Anhedonia Relates to the Altered Global and Local Grey Matter Network Properties in Schizophrenia
Abstract
1. Introduction
2. Methods
2.1. Subject Demographics and Scale Measurements
2.2. Image Acquisition and Anatomical Processing
2.3. Construction of the Networks and Measurement of the Network Small-World Property
2.4. Global-Scale Statistical Analysis
2.5. Local-Scale Statistical Analyses
3. Results
3.1. Subject Demographics and Scale Measurements
3.2. Global Network’s Small Worldness
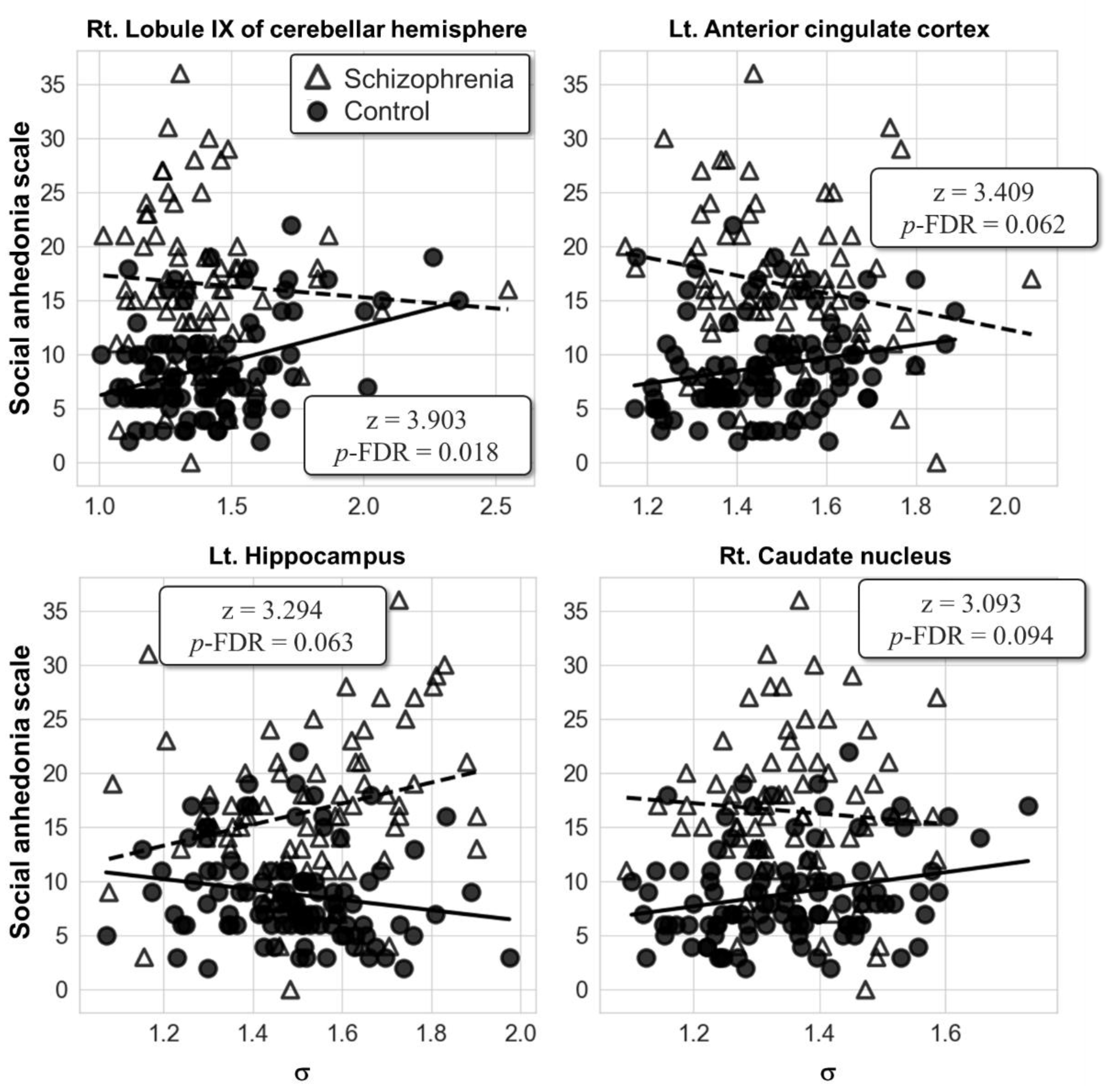
3.3. Network’s Small-World Metric at the Local Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub.: Washington, DC, USA, 2013. [Google Scholar]
- Blanchard, J.J.; Cohen, A.S. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr. Bull. 2006, 32, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, W.T.; Blanchard, J.J.; Kirkpatrick, B. New standards for negative symptom assessment. Schizophr. Bull. 2015, 42, 1–3. [Google Scholar]
- Milev, P.; Ho, B.C.; Arndt, S.; Andreasen, N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 2005, 162, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.R.; Prestia, D.; Twamley, E.W.; Patterson, T.L.; Bowie, C.R.; Harvey, P.D. Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophr. Res. 2014, 160, 136–141. [Google Scholar] [CrossRef]
- Bègue, I.; Kaiser, S.; Kirschner, M. Pathophysiology of negative symptom dimensions of schizophrenia–current developments and implications for treatment. Neurosci. Biobehav. Rev. 2020, 116, 74–88. [Google Scholar] [CrossRef]
- Strauss, G.P.; Gold, J.M. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry 2012, 169, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Horan, W.P.; Kring, A.M.; Blanchard, J.J. Anhedonia in schizophrenia: A review of assessment strategies. Schizophr. Bull. 2006, 32, 259–273. [Google Scholar] [CrossRef]
- Höflich, A.; Michenthaler, P.; Kasper, S.; Lanzenberger, R. Circuit mechanisms of reward, anhedonia, and depression. Int. J. Neuropsychopharmacol. 2019, 22, 105–118. [Google Scholar] [CrossRef]
- Der-Avakian, A.; Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, S.; Park, I.H.; Kim, J.J. Neural basis of anhedonia and amotivation in patients with schizophrenia: The role of reward system. Curr. Neuropharmacol. 2015, 13, 750–759. [Google Scholar] [CrossRef]
