Recent Progress in Oculopharyngeal Muscular Dystrophy
Abstract
1. Introduction
2. Epidemiology
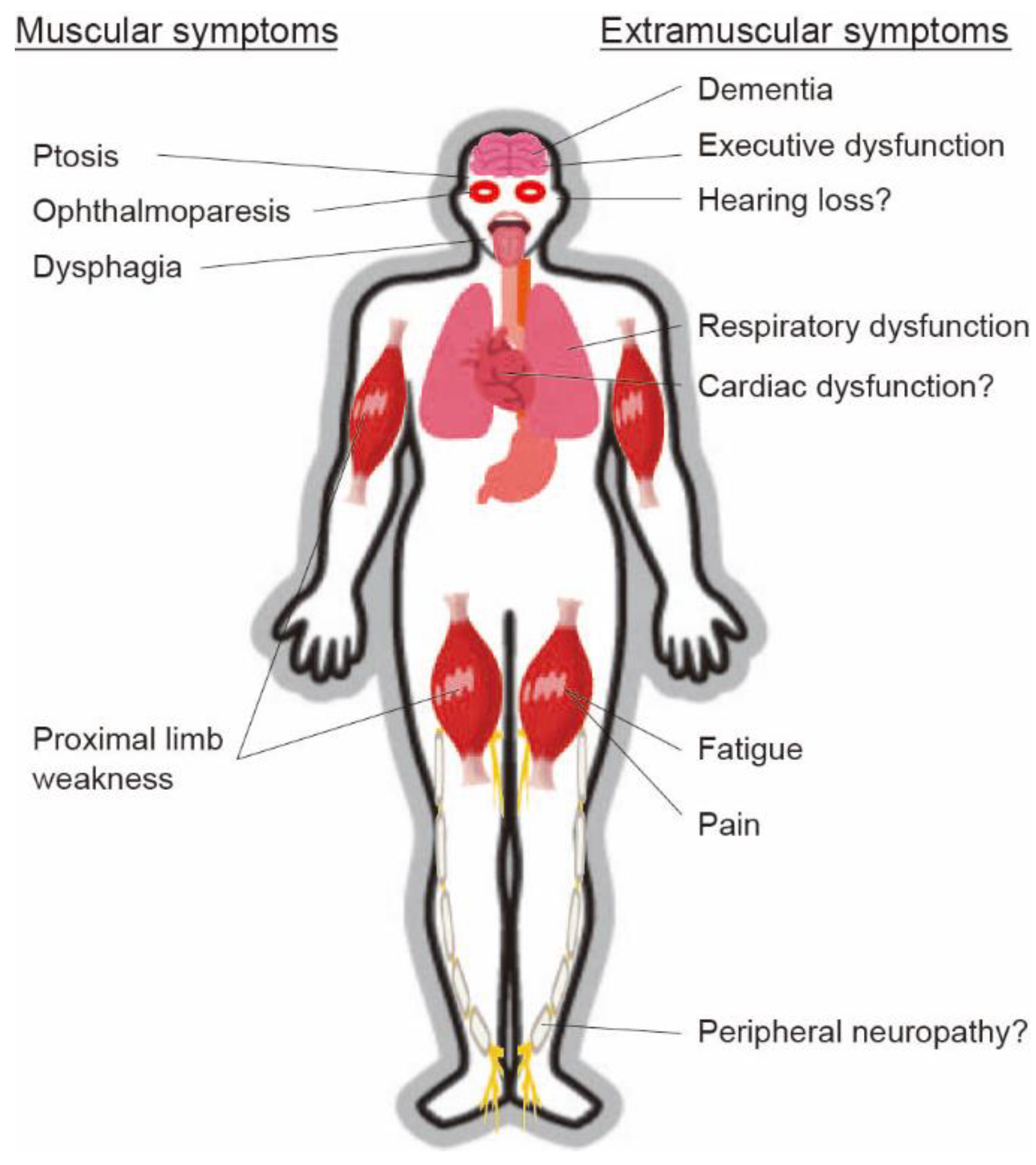
3. Presentation
4. Diagnosis
4.1. Blood Examination
4.2. Electrophysiology
4.3. Histology
4.4. Radiology
4.5. Swallowing Examination
4.6. Genetic Examination
4.7. Diagnosis
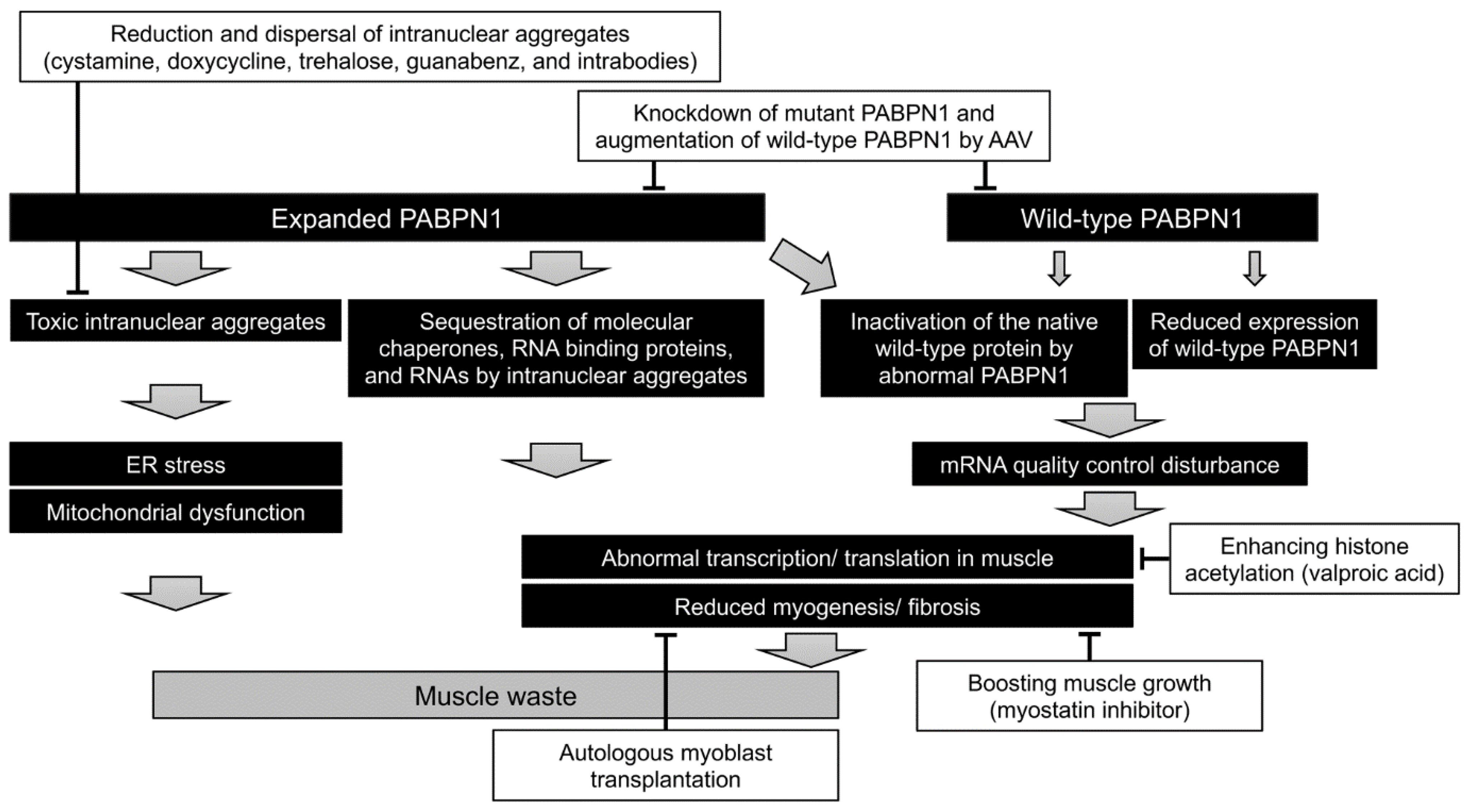
5. Pathogenesis and Therapeutic Approach
5.1. Knockdown of Mutant PABPN1 and/or Augmentation of Wild-Type PABPN1
5.2. Boosting of Muscle Growth
5.3. Reduction and Dispersal of Intranuclear Aggregates
5.4. Autologous Myoblast Transplantation
5.5. Mitochondrial Restoration
6. Current Treatment and Prognosis
7. Patient Registry
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brais, B.; Bouchard, J.P.; Xie, Y.G.; Rochefort, D.L.; Chretien, N.; Tome, F.M.; Lafreniere, R.G.; Rommens, J.M.; Uyama, E.; Nohira, O.; et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat. Genet. 1998, 18, 164–167. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=Oculopharyngeal+Muscular+Dystrophy&term=&cntry=&state=&city=&dist= (accessed on 23 February 2021).
