and temperature dependence. J. Physiol. 1996, 495 Pt 2, 573–586. [Google Scholar] [CrossRef]
- Weiss, A.; McDonough, D.; Wertman, B.; Acakpo-Satchivi, L.; Montgomery, K.; Kucherlapati, R.; Leinwand, L.; Krauter, K. Organization of human and mouse skeletal myosin heavy chain gene clusters is highly conserved. Proc. Natl. Acad. Sci. USA 1999, 96, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Korfage, J.A.; Van Eijden, T.M. Myosin isoform composition of the human medial and lateral pterygoid muscles. J. Dent. Res. 2000, 79, 1618–1625. [Google Scholar] [CrossRef]
- Poluha, R.L.; Canales, G.D.l.T.; Costa, Y.M.; Grossmann, E.; Bonjardim, L.R.; Conti, P.C.R. Temporomandibular joint disc displacement with reduction: A review of mechanisms and clinical presentation. J. Appl. Oral Sci. 2019, 27, e20180433. [Google Scholar] [CrossRef]
- Hu, Y.K.; Yang, C.; Xie, Q.Y. Changes in disc status in the reducing and nonreducing anterior disc displacement of temporomandibular joint: A longitudinal retrospective study. Sci. Rep. 2016, 6, 34253. [Google Scholar] [CrossRef]
- Al-Baghdadi, M.; Durham, J.; Araujo-Soares, V.; Robalino, S.; Errington, L.; Steele, J. TMJ Disc Displacement without Reduction Management: A Systematic Review. J. Dent. Res. 2014, 93, 37S–51S. [Google Scholar] [CrossRef]
- Howard, R.P.; Benedict, J.V.; Raddin, J.H., Jr.; Smith, H.L. Assessing neck extension-flexion as a basis for temporomandibular joint dysfunction. J. Oral Maxillofac. Surg. 1991, 49, 1210–1213. [Google Scholar] [CrossRef]
- Chimenti, R.L.; Frey-Law, L.A.; Sluka, K.A. A Mechanism-Based Approach to Physical Therapist Management of Pain. Phys. Ther. 2018, 98, 302–314. [Google Scholar] [CrossRef]
- La Mantia, L.; Erbetta, A. Headache and inflammatory disorders of the central nervous system. Neurol. Sci. 2004, 25 (Suppl. 3), S148–S153. [Google Scholar] [CrossRef] [PubMed]
- Dyken, M.E.; Afifi, A.K.; Lin-Dyken, D.C. Sleep-related problems in neurologic diseases. Chest 2012, 141, 528–544. [Google Scholar] [CrossRef]
- de Morree, H.M.; Szabó, B.M.; Rutten, G.J.; Kop, W.J. Central nervous system involvement in the autonomic responses to psychological distress. Neth. Heart J. 2013, 21, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.P.; Martin, P.R.; Boschen, M.J. Psychological Sleep Interventions for Migraine and Tension-Type Headache: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 6411. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.C.; Charles, A. Sleep and headache. Semin. Neurol. 