- Dowd, E.C.; Barch, D.M. Pavlovian reward prediction and receipt in schizophrenia: Relationship to anhedonia. PLoS ONE 2012, 7, e35622. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Vignapiano, A.; Mucci, A.; Ford, J.; Montefusco, V.; Plescia, G.M.; Bucci, P.; Galderisi, S. Reward anticipation and trait anhedonia: An electrophysiological investigation in subjects with schizophrenia. Clin. Neurophysiol. 2016, 127, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lin, P.; Shi, H.; Öngür, D.; Auerbach, R.P.; Wang, X.; Yao, S.; Wang, X. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: An ALE meta-analysis. Brain Imaging Behav. 2016, 10, 920–939. [Google Scholar] [CrossRef]
- Blanchard, J.J.; Mueser, K.T.; Bellack, A.S. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr. Bull. 1998, 24, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Honea, R.; Crow, T.J.; Passingham, D.; Mackay, C.E. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 2005, 162, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, H.J.; Chun, J.W.; Seok, J.H.; Park, I.H.; Park, B.; Kim, J.J. Neuroanatomical correlates of trait anhedonia in patients with schizophrenia: A voxel-based morphometric study. Neurosci. Lett. 2011, 489, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Yücel, M.; Wood, S.J.; Adamson, C.; Velakoulis, D.; Saling, M.M.; McGorry, P.D.; Pantelis, C. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum. Brain Mapp. 2008, 29, 478–489. [Google Scholar] [CrossRef]
- Schultz, C.C.; Koch, K.; Wagner, G.; Roebel, M.; Nenadic, I.; Gaser, C.; Schachtzabel, C.; Reichenbach, J.; Sauer, H.; Schlösser, R.G. Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophr. Res. 2010, 123, 137–144. [Google Scholar] [CrossRef]
- Tijms, B.M.; Seriès, P.; Willshaw, D.J.; Lawrie, S.M. Similarity-based extraction of individual networks from gray matter MRI scans. Cereb. Cortex 2012, 22, 1530–1541. [Google Scholar] [CrossRef]