- Blumen, S.C.; Nisipeanu, P.; Sadeh, M.; Asherov, A.; Tome, F.M.; Korczyn, A.D. Clinical features of oculopharyngeal muscular dystrophy among Bukhara Jews. Neuromuscul. Disord. 1993, 3, 575–577. [Google Scholar] [CrossRef]
- Bouchard, J.P. Andre Barbeau and the oculopharyngeal muscular dystrophy in French Canada and North America. Neuromuscul. Disord. 1997, 7 (Suppl. 1), S5–S11. [Google Scholar] [CrossRef]
- Becher, M.W.; Morrison, L.; Davis, L.E.; Maki, W.C.; King, M.K.; Bicknell, J.M.; Reinert, B.L.; Bartolo, C.; Bear, D.G. Oculopharyngeal muscular dystrophy in Hispanic New Mexicans. JAMA 2001, 286, 2437–2440. [Google Scholar] [CrossRef] [PubMed]
- Brais, B.; Rouleau, G.A.; Bouchard, J.P.; Fardeau, M.; Tome, F.M. Oculopharyngeal muscular dystrophy. Semin. Neurol. 1999, 19, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Deenen, J.C.; Horlings, C.G.; Verschuuren, J.J.; Verbeek, A.L.; van Engelen, B.G. The Epidemiology of Neuromuscular Disorders: A Comprehensive Overview of the Literature. J. Neuromuscul. Dis. 2015, 2, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Meola, G.; Sansone, V.; Rotondo, G.; Tome, F.M.; Bouchard, J.P. Oculopharyngeal muscular dystrophy in Italy. Neuromuscul. Disord. 1997, 7 (Suppl. 1), S53–S56. [Google Scholar] [CrossRef]
- Perie, S.; Eymard, B.; Laccourreye, L.; Chaussade, S.; Fardeau, M.; Lacau St Guily, J. Dysphagia in oculopharyngeal muscular dystrophy: A series of 22 French cases. Neuromuscul. Disord. 1997, 7 (Suppl. 1), S96–S99. [Google Scholar] [CrossRef]
- Porschke, H.; Kress, W.; Reichmann, H.; Goebel, H.H.; Grimm, T. Oculopharyngeal muscular dystrophy in a northern German family linked to chromosome 14q, and presenting carnitine deficiency. Neuromuscul. Disord. 1997, 7 (Suppl. 1), S57–S62. [Google Scholar] [CrossRef]
- Medici, M.; Pizzarossa, C.; Skuk, D.; Yorio, D.; Emmanuelli, G.; Mesa, R. Oculopharyngeal muscular dystrophy in Uruguay. Neuromuscul. Disord. 1997, 7 (Suppl. 1), S50–S52. [Google Scholar] [CrossRef]
- Hill, M.E.; Creed, G.A.; McMullan, T.F.; Tyers, A.G.; Hilton-Jones, D.; Robinson, D.O.; Hammans, S.R. Oculopharyngeal muscular dystrophy: Phenotypic and genotypic studies in a UK population. Brain 2001, 124, 522–526. [Google Scholar] [CrossRef]
- Van Der Sluijs, B.M.; Hoefsloot, L.H.; Padberg, G.W.; Van Der Maarel, S.M.; Van Engelen, B.G. Oculopharyngeal muscular dystrophy with limb girdle weakness as major complaint. J. Neurol. 2003, 250, 1307–1312. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Mansfield, D.C.; Mechan, D.; Al-Shahi Salman, R.; Davenport, R.J.; Connor, M.; Metcalfe, R.; Petty, R. Delayed diagnosis of oculopharyngeal muscular dystrophy in Scotland. Br. J. Ophthalmol. 2012, 96, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Tondo, M.; Gamez, J.; Gutierrez-Rivas, E.; Medel-Jimenez, R.; Martorell, L. Genotype and phenotype study of 34 Spanish patients diagnosed with oculopharyngeal muscular dystrophy. J. Neurol. 2012, 259, 1546–1552. [Google Scholar] [CrossRef]
- Mensah, A.; Witting, N.; Duno, M.; Milea, D.; Vissing, J. Delayed diagnosis of oculopharyngeal muscular dystrophy in Denmark: From initial ptosis to genetic testing. Acta Ophthalmol. 2014, 92, e247–e249. [Google Scholar] [CrossRef] [PubMed]