2009, 29, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Tchivileva, I.E.; Ohrbach, R.; Fillingim, R.B.; Greenspan, J.D.; Maixner, W.; Slade, G.D. Temporal change in headache and its contribution to the risk of developing first-onset temporomandibular disorder in the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study. Pain 2017, 158, 120–129. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Vicenzino, B.; Kenardy, J.; Darnell, R. Physical and psychological factors predict outcome following whiplash injury. Pain 2005, 114, 141–148. [Google Scholar] [CrossRef]
- Ohrbach, R. Assessment and Further Development of RDC/TMD Axis II Biobehavioral Instruments: A Research Program Progress Report. J. Oral Rehabil. 2010, 37, 784–798. [Google Scholar] [CrossRef]
- Koolstra, J.H.; van Eijden, T.M. Dynamics of the human masticatory muscles during a jaw open-close movement. J. Biomech. 1997, 30, 883–889. [Google Scholar] [CrossRef]
- Devoize, L.; Doméjean, S.; Melin, C.; Raboisson, P.; Artola, A.; Dallel, R. Organization of projections from the spinal trigeminal subnucleus oralis to the spinal cord in the rat: A neuroanatomical substrate for reciprocal orofacial-cervical interactions. Brain Res. 2010, 1343, 75–82. [Google Scholar] [CrossRef]
- Giannakopoulos, N.N.; Schindler, H.J.; Hellmann, D. Co-contraction behaviour of masticatory and neck muscles during tooth grinding. J. Oral Rehabil. 2018, 45, 504–511. [Google Scholar] [CrossRef] [PubMed]


| wTMD Group Mean ± SD or n (%) (n = 45) | iTMD Group Mean ± SD or n (%) (n = 45) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age, years a | 37.94 ± 12.27 | 40.77 ± 18.02 | 0.387 |
| Female b | 31 (68.9%) | 33 (73.3%) | 0.816 |
| Pain characteristics | |||
| Symptom duration, days a | 66.27 ± 58.06 | 57.78 ± 48.33 | 0.453 |
| VAS a | 6.73 ±1.84 | 4.32 ± 2.80 | <0.001 *** |
| TMD indexes | |||
| PI a | 0.27 ± 0.21 | 0.16 ± 0.18 | 0.0086 ** |
| Neck PI a | 0.45 ± 0.31 | 0.27 ± 0.23 | 0.003 ** |
| wTMD Group | iTMD Group | ||||
|---|---|---|---|---|---|
| n = 45 | Column (%) | n = 45 | Column (%) | p-Value | |
| Bruxism a | |||||
| No | 40 | (88.9) | 40 | (88.9) | 1.000 |
| Yes | 5 | (11.1) | 5 | (11.1) | |
| Clenching b | |||||
| No | 41 | (91.1) | 33 | (73.3) | 0.026 * |
| Yes | 4 | (8.9) | 12 | (26.7) | |
| Tinnitus a | |||||
| No | 30 | (66.7) | 35 | (77.8) | 0.347 |
| Yes | 15 | (33.3) | 10 | (22.2) | |
| Headache a | |||||