- Tijms, B.M.; Sprooten, E.; Job, D.; Johnstone, E.C.; Owens, D.G.; Willshaw, D.; Seriès, P.; Lawrie, S.M. Grey matter networks in people at increased familial risk for schizophrenia. Schizophr. Res. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, D.; Keedy, S.K.; Ivleva, E.I.; Eum, S.; Yao, L.; Tamminga, C.A.; Clementz, B.A.; Keshavan, M.S.; Pearlson, G.D.; et al. Brain gray matter network organization in psychotic disorders. Neuropsychopharmacology 2020, 45, 666–674. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, M.; Zhou, Y.; He, Y.; Hao, Y.; Song, M.; Yu, C.; Liu, H.; Liu, Z.; Jiang, T. Disrupted small-world networks in schizophrenia. Brain 2008, 131, 945–961. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Su, T.W.; Hsu, T.W.; Lin, Y.C.; Lin, C.P. Schizophrenia symptoms and brain network efficiency: A resting-state fMRI study. Psychiatry Res. Neuroimaging 2015, 234, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Su, T.P.; Zhou, Y.; Chou, K.H.; Chen, I.Y.; Jiang, T.; Lin, C.P. Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage 2012, 59, 1085–1093. [Google Scholar] [CrossRef]
- Yan, H.; Tian, L.; Wang, Q.; Zhao, Q.; Yue, W.; Yan, J.; Liu, B.; Zhang, D. Compromised small-world efficiency of structural brain networks in schizophrenic patients and their unaffected parents. Neurosci. Bull. 2015, 31, 275–287. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Lee, R.; Bezerianos, A.; Collinson, S.L.; Sim, K. Disruption of brain anatomical networks in schizophrenia: A longitudinal, diffusion tensor imaging based study. Schizophr. Res. 2016, 171, 149–157. [Google Scholar] [CrossRef]
- Hadley, J.A.; Kraguljac, N.V.; White, D.M.; Ver Hoef, L.; Tabora, J.; Lahti, A.C. Change in brain network topology as a function of treatment response in schizophrenia: A longitudinal resting-state fMRI study using graph theory. NPJ Schizophr. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, Z.; Mantini, D.; Xu, Q.; Wang, Z.; Chen, G.; Jiao, Q.; Zang, Y.F.; Lu, G. Relationship between large-scale functional and structural covariance networks in idiopathic generalized epilepsy. Brain Connect. 2013, 3, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, B.A.; Gennatas, E.D.; Zhou, J.; Seeley, W.W. Network-level structural covariance in the developing brain. Proc. Natl. Acad. Sci. USA 2010, 107, 18191–18196. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Gibbon, M.; Spitzer, R.L.; Williams, J.B.W. Structured Clinical Interview for DSM-IV Axis I Disorders; New York State Psychiatric Institute Biometric Research: New York, NY, USA, 1996. [Google Scholar]