- Blumen, S.C.; Kesler, A.; Dabby, R.; Shalev, S.; Khayat, M.; Almog, Y.; Zoldan, J.; Benninger, F.; Drory, V.E.; Gurevich, M.; et al. Oculopharyngeal muscular dystrophy among Bulgarian Jews: A new cluster? Isr. Med. Assoc. J. 2013, 15, 748–752. [Google Scholar] [PubMed]
- Chien, Y.Y. Oculopharyngeal muscular dystrophy—An under-diagnosed disease in China? Report a China-born Chinese with PABPN1 mutation and epidemiology review of the literature. J. Formos. Med. Assoc. 2012, 111, 397–402. [Google Scholar] [CrossRef]
- Shan, J.; Chen, B.; Lin, P.; Li, D.; Luo, Y.; Ji, K.; Zheng, J.; Yuan, Y.; Yan, C. Oculopharyngeal muscular dystrophy: Phenotypic and genotypic studies in a Chinese population. Neuromolecular. Med. 2014, 16, 782–786. [Google Scholar] [CrossRef]
- Huang, C.L.; Wu, S.L.; Lai, S.C.; Lu, C.S.; Wu-Chou, Y.H. Oculopharyngeal muscular dystrophy—A genetically verified taiwanese family. Chang Gung Med. J. 2010, 33, 44–50. [Google Scholar]
- Luk, H.M.; Lo, I.F.; Fu, K.H.; Lui, C.H.; Tong, T.M.; Chan, D.H.; Lam, S.T. Oculopharyngeal muscular dystrophy: Underdiagnosed disease in Hong Kong. Hong Kong Med. J. 2013, 19, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Ki, C.S.; Kim, J.W.; Kim, B.J. Identification of a novel mutation in a Korean patient with oculopharyngeal muscular dystrophy. J. Clin. Neurosci. 2007, 14, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Pulkes, T.; Papsing, C.; Busabaratana, M.; Dejthevaporn, C.; Witoonpanich, R. Mutation and haplotype analysis of oculopharyngeal muscular dystrophy in Thai patients. J. Clin. Neurosci. 2011, 18, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.J.; Wong, K.T.; Nishino, I.; Minami, N.; Nonaka, I. Oculopharyngeal muscular dystrophy with PABPN1 mutation in a Chinese Malaysian woman. Neuromuscul. Disord. 2005, 15, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Tan, N.C.; Chai, J. Oculopharyngeal Muscular Dystrophy in Singapore: Not So Rare. Ann. Acad. Med. Singap. 2018, 47, 349–352. [Google Scholar] [PubMed]
- Uyama, E.; Nohira, O.; Chateau, D.; Tokunaga, M.; Uchino, M.; Okabe, T.; Ando, M.; Tome, F.M. Oculopharyngeal muscular dystrophy in two unrelated Japanese families. Neurology 1996, 46, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Uyama, E.; Nohira, O.; Tome, F.M.; Chateau, D.; Tokunaga, M.; Ando, M.; Maki, M.; Okabe, T.; Uchino, M. Oculopharyngeal muscular dystrophy in Japan. Neuromuscul. Disord. 1997, 7 (Suppl. 1), S41–S49. [Google Scholar] [CrossRef]
- Nagashima, T.; Kato, H.; Kase, M.; Maguchi, S.; Mizutani, Y.; Matsuda, K.; Chuma, T.; Mano, Y.; Goto, Y.; Minami, N.; et al. Oculopharyngeal muscular dystrophy in a Japanese family with a short GCG expansion (GCG)(11) in PABP2 gene. Neuromuscul. Disord. 2000, 10, 173–177. [Google Scholar] [CrossRef]
- Richard, P.; Trollet, C.; Stojkovic, T.; de Becdelievre, A.; Perie, S.; Pouget, J.; Eymard, B. Neurologists of French Neuromuscular Reference Centers. Correlation between PABPN1 genotype and disease severity in oculopharyngeal muscular dystrophy. Neurology 2017, 88, 359–365. [Google Scholar] [CrossRef]
