High Incidence of Adverse Outcomes in Haemodialysis Patients with Diabetes with or without Diabetic Foot Syndrome: A 5-Year Observational Study in Lleida, Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Studied Variables
2.3. Statistical Analysis
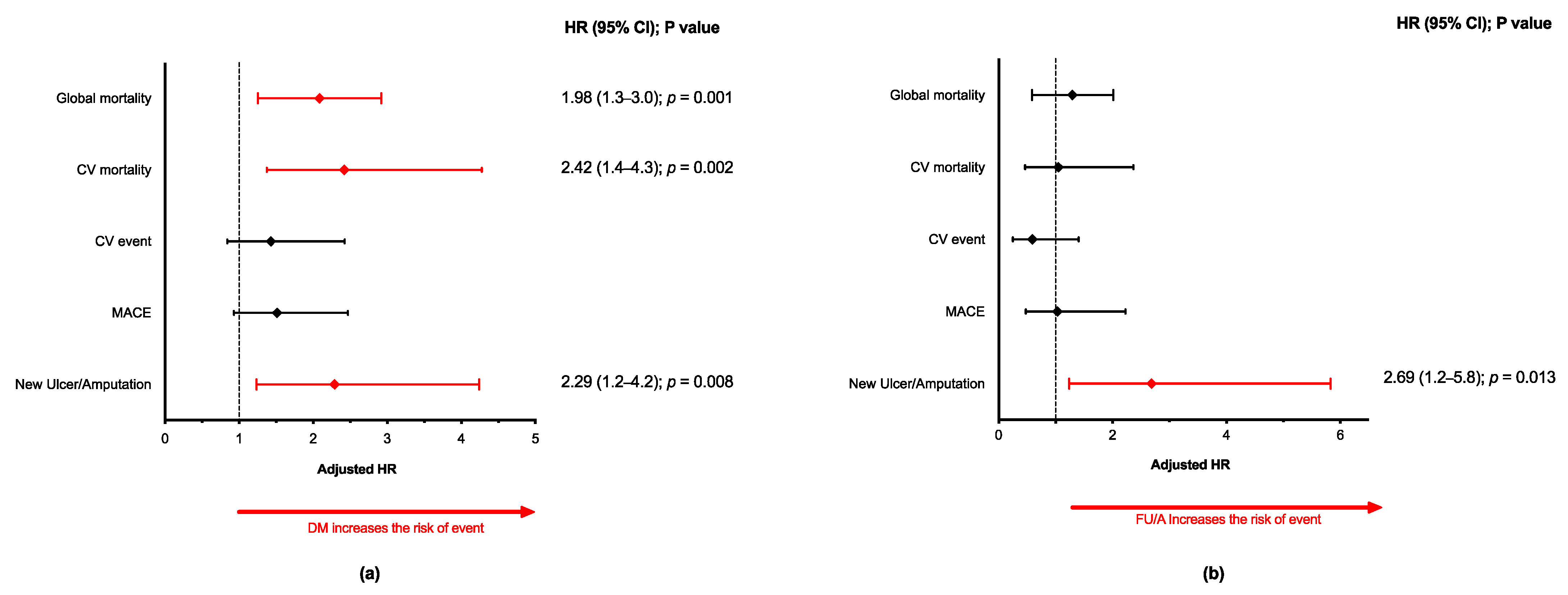
3. Results
3.1. Outcomes at 5-Years
3.2. Predictors of All-Cause Mortality and CV Mortality
3.2.1. Overall Dialysis Population
3.2.2. DM Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef]
- Ghaderian, S.B.; Hayati, F.; Shayanpour, S.; Beladi Mousavi, S.S. Diabetes and end-stage renal disease; a review article on new concepts. J Ren. Inj. Prev. 2015, 4, 28–33. [Google Scholar] [PubMed]
- Al-Thani, H.; El-Menyar, A.; Koshy, V.; Hussein, A.; Sharaf, A.; Asim, M.; Sadek, A. Implications of foot ulceration in hemodialysis patients: A 5-year observational study. J. Diabetes Res. 2014, 2014, 945075. [Google Scholar] [CrossRef]
- Orimoto, Y.; Ohta, T.; Ishibashi, H.; Sugimoto, I.; Iwata, H.; Yamada, T.; Tadakoshi, M.; Hida, N. The prognosis of patients on hemodialysis with foot lesions. J. Vasc. Surg. 2013, 58, 1291–1299. [Google Scholar] [CrossRef]
- Dietrich, I.; Braga, G.A.; de Melo, F.G.; da Costa Silva Silva, A.C.C. The Diabetic Foot as a Proxy for Cardiovascular Events and Mortality Review. Curr. Atheroscler. Rep. 2017, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.W.; Hoffstad, O.J.; Sullivan, M.O.; Margolis, D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet. Med. 2016, 33, 1493–1498. [Google Scholar] [CrossRef]
- Iversen, M.M.; Tell, G.S.; Riise, T.; Hanestad, B.R.; Ostbye, T.; Graue, M.; Midthjell, K. History of foot ulcer increases mortality among individuals with diabetes: Ten-year follow-up of the Nord-Trondelag Health Study, Norway. Diabetes Care 2009, 32, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.D.; Newton, K.; Blough, D.; McCulloch, D.K.; Sandhu, N.; Reiber, G.E.; Wagner, E.H. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999, 22, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Boyko, E.J.; Ahroni, J.H.; Smith, D.G.; Davignon, D. Increased mortality associated with diabetic foot ulcer. Diabetes Med. 1996, 13, 967–972. [Google Scholar] [CrossRef]
- Moulik, P.K.; Mtonga, R.; Gill, G.V. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003, 26, 491–494. [Google Scholar] [CrossRef]
- Hoffstad, O.; Mitra, N.; Walsh, J.; Margolis, D.J. Diabetes, lower-extremity amputation, and death. Diabetes Care 2015, 38, 1852–1857. [Google Scholar] [CrossRef]