- Chapman, L.J.; Chapman, J.P.; Raulin, M.L. Scales for physical and social anhedonia. J. Abnorm. Psychol. 1976, 85, 374–382. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9, 386. [Google Scholar]
- Miller, G.A.; Chapman, J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001, 110, 40–48. [Google Scholar] [CrossRef]
- Schaefer, A.; Kong, R.; Gordon, E.M.; Laumann, T.O.; Zuo, X.; Holmes, A.J.; Eickhoff, S.B.; Yeo, B.T.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 2018, 28, 3095–3114. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Micheloyannis, S.; Pachou, E.; Stam, C.J.; Breakspear, M.; Bitsios, P.; Vourkas, M.; Erimaki, S.; Zervakis, M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr. Res. 2006, 87, 60–66. [Google Scholar] [CrossRef]
- Rubinov, M.; Knock, S.A.; Stam, C.J.; Micheloyannis, S.; Harris, A.W.; Williams, L.M.; Breakspear, M. Small-world properties of nonlinear brain activity in schizophrenia. Hum. Brain Mapp. 2009, 30, 403–416. [Google Scholar] [CrossRef]
- Camchong, J.; MacDonald III, A.W.; Bell, C.; Mueller, B.A.; Lim, K.O. Altered functional and anatomical connectivity in schizophrenia. Schizophr. Bull. 2011, 37, 640–650. [Google Scholar] [CrossRef]
- Hu, M.L.; Zong, X.F.; Mann, J.J.; Zheng, J.J.; Liao, Y.H.; Li, Z.C.; He, Y.; Chen, X.; Tang, J.S. A review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 2017, 33, 73–84. [Google Scholar] [CrossRef]
- Salgado-Pineda, P.; Fakra, E.; Delaveau, P.; McKenna, P.J.; Pomarol-Clotet, E.; Blin, O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr. Res. 2011, 125, 101–109. [Google Scholar] [CrossRef]
- Garrity, A.G.; Pearlson, G.D.; McKiernan, K.; Lloyd, D.; Kiehl, K.A.; Calhoun, V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry 2007, 164, 450–457. [Google Scholar] [CrossRef]
- Dodell-Feder, D.; Tully, L.M.; Lincoln, S.H.; Hooker, C.I. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. Neuroimage Clin. 2014, 4, 154–163. [Google Scholar] [CrossRef]