- Witting, N.; Mensah, A.; Kober, L.; Bundgaard, H.; Petri, H.; Duno, M.; Milea, D.; Vissing, J. Ocular, bulbar, limb, and cardiopulmonary involvement in oculopharyngeal muscular dystrophy. Acta Neurol. Scand. 2014, 130, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, Y.; Yamamoto, T.; Minami, N.; Ohkuma, A.; Nonaka, I.; Nishino, I.; Tamura, N.; Amano, T.; Araki, N. Oculopharyngeal muscular dystrophy associated with dementia. Intern. Med. 2011, 50, 2409–2412. [Google Scholar] [CrossRef]
- Nisbet, M.K.; Marshall, L. Oculopharyngeal muscular dystrophy (OPMD) and dementia in a 75-year-old female. BMJ Case Rep. 2019, 12. [Google Scholar] [CrossRef]
- Dubbioso, R.; Moretta, P.; Manganelli, F.; Fiorillo, C.; Iodice, R.; Trojano, L.; Santoro, L. Executive functions are impaired in heterozygote patients with oculopharyngeal muscular dystrophy. J. Neurol. 2012, 259, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Boukriche, Y.; Maisonobe, T.; Masson, C. Neurogenic involvement in a case of oculopharyngeal muscular dystrophy. Muscle Nerve 2001, 25, 98–101. [Google Scholar] [CrossRef]
- Van der Sluijs, B.M.; Knoop, H.; Bleijenberg, G.; van Engelen, B.G.; Voermans, N.C. The Dutch patients’ perspective on oculopharyngeal muscular dystrophy: A questionnaire study on fatigue, pain and impairments. Neuromuscul. Disord. 2016, 26, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Kress, W.; Vielhaber, S.; Schroder, R.; Kornblum, C. Variability of the recessive oculopharyngeal muscular dystrophy phenotype. Muscle Nerve 2007, 35, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.K., Jr.; Harper, C.M. Clinical and electrophysiologic features of oculopharyngeal muscular dystrophy: Lack of evidence for an associated peripheral neuropathy. Clin. Neurophysiol. 2010, 121, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Luigetti, M.; Lo Monaco, M.; Mirabella, M.; Primiano, G.; Lucchini, M.; Monforte, M.; Servidei, S. Oculopharyngeal muscular dystrophy: Clinical and neurophysiological features. Clin. Neurophysiol. 2015, 126, 2406–2408. [Google Scholar] [CrossRef]
- Tome, F.M.; Chateau, D.; Helbling-Leclerc, A.; Fardeau, M. Morphological changes in muscle fibers in oculopharyngeal muscular dystrophy. Neuromuscul. Disord. 1997, 7 (Suppl. 1), S63–S69. [Google Scholar] [CrossRef]
- Pratt, M.F.; Meyers, P.K. Oculopharyngeal muscular dystrophy: Recent ultrastructural evidence for mitochondrial abnormalities. Laryngoscope 1986, 96, 368–373. [Google Scholar] [CrossRef]
- Pauzner, R.; Blatt, I.; Mouallem, M.; Ben-David, E.; Farfel, Z.; Sadeh, M. Mitochondrial abnormalities in oculopharyngeal muscular dystrophy. Muscle Nerve 1991, 14, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Gambelli, S.; Malandrini, A.; Ginanneschi, F.; Berti, G.; Cardaioli, E.; De Stefano, R.; Franci, M.; Salvadori, C.; Mari, F.; Bruttini, M.; et al. Mitochondrial abnormalities in genetically assessed oculopharyngeal muscular dystrophy. Eur. Neurol. 2004, 51, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Tironi, R.; Lerario, A.; Scali, M.; Del Bo, R.; Rodolico, C.; Brizzi, T.; Gibertini, S.; Maggi, L.; Mora, M.; et al. Value of insoluble PABPN1 accumulation in the diagnosis of oculopharyngeal muscular dystrophy. Eur. J. Neurol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fischmann, A.; Gloor, M.; Fasler, S.; Haas, T.; Rodoni Wetzel, R.; Bieri, O.; Wetzel, S.; Heinimann, K.; Scheffler, K.; Fischer, D. Muscular involvement assessed by MRI correlates to motor function measurement values in oculopharyngeal muscular dystrophy. J. Neurol. 2011, 258, 1333–1340. [Google Scholar] [CrossRef]