- Brownrigg, J.R.; Davey, J.; Holt, P.J.; Davis, W.A.; Thompson, M.M.; Ray, K.K.; Hinchliffe, R.J. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: A meta-analysis. Diabetologia 2012, 55, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, K.; Berhane, T.; Hamilton, M.; Chandra, A.P.; Falhammar, H. Mortality in patients with diabetic foot ulcer: A retrospective study of 513 cases from a single Centre in the Northern Territory of Australia. BMC Endocr. Disord. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Valabhji, J. Foot problems in patients with diabetes and chronic kidney disease. J Ren. Care 2012, 38 (Suppl. S1), 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ndip, A.; Lavery, L.A.; Lafontaine, J.; Rutter, M.K.; Vardhan, A.; Vileikyte, L.; Boulton, A.J. High levels of foot ulceration and amputation risk in a multiracial cohort of diabetic patients on dialysis therapy. Diabetes Care 2010, 33, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Ndip, A.; Rutter, M.K.; Vileikyte, L.; Vardhan, A.; Asari, A.; Jameel, M.; Tahir, H.A.; Lavery, L.A.; Boulton, A.J. Dialysis treatment is an independent risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care 2010, 33, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Jaar, B.G.; Astor, B.C.; Berns, J.S.; Powe, N.R. Predictors of amputation and survival following lower extremity revascularization in hemodialysis patients. Kidney Int. 2004, 65, 613–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilhotra, R.A.; Rodrigues, B.T.; Vangaveti, V.N.; Malabu, U.H. Prevalence and Risk Factors of Lower Limb Amputation in Patients with End-Stage Renal Failure on Dialysis: A Systematic Review. Int. J. Nephrol. 2016, 2016, 4870749. [Google Scholar] [CrossRef]
- Combe, C.; Albert, J.M.; Bragg-Gresham, J.L.; Andreucci, V.E.; Disney, A.; Fukuhara, S.; Goodkin, D.A.; Gillespie, B.W.; Saito, A.; Jadoul, M.; et al. The burden of amputation among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2009, 54, 680–692. [Google Scholar] [CrossRef]
- Lavery, L.A.; Lavery, D.C.; Hunt, N.A.; La Fontaine, J.; Ndip, A.; Boulton, A.J. Amputations and foot-related hospitalisations disproportionately affect dialysis patients. Int. Wound J. 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Kaminski, M.; Frescos, N.; Tucker, S. Prevalence of risk factors for foot ulceration in patients with end-stage renal disease on haemodialysis. Intern. Med. J. 2012, 42, e120–e128. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.R.; Raspovic, A.; McMahon, L.P.; Strippoli, G.F.; Palmer, S.C.; Ruospo, M.; Dallimore, S.; Landorf, K.B. Risk factors for foot ulceration and lower extremity amputation in adults with end-stage renal disease on dialysis: A systematic review and meta-analysis. Nephrol. Dial. Transpl. 2015, 30, 1747–1766. [Google Scholar] [CrossRef]
- Jones, N.J.; Chess, J.; Cawley, S.; Phillips, A.O.; Riley, S.G. Prevalence of risk factors for foot ulceration in a general haemodialysis population. Int. Wound J. 2013, 10, 683–688. [Google Scholar] [CrossRef]
- Garimella, P.S.; Wang, W.; Lin, S.F.; Hymes, J.; Lacson, E., Jr. Incident diabetic foot ulcers and mortality in hemodialysis patients. Hemodial. Int. 2017, 21, 145–147. [Google Scholar] [CrossRef]
- Meloni, M.; Giurato, L.; Izzo, V.; Stefanini, M.; Pampana, E.; Gandini, R.; Uccioli, L. Long term outcomes of diabetic haemodialysis patients with critical limb ischemia and foot ulcer. Diabetes Res. Clin. Pr. 2016, 116, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Doria, M.; Rosado, V.; Pacheco, L.R.; Hernandez, M.; Betriu, A.; Valls, J.; Franch-Nadal, J.; Fernandez, E.; Mauricio, D. Prevalence of Diabetic Foot Disease in Patients with Diabetes Mellitus under Renal Replacement Therapy in Lleida, Spain. BioMed Res. Int. 2016, 2016, 7217586. [Google Scholar] [CrossRef] [PubMed]
- Junyent, M.; Gilabert, R.; Nunez, I.; Corbella, E.; Vela, M.; Zambon, D.; Ros, E. Carotid ultrasound in the assessment of preclinical atherosclerosis. Distribution of intima-media thickness values and plaque frequency in a Spanish community cohort. Med. Clin. 2005, 125, 770–774. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Beladi Mousavi, S.S.; Hayati, F.; Alemzadeh Ansari, M.J.; Valavi, E.; Cheraghian, B.; Shahbazian, H.; Golzari, K.; Ghorbani, A.; Rashidi, H.; Payami, P.; et al. Survival at 1, 3, and 5 years in diabetic and nondiabetic patients on hemodialysis. Iran. J. Kidney Dis. 2010, 4, 74–77. [Google Scholar]
- Beladi-Mousavi, S.S.; Alemzadeh-Ansari, M.J.; Alemzadeh-Ansari, M.H.; Beladi-Mousavi, M. Long-term survival of patients with end-stage renal disease on maintenance hemodialysis: A multicenter study in Iran. Iran. J. Kidney Dis. 2012, 6, 452–456. [Google Scholar]
- United States Renal Data System (USRDS). 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Available online: https://www.usrds.org/media/2371/2019-executive-summary.pdf (accessed on 1 October 2020).