- Fox, J.M.; Abram, S.V.; Reilly, J.L.; Eack, S.; Goldman, M.B.; Csernansky, J.G.; Lie, W.; Smith, M.J. Default mode functional connectivity is associated with social functioning in schizophrenia. J. Abnorm. Psychol. 2017, 126, 392–405. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.K.; Park, K.; Kim, C.E.; Ryu, S. Default mode network connectivity is associated with long-term clinical outcome in patients with schizophrenia. Neuroimage Clin. 2019, 22, 101805. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, J.J.; Chun, J.; Jung, Y.C.; Seok, J.H.; Park, H.J.; Lee, J.D. Medial prefrontal default-mode hypoactivity affecting trait physical anhedonia in schizophrenia. Psychiatry Res. Neuroimaging 2009, 171, 155–165. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhang, R.T.; Li, Y.; Wang, Y.; Wang, Y.M.; Wang, S.K.; Öngür, D.; Cheung, E.F.C.; Chan, R.C.K. Functional connectivity of the default mode network is associated with prospection in schizophrenia patients and individuals with social anhedonia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 412–420. [Google Scholar] [CrossRef]
- Hare, S.M.; Ford, J.M.; Mathalon, D.H.; Damaraju, E.; Bustillo, J.; Belger, A.; Lee, H.J.; Mueller, B.A.; Lim, K.O.; Brown, G.G.; et al. Salience-default mode functional network connectivity linked to positive and negative symptoms of schizophrenia. Schizophr. Bull. 2019, 45, 892–901. [Google Scholar] [CrossRef]
- Orliac, F.; Naveau, M.; Joliot, M.; Delcroix, N.; Razafimandimby, A.; Brazo, P.; Dollfus, S.; Delamillieure, P. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr. Res. 2013, 148, 74–80. [Google Scholar] [CrossRef]
- Gradin, V.B.; Waiter, G.; O’Connor, A.; Romaniuk, L.; Stickle, C.; Matthews, K.; Hall, J.; Steele, J.D. Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. Neuroimaging 2013, 211, 104–111. [Google Scholar] [CrossRef]
- Kim, B.-H.; Shin, Y.B.; Kyeong, S.; Lee, S.K.; Kim, J.-J. Disrupted salience processing involved in motivational deficits for real-life activities in patients with schizophrenia. Schizophr. Res. 2018, 197, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bodapati, A.S.; Jenkins, L.M.; Sharma, R.P.; Rosen, C. Visual memory uniquely predicts anhedonia in schizophrenia but not bipolar disorder. J. Neuropsychol. 2019, 13, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Baetens, K.; Mariën, P.; Vandekerckhove, M. Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. Neuroimage 2014, 86, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Hoche, F.; Guell, X.; Sherman, J.C.; Vangel, M.G.; Schmahmann, J.D. Cerebellar contribution to social cognition. Cerebellum 2016, 15, 732–743. [Google Scholar] [CrossRef]