- King, M.K.; Lee, R.R.; Davis, L.E. Magnetic resonance imaging and computed tomography of skeletal muscles in oculopharyngeal muscular dystrophy. J. Clin. Neuromuscul. Dis. 2005, 6, 103–108. [Google Scholar] [CrossRef]
- Alonso-Jimenez, A.; Kroon, R.; Alejaldre-Monforte, A.; Nunez-Peralta, C.; Horlings, C.G.C.; van Engelen, B.G.M.; Olive, M.; Gonzalez, L.; Verges-Gil, E.; Paradas, C.; et al. Muscle MRI in a large cohort of patients with oculopharyngeal muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 2019, 90, 576–585. [Google Scholar] [CrossRef]
- Castell, J.A.; Castell, D.O.; Duranceau, C.A.; Topart, P. Manometric characteristics of the pharynx, upper esophageal sphincter, esophagus, and lower esophageal sphincter in patients with oculopharyngeal muscular dystrophy. Dysphagia 1995, 10, 22–26. [Google Scholar] [CrossRef]
- Waito, A.A.; Steele, C.M.; Peladeau-Pigeon, M.; Genge, A.; Argov, Z. A Preliminary Videofluoroscopic Investigation of Swallowing Physiology and Function in Individuals with Oculopharyngeal Muscular Dystrophy (OPMD). Dysphagia 2018, 33, 789–802. [Google Scholar] [CrossRef]
- Tabor, L.C.; Plowman, E.K.; Romero-Clark, C.; Youssof, S. Oropharyngeal dysphagia profiles in individuals with oculopharyngeal muscular dystrophy. Neurogastroenterol. Motil. 2018, 30, e13251. [Google Scholar] [CrossRef] [PubMed]
- Abu-Baker, A.; Rouleau, G.A. Oculopharyngeal muscular dystrophy: Recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochim. Biophys. Acta 2007, 1772, 173–185. [Google Scholar] [CrossRef]
- Banerjee, A.; Apponi, L.H.; Pavlath, G.K.; Corbett, A.H. PABPN1: Molecular function and muscle disease. FEBS J. 2013, 280, 4230–4250. [Google Scholar] [CrossRef]
- Malerba, A.; Klein, P.; Bachtarzi, H.; Jarmin, S.A.; Cordova, G.; Ferry, A.; Strings, V.; Espinoza, M.P.; Mamchaoui, K.; Blumen, S.C.; et al. PABPN1 gene therapy for oculopharyngeal muscular dystrophy. Nat. Commun. 2017, 8, 14848. [Google Scholar] [CrossRef]
- Malerba, A.; Klein, P.; Lu-Nguyen, N.; Cappellari, O.; Strings-Ufombah, V.; Harbaran, S.; Roelvink, P.; Suhy, D.; Trollet, C.; Dickson, G. Established PABPN1 intranuclear inclusions in OPMD muscle can be efficiently reversed by AAV-mediated knockdown and replacement of mutant expanded PABPN1. Hum. Mol. Genet. 2019, 28, 3301–3308. [Google Scholar] [CrossRef] [PubMed]
- Harish, P.; Malerba, A.; Lu-Nguyen, N.; Forrest, L.; Cappellari, O.; Roth, F.; Trollet, C.; Popplewell, L.; Dickson, G. Inhibition of myostatin improves muscle atrophy in oculopharyngeal muscular dystrophy (OPMD). J. Cachexia Sarcopenia Muscle 2019, 10, 1016–1026. [Google Scholar] [CrossRef]
- Harish, P.; Forrest, L.; Herath, S.; Dickson, G.; Malerba, A.; Popplewell, L. Inhibition of Myostatin Reduces Collagen Deposition in a Mouse Model of Oculopharyngeal Muscular Dystrophy (OPMD) with Established Disease. Front. Physiol. 2020, 11, 184. [Google Scholar] [CrossRef]
- Davies, J.E.; Rose, C.; Sarkar, S.; Rubinsztein, D.C. Cystamine suppresses polyalanine toxicity in a mouse model of oculopharyngeal muscular dystrophy. Sci. Transl. Med. 2010, 2, 34–40. [Google Scholar] [CrossRef]