| No | 13 | (28.9) | 27 | (60.0) | 0.006 ** |
| Yes | 32 | (71.1) | 18 | (40.0) | |
| Sleep problem a | |||||
| No | 29 | (64.4) | 19 | (42.2) | 0.028 * |
| Yes | 26 | (57.8) | 16 | (35.6) | |
| Psychological distress a | |||||
| No | 39 | (86.7) | 30 | (66.7) | 0.045 * |
| Yes | 15 | (33.3) | 6 | (13.3) | |
| wTMD Group | iTMD Group | |||||
|---|---|---|---|---|---|---|
| n = 45 | Column (%) | n = 45 | Column (%) | p-Value | ||
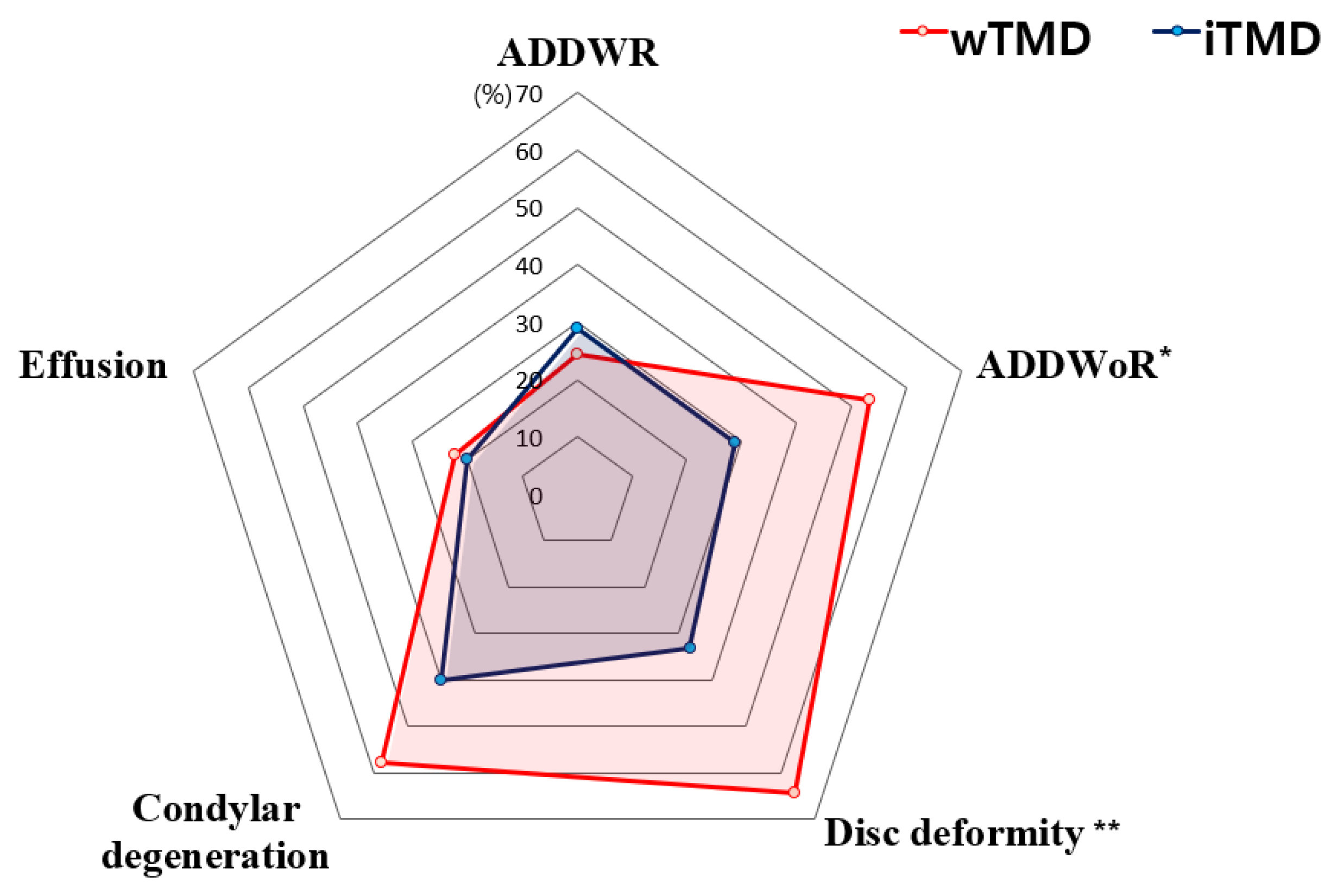
| ADDWR | No | 34 | (75.6) | 32 | (71.1) | 0.812 |
| Yes | 11 | (24.4) | 13 | (28.9) | ||
| ADDWoR | No | 21 | (46.7) | 32 | (71.1) | 0.032 * |
| Yes | 24 | (53.3) | 13 | (28.9) | ||
| Disc deformity | No | 16 | (35.6) | 30 | (66.7) | 0.006 ** |
| Yes | 29 | (64.4) | 15 | (33.3) | ||
| Condylar degeneration | No | 19 | (42.2) | 27 | (60.0) | 0.140 |
| Yes | 26 | (57.8) | 18 | (40.0) | ||
| Effusion | No | 35 | (77.8) | 36 | (80.0) | 1.000 |
| Yes | 10 | (22.2) | 9 | (20.0) | ||
| wTMD Group | iTMD Group | ||||||
|---|---|---|---|---|---|---|---|
| n = 45 | Column (%) | n = 45 | Column (%) | p-Value | |||
| Masseter muscle a | VC | No | 45 | (100.0) | 45 | (100.0) | |
| Yes | 0 | (0.0) | 0 | (0.0) | |||
| SC | No | 45 | (100.0) | 45 | (100.0) | ||
| Yes | 0 | (0.0) | 0 | (0.0) | |||
| Temporalis muscle a | VC | No | 44 | (97.8) | 44 | (97.8) | 1.000 |
| Yes | 1 | (2.2) | 1 | (2.2) | |||
| SC | No | 44 | (97.8) | 44 | (97.8) | 1.000 | |
| Yes | 1 | (2.2) | 1 | (2.2) | |||
| LPM b | VC | No | 19 | (42.2) | 37 | (82.2) | <0.0001 *** |
| Yes | 26 | (57.8) | 8 | (17.8) | |||
| SC | No | 26 | (57.8) | 38 | (84.4) | 0.010 ** | |
| Yes | 19 | (42.2) | 7 | (15.6) | |||
| MPM a | VC | No | 45 | (100.0) | 43 | (95.6) | 0.494 |
| Yes | 0 | (0.0) | 2 | (4.4) | |||
| SC | No | 45 | (100.0) | 43 | (95.6) | 0.494 | |
| Yes | 0 | (0.0) | 2 | (4.4) | |||
| wTMD Group | iTMD Group | ||||
|---|---|---|---|---|---|
| n = 45 | Column (%) | n = 45 | Column (%) | p-Value | |
| Masseter muscle tenderness a | |||||
| No | 13 | (28.9) | 16 | (35.6) | 0.652 |
| Yes | 32 | (71.1) | 29 | (64.4) | |
| Temporalis muscle tenderness a | |||||
| No | 18 | (40.0) | 29 | (64.4) | 0.034 * |
| Yes | 27 | (60.0) | 16 | (35.6) | |
| LPM tenderness b | |||||
| No | 35 | (77.8) | 44 | (97.8) | 0.007 ** |
| Yes | 10 | (22.2) | 1 | (2.2) | |
| MPM tenderness b | |||||
| No | 38 | (84.4) | 45 | (100.0) | 0.012 * |
| Yes | 7 | (15.6) | 0 | (0.0) | |