- Jupiter, D.C.; Thorud, J.C.; Buckley, C.J.; Shibuya, N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int. Wound J. 2016, 13, 892–903. [Google Scholar] [CrossRef]
- Brennan, M.B.; Hess, T.M.; Bartle, B.; Cooper, J.M.; Kang, J.; Huang, E.S.; Smith, M.; Sohn, M.W.; Crnich, C. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J. Diabetes Complicat. 2017, 31, 556–561. [Google Scholar] [CrossRef]
- Morbach, S.; Furchert, H.; Groblinghoff, U.; Hoffmeier, H.; Kersten, K.; Klauke, G.T.; Klemp, U.; Roden, T.; Icks, A.; Haastert, B.; et al. Long-term prognosis of diabetic foot patients and their limbs: Amputation and death over the course of a decade. Diabetes Care 2012, 35, 2021–2027. [Google Scholar] [CrossRef]
- Rubio, J.A.; Jimenez, S.; Alvarez, J. Clinical characteristics and mortality in patients treated in a Multidisciplinary Diabetic Foot Unit. Endocrinol. Diabetes Nutr. 2017, 64, 241–249. [Google Scholar] [CrossRef]
- Rigor, J.; Martins-Mendes, D.; Monteiro-Soares, M. Risk factors for mortality in patients with a diabetic foot ulcer: A cohort study. Eur. J. Intern. Med. 2020, 71, 107–110. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef]
- Karame, A.; Labeeuw, M.; Trolliet, P.; Caillette-Beaudoin, A.; Cahen, R.; Ecochard, R.; Galland, R.; Hallonet, P.; Pouteil-Noble, C.; Villar, E. The Impact of type 2 diabetes on mortality in end-stage renal disease patients differs between genders. Nephron Clin. Pr. 2009, 112, c268–c275. [Google Scholar]
- Triebswetter, S.; Gutjahr-Lengsfeld, L.J.; Schmidt, K.R.; Drechsler, C.; Wanner, C.; Krane, V. Long-Term Survivor Characteristics in Hemodialysis Patients with Type 2 Diabetes. Am. J. Nephrol. 2018, 47, 30–39. [Google Scholar] [CrossRef] [PubMed]
- El-Menyar, A.; Al Thani, H.; Hussein, A.; Sadek, A.; Sharaf, A.; Al Suwaidi, J. Diabetic retinopathy: A new predictor in patients on regular hemodialysis. Curr. Med. Res. Opin. 2012, 28, 999–1055. [Google Scholar] [CrossRef] [PubMed]
- Crawford, F.; Inkster, M.; Kleijnen, J.; Fahey, T. Predicting foot ulcers in patients with diabetes: A systematic review and meta-analysis. QJM 2007, 100, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Boyko, E.J.; Ribeiro, J.; Ribeiro, I.; Dinis-Ribeiro, M. Risk stratification systems for diabetic foot ulcers: A systematic review. Diabetologia 2011, 54, 1190–1199. [Google Scholar] [CrossRef]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Ghanassia, E.; Villon, L.; Thuan Dit Dieudonne, J.F.; Boegner, C.; Avignon, A.; Sultan, A. Long-term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: A 6.5-year follow-up study. Diabetes Care 2008, 31, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Sargen, M.R.; Hoffstad, O.; Margolis, D.J. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J. Diabetes Complicat. 2013, 27, 128–133. [Google Scholar] [CrossRef] [PubMed]

| Patients with DM (n = 85) | p-Value No DM vs. DM | p-Value No DF vs. DF | ||||
|---|---|---|---|---|---|---|