- Davies, J.E.; Wang, L.; Garcia-Oroz, L.; Cook, L.J.; Vacher, C.; O’Donovan, D.G.; Rubinsztein, D.C. Doxycycline attenuates and delays toxicity of the oculopharyngeal muscular dystrophy mutation in transgenic mice. Nat. Med. 2005, 11, 672–677. [Google Scholar] [CrossRef]
- Davies, J.E.; Sarkar, S.; Rubinsztein, D.C. Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 2006, 15, 23–31. [Google Scholar] [CrossRef]
- Malerba, A.; Roth, F.; Harish, P.; Dhiab, J.; Lu-Nguyen, N.; Cappellari, O.; Jarmin, S.; Mahoudeau, A.; Ythier, V.; Laine, J.; et al. Pharmacological modulation of the ER stress response ameliorates oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 2019, 28, 1694–1708. [Google Scholar] [CrossRef]
- Chartier, A.; Raz, V.; Sterrenburg, E.; Verrips, C.T.; van der Maarel, S.M.; Simonelig, M. Prevention of oculopharyngeal muscular dystrophy by muscular expression of Llama single-chain intrabodies in vivo. Hum. Mol. Genet. 2009, 18, 1849–1859. [Google Scholar] [CrossRef]
- Messaed, C.; Dion, P.A.; Abu-Baker, A.; Rochefort, D.; Laganiere, J.; Brais, B.; Rouleau, G.A. Soluble expanded PABPN1 promotes cell death in oculopharyngeal muscular dystrophy. Neurobiol. Dis. 2007, 26, 546–557. [Google Scholar] [CrossRef]
- Kim, Y.J.; Noguchi, S.; Hayashi, Y.K.; Tsukahara, T.; Shimizu, T.; Arahata, K. The product of an oculopharyngeal muscular dystrophy gene, poly(A)-binding protein 2, interacts with SKIP and stimulates muscle-specific gene expression. Hum. Mol. Genet. 2001, 10, 1129–1139. [Google Scholar] [CrossRef]
- Abu-Baker, A.; Parker, A.; Ramalingam, S.; Laganiere, J.; Brais, B.; Neri, C.; Dion, P.; Rouleau, G. Valproic acid is protective in cellular and worm models of oculopharyngeal muscular dystrophy. Neurology 2018, 91, e551–e561. [Google Scholar] [CrossRef] [PubMed]
- Perie, S.; Mamchaoui, K.; Mouly, V.; Blot, S.; Bouazza, B.; Thornell, L.E.; St Guily, J.L.; Butler-Browne, G. Premature proliferative arrest of cricopharyngeal myoblasts in oculo-pharyngeal muscular dystrophy: Therapeutic perspectives of autologous myoblast transplantation. Neuromuscul. Disord. 2006, 16, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Perie, S.; Trollet, C.; Mouly, V.; Vanneaux, V.; Mamchaoui, K.; Bouazza, B.; Marolleau, J.P.; Laforet, P.; Chapon, F.; Eymard, B.; et al. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: A phase I/IIa clinical study. Mol. Ther. 2014, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Vest, K.E.; Phillips, B.L.; Banerjee, A.; Apponi, L.H.; Dammer, E.B.; Xu, W.; Zheng, D.; Yu, J.; Tian, B.; Pavlath, G.K.; et al. Novel mouse models of oculopharyngeal muscular dystrophy (OPMD) reveal early onset mitochondrial defects and suggest loss of PABPN1 may contribute to pathology. Hum. Mol. Genet. 2017, 26, 3235–3252. [Google Scholar] [CrossRef] [PubMed]
- Doki, T.; Yamashita, S.; Wei, F.Y.; Hara, K.; Yamamoto, T.; Zhang, Z.; Zhang, X.; Tawara, N.; Hino, H.; Uyama, E.; et al. Mitochondrial localization of PABPN1 in oculopharyngeal muscular dystrophy. Lab. Invest. 2019, 99, 1728–1740. [Google Scholar] [CrossRef]
- Little, B.W.; Perl, D.P. Oculopharyngeal muscular dystrophy. An autopsied case from the French-Canadian kindred. J. Neurol. Sci. 1982, 53, 145–158. [Google Scholar] [CrossRef]