- Schmahmann, J.D. An emerging concept: The cerebellar contribution to higher function. Arch. Neurol. 1991, 48, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn. Sci. 1998, 2, 362–371. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Pierson, R. The role of the cerebellum in schizophrenia. Bio. Psychiatry 2008, 64, 81–88. [Google Scholar] [CrossRef]
- Picard, H.; Amado, I.; Mouchet-Mages, S.; Olié, J.P.; Krebs, M.O. The role of the cerebellum in schizophrenia: An update of clinical, cognitive, and functional evidences. Schizophr. Bull. 2008, 34, 155–172. [Google Scholar] [CrossRef]
- Lungu, O.; Barakat, M.; Laventure, S.; Debas, K.; Proulx, S.; Luck, D.; Stip, E. The incidence and nature of cerebellar findings in schizophrenia: A quantitative review of fMRI literature. Schizophr. Bull. 2013, 39, 797–806. [Google Scholar] [CrossRef]
- Brady, R.O., Jr.; Gonsalvez, I.; Lee, I.; Öngür, D.; Seidman, L.J.; Schmahmann, J.D.; Eack, S.M.; Keshavan, M.S.; Pascual-Leone, A.; Halko, M.A. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am. J. Psychiatry 2019, 176, 512–520. [Google Scholar] [CrossRef]
- Wang, L.; Zou, F.; Shao, Y.; Ye, E.; Jin, X.; Tan, S.; Hu, D.; Yang, Z. Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr. Res. 2014, 160, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kring, A.M.; Barch, D.M. The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. Eur. Neuropsychopharmacol. 2014, 24, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Kring, A.M.; Gur, R.E.; Blanchard, J.J.; Horan, W.P.; Reise, S.P. The clinical assessment interview for negative symptoms (CAINS): Final development and validation. Am. J. Psychiatry 2013, 170, 165–172. [Google Scholar] [CrossRef]
- Keedwell, P.A.; Andrew, C.; Williams, S.C.R.; Brammer, M.J.; Phillips, M.L. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry 2005, 58, 843–853. [Google Scholar] [CrossRef] [PubMed]
| Schizophrenia (n = 121) | Control (n = 160) | t | df | p | |