| wTMD | iTMD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS | PI | Neck PI | VAS | PI | Neck PI | |||||||
| r | p-Value | r | p-value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| TMD index | ||||||||||||
| PI | 0.174 | 0.252 | 1.000 | 0.866 *** | <0.001 | 0.300 * | 0.045 | 1.000 | 0.764 *** | <0.001 | ||
| Neck PI | 0.081 | 0.597 | 0.866 *** | <0.001 | 1.000 | 0.301 * | 0.044 | 0.764 *** | <0.001 | 1.000 | ||
| Abnormality of masticatory muscle | ||||||||||||
| Temp. VC | −0.124 | 0.416 | −0.180 | 0.236 | −0.006 | 0.970 | −0.029 | 0.849 | −0.059 | 0.703 | −0.035 | 0.820 |
| Temp. SC | −0.124 | 0.416 | −0.180 | 0.236 | −0.006 | 0.970 | −0.029 | 0.849 | −0.059 | 0.703 | −0.035 | 0.820 |
| LPM VC | −0.254 | 0.092 | −0.089 | 0.563 | 0.094 | 0.540 | −0.135 | 0.377 | −0.210 | 0.167 | −0.092 | 0.547 |
| LPM SC | 0.346 * | 0.020 | 0.069 | 0.650 | 0.229 | 0.130 | −0.185 | 0.224 | 0.061 | 0.689 | −0.171 | 0.262 |
| MPM VC | - | - | - | - | - | - | −0.217 | 0.153 | −0.293 | 0.051 | −0.064 | 0.674 |
| MPM SC | - | - | - | - | - | - | −0.217 | 0.153 | −0.293 | 0.051 | −0.064 | 0.674 |
| Abnormality of TMJ | ||||||||||||
| ADDWR | −0.043 | 0.781 | 0.208 | 0.171 | 0.130 | 0.395 | 0.064 | 0.674 | −0.202 | 0.184 | −0.178 | 0.241 |
| ADDWoR | 0.040 | 0.793 | −0.158 | 0.299 | −0.084 | 0.582 | 0.325 * | 0.029 | 0.124 | 0.418 | 0.169 | 0.268 |
| Disc deformity | −0.175 | 0.251 | 0.000 | 1.000 | −0.029 | 0.852 | 0.200 | 0.187 | −0.082 | 0.591 | −0.007 | 0.962 |
| Condylar degeneration | −0.053 | 0.730 | −0.089 | 0.563 | −0.144 | 0.345 | 0.297 * | 0.047 | −0.070 | 0.646 | 0.098 | 0.521 |
| Effusion | 0.021 | 0.891 | −0.274 | 0.068 | −0.134 | 0.379 | 0.180 | 0.236 | 0.181 | 0.234 | 0.067 | 0.664 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Lee, K.M.; Auh, Q.-S. MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury. J. Clin. Med. 2021, 10, 1404. https://doi.org/10.3390/jcm10071404
Lee Y-H, Lee KM, Auh Q-S. MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury. Journal of Clinical Medicine. 2021; 10(7):1404. https://doi.org/10.3390/jcm10071404
Chicago/Turabian StyleLee, Yeon-Hee, Kyung Mi Lee, and Q-Schick Auh. 2021. "MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury" Journal of Clinical Medicine 10, no. 7: 1404. https://doi.org/10.3390/jcm10071404
APA StyleLee, Y.-H., Lee, K. M., & Auh, Q.-S. (2021). MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury. Journal of Clinical Medicine, 10(7), 1404. https://doi.org/10.3390/jcm10071404