- Youssof, S. The relationship between physical symptoms and health-related quality of life in oculopharyngeal muscular dystrophy. Muscle Nerve 2016, 53, 694–699. [Google Scholar] [CrossRef]
- Youssof, S.; Romero-Clark, C.; Warner, T.; Plowman, E. Dysphagia-related quality of life in oculopharyngeal muscular dystrophy: Psychometric properties of the SWAL-QOL instrument. Muscle Nerve 2017, 56, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Youssof, S.; Schrader, R.M.; Romero-Clark, C.; Roy, G.; Spafford, M. Safety of botulinum toxin for dysphagia in oculopharyngeal muscular dystrophy. Muscle Nerve 2014, 49, 601–603. [Google Scholar] [CrossRef][Green Version]
- Molgat, Y.M.; Rodrigue, D. Correction of blepharoptosis in oculopharyngeal muscular dystrophy: Review of 91 cases. Can. J. Ophthalmol. 1993, 28, 11–14. [Google Scholar] [PubMed]
- Gliklich, R.; Dreyer, N. Registries for Evaluating Patient Outcomes: A User’s Guide. In Agency for Healthcare Research and Quality; AHRQ Publication: Rockville, MD, USA, 2010; Volume 10. [Google Scholar]
- Nakamura, H.; Kimura, E.; Mori-Yoshimura, M.; Komaki, H.; Matsuda, Y.; Goto, K.; Hayashi, Y.K.; Nishino, I.; Takeda, S.; Kawai, M. Characteristics of Japanese Duchenne and Becker muscular dystrophy patients in a novel Japanese national registry of muscular dystrophy (Remudy). Orphanet J. Rare. Dis. 2013, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Daneshvari, S.; Youssof, S.; Kroth, P.J. The NIH Office of Rare Diseases Research patient registry Standard: A report from the University of New Mexico’s Oculopharyngeal Muscular Dystrophy Patient Registry. AMIA Annu. Symp. Proc. 2013, 2013, 269–277. [Google Scholar] [PubMed]
| Basic Items | Name |
|---|---|
| Date of birth | |
| Nationality | |
| Address | |
| Participation in patients’ association | |
| Proposal for clinical trials | |
| Clinical items | Presence or absence of family history |
| Consanguinity | |
| Muscle histology (rimmed vacuoles, intranuclear aggregates) | |
| (GCN)n repeat-length | |
| Height and weight | |
| Age at onset | |
| Initial symptoms | |
| Age at ptosis | |
| Age at diplopia | |
| Age at dysarthria | |
| Age at dysphagia | |
| Age at diet restrictions/alterations | |
| Age at tube feeding | |
| Age at lower proximal weakness | |
| Age at gait disorder | |
| Age at upper proximal weakness | |
| Any other complications including neuropathy | |
| Respiratory (% vital capacity and % forced vital capacity) | |
| Cardiac functions (% ejection fraction and % fractional shortening) | |
| Electrocardiography | |
| Serum CK levels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, S. Recent Progress in Oculopharyngeal Muscular Dystrophy. J. Clin. Med. 2021, 10, 1375. https://doi.org/10.3390/jcm10071375
Yamashita S. Recent Progress in Oculopharyngeal Muscular Dystrophy. Journal of Clinical Medicine. 2021; 10(7):1375. https://doi.org/10.3390/jcm10071375
Chicago/Turabian StyleYamashita, Satoshi. 2021. "Recent Progress in Oculopharyngeal Muscular Dystrophy" Journal of Clinical Medicine 10, no. 7: 1375. https://doi.org/10.3390/jcm10071375
APA StyleYamashita, S. (2021). Recent Progress in Oculopharyngeal Muscular Dystrophy. Journal of Clinical Medicine, 10(7), 1375. https://doi.org/10.3390/jcm10071375