|---|---|---|---|---|---|
| Age, years | 34.5 ± 8.8 | 33.4 ± 6.8 | 1.06 | 224.18 | 0.291 |
| Education, years | 13.3 ± 2.2 | 16.2 ± 2.8 | −9.47 | 273.69 | <0.001 |
| Sex, female/male | 55/68 | 73/87 | 0.001 | - | 0.975 |
| Grey matter volume, cm3 | 585.8 ± 60.5 | 600.0 ± 59.3 | −1.97 | 259.92 | 0.049 |
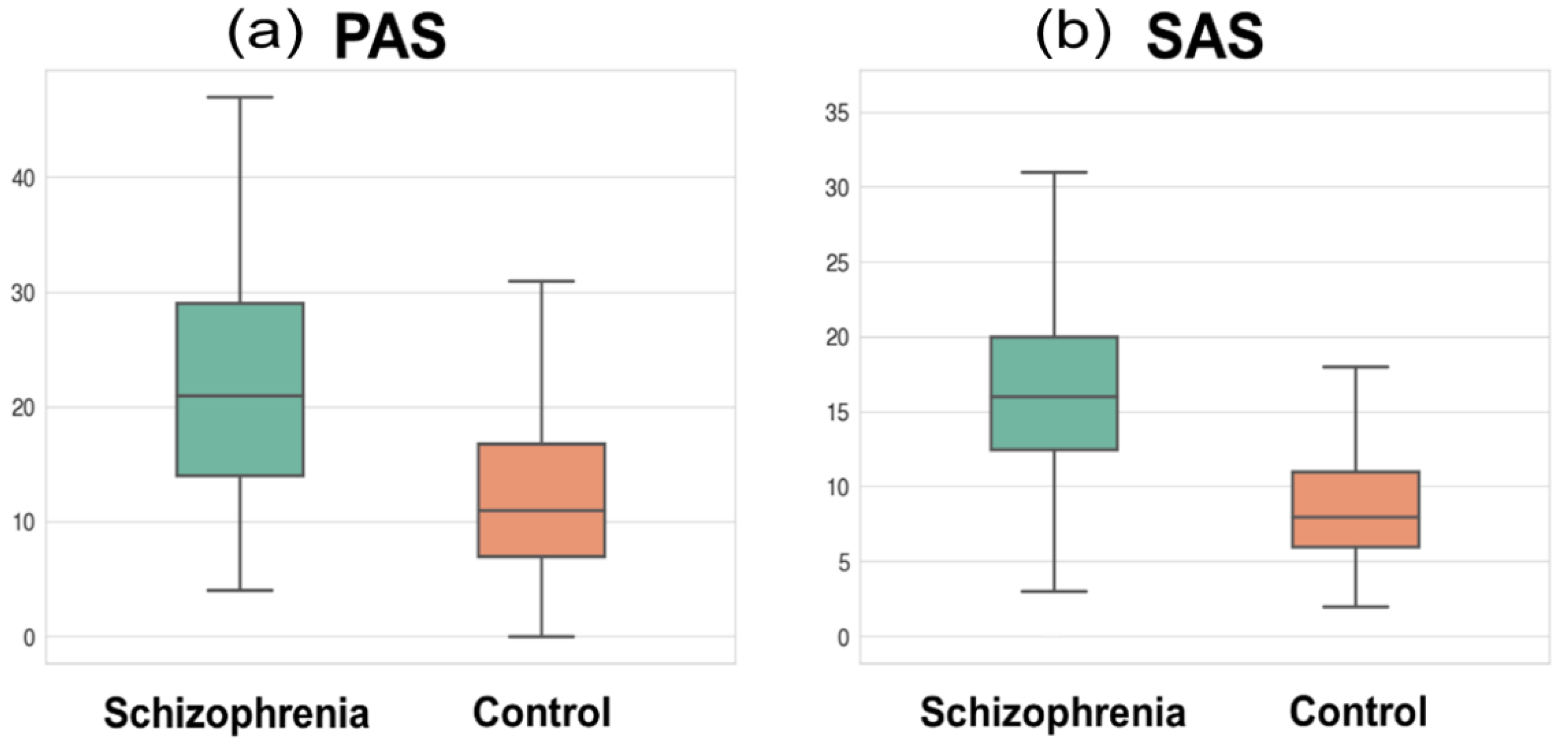
| Physical Anhedonia Scale score | 21.5 ± 9.6 | 12.4 ± 7.5 | 8.66 | 227.02 | <0.001 |
| Social Anhedonia Scale score | 16.4 ± 6.9 | 8.9 ± 4.4 | 8.44 | 120.37 | <0.001 |
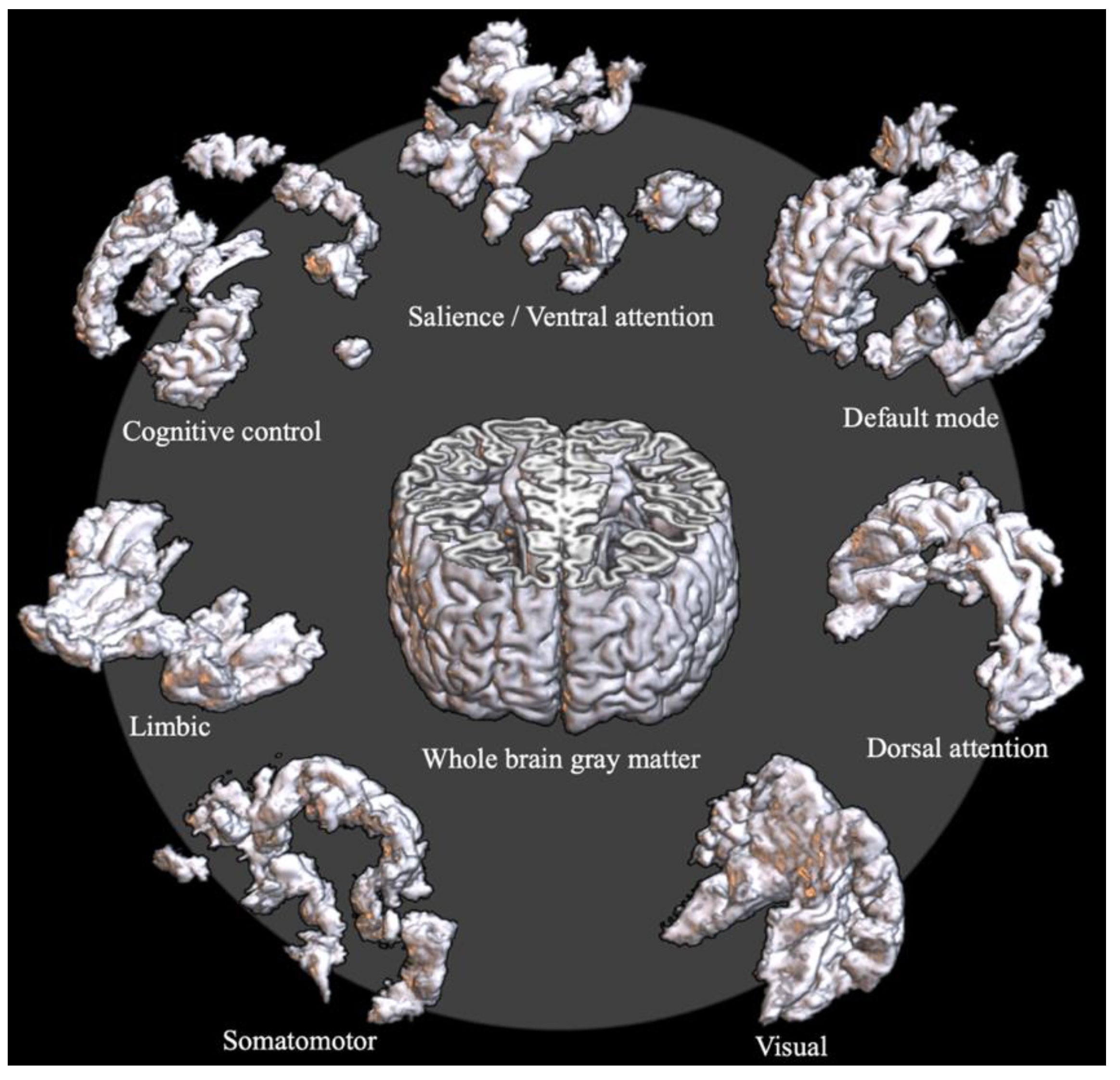
| Intrinsic Connectivity Network | σ | F | p-unc | p-FDR | |
|---|---|---|---|---|---|
| Schizophrenia (n = 121) | Control (n = 160) | ||||
| Default mode network | 1.298 ± 0.038 | 1.316 ± 0.044 | 11.03 | 0.001 | 0.002 |
| Cognitive control network | 1.227 ± 0.042 | 1.234 ± 0.037 | 2.57 | 0.110 | 0.128 |
| Salience/ventral attention network | 1.284 ± 0.050 | 1.312 ± 0.049 | 20.38 | <0.001 | <0.001 |
| Dorsal attention network | 1.207 ± 0.045 | 1.216 ± 0.043 | 3.04 | 0.083 | 0.116 |
| Limbic network | 1.326 ± 0.051 | 1.339 ± 0.050 | 3.41 | 0.066 | 0.116 |
| Somatomotor network | 1.294 ± 0.044 | 1.299 ± 0.042 | 0.89 | 0.346 | 0.346 |
| Visual network | 1.295 ± 0.053 | 1.320 ± 0.047 | 15.10 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-H.; Kim, H.E.; Lee, J.S.; Kim, J.-J. Anhedonia Relates to the Altered Global and Local Grey Matter Network Properties in Schizophrenia. J. Clin. Med. 2021, 10, 1395. https://doi.org/10.3390/jcm10071395
Kim B-H, Kim HE, Lee JS, Kim J-J. Anhedonia Relates to the Altered Global and Local Grey Matter Network Properties in Schizophrenia. Journal of Clinical Medicine. 2021; 10(7):1395. https://doi.org/10.3390/jcm10071395
Chicago/Turabian StyleKim, Byung-Hoon, Hesun Erin Kim, Jung Suk Lee, and Jae-Jin Kim. 2021. "Anhedonia Relates to the Altered Global and Local Grey Matter Network Properties in Schizophrenia" Journal of Clinical Medicine 10, no. 7: 1395. https://doi.org/10.3390/jcm10071395
APA StyleKim, B.-H., Kim, H. E., Lee, J. S., & Kim, J.-J. (2021). Anhedonia Relates to the Altered Global and Local Grey Matter Network Properties in Schizophrenia. Journal of Clinical Medicine, 10(7), 1395. https://doi.org/10.3390/jcm10071395