| Variable | All HD Patients | Patients without DM (n = 135) | No Diabetic Foot * (n = 55) | Diabetic Foot (n = 30) | ||
| Gender, male, n (%) | 132 (60) | 80 (59.3) | 31 (56.4) | 21 (70.0) | 0.888 | 0.317 |
| Age, years, mean (SD), n (%) | 67.5 (16.2) | 66.7 (17.6) | 68.5 (13.2) | 69.1 (14.3) | 0.332 | 0.856 |
| Diabetes type, n (%) | - | 0.508 | ||||
| Type 1 | 11 (5.0) | - | 6 (10.9) | 5 (16.7) | ||
| Type 2 | 74 (33.6) | - | 49 (89.1) | 25 (83.3) | ||
| Duration of DM, years, median (25th, 75th percentile) | 19.7 (13.2; 29.7] | - | 20.1 (11.5; 29.7) | 18.7 (16.1; 30.7) | - | 0.530 |
| Smoking status, n (%) | 0.037 | 0.206 | ||||
| Current | 28 (17.2) | 22 (16.3) | 3 (5.5) | 3 (10) | ||
| Past | 63 (28.6) | 32 (23.7) | 17 (30.9) | 14 (46.7) | ||
| Never smoked | 129 (58.6) | 81 (60.0) | 35 (63.5) | 13 (43.3) | ||
| Hypertension, n (%) | 189 (85.9) | 117 (86.7) | 47 (85.5) | 25 (83.3) | 0.835 | 0.764 |
| Dyslipidemia, n (%) | 107 (48.6) | 46 (34.1) | 41 (74.5) | 20 (66.7) | <0.001 | 0.604 |
| Clinical history, n (%) | ||||||
| CHD | 51 (23.2) | 23 (17.0) | 17 (30.9) | 11 (36.7) | 0.011 | 0.765 |
| CVD | 28 (12.7) | 10 (7.4) | 7 (12.7) | 11 (36.7) | 0.006 | 0.021 |
| Cardiac arrhythmia | 21 (9.5) | 13 (9.6) | 3 (5.5) | 5 (16.7) | 1.00 | 0.124 |
| Heart failure | 13 (5.9) | 5 (3.7) | 5 (9.1) | 3 (10.0) | 0.146 | 1.000 |
| PAD, n (%) | 110 (50.0) | 51 (37.8) | 37 (67.3) | 22 (73.3) | <0.001 | 0.461 |
| Foot ulcer, n (%) | ||||||
| Previous | 22 (10) | 4 (3.0) | 0 (0.0) | 18 (60.0) | <0.001 | <0.001 |
| Current | 20 (9.1) | 4 (3.0) | 0 (0.0) | 16 (53.3) | <0.001 | <0.001 |
| Previous amputation, n (%) | <0.001 | <0.001 | ||||
| No amputation | 202 (91.8) | 131 (97.0) | 55 (100) | 16 (53.3) | ||
| Major | 7 (3.2) | 1 (0.7) | 0 (0.0) | 6 (20.0) | ||
| All-Cause Mortality | CV Mortality | |
|---|---|---|
| Variable | Hazard Ratio (95% CI); p-Value | Hazard Ratio (95% CI); p-Value |
| Gender (female) | 0.69 (0.48–1.00); 0.051 | 0.45 (0.24–0.86); 0.015 |
| Age (years) | 1.05 (1.03–1.07); <0.001 | 1.04 (1.01–1.06); 0.001 |
| DM | 1.90 (1.33–2.71); <0.001 | 3.15 (1.76–5.63); <0.001 |
| DM duration (years) | 1.00 (0.98–1.03); 0.826 | 1.01 (0.98–1.04); 0.659 |
| Hypertension | 1.06 (0.63–1.79); 0.832 | 0.69 (0.33–1.42); 0.314 |
| Dyslipidaemia | 1.06 (0.75–1.52); 0.728 | 1.48 (0.83–2.62); 0.183 |
| Smoker | 0.76 (0.42–1.36); 0.353 | 0.79 (0.30–2.06); 0.630 |
| CHD | 1.54 (1.04–2.28); 0.031 | 2.19 (1.21–3.95); 0.010 |
| CVD | 1.70 (1.06–2.72); 0.027 | 2.52 (1.28–4.95); 0.007 |
| Arrhythmia | 1.92 (1.14–3.26); 0.015 | 2.28 (1.02–5.09); 0.045 |
| HF | 2.39 (1.31–4.37); 0.004 | 2.68 (1.05–6.84); 0.038 |
| PAD | 2.09 (1.32–3.32); 0.002 | 2.27 (1.10–4.68); 0.027 |
| Diabetic retinopathy | 2.32 (1.56–3.43); <0.001 | 4.09 (2.20–7.59); <0.001 |
| Diabetic neuropathy | 1.92 (1.34–2.76); <0.001 | 3.04 (1.69–5.46); <0.001 |
| Previous FU | 2.10 (1.27–3.47); 0.004 | 2.22 (0.99–4.95); 0.053 |
| Current FU | 1.69 (0.98–2.90); 0.058 | 2.12 (0.95–4.73); 0.066 |
| Previous minor amputation | 2.43 (1.27–4.67); 0.007 | 2.58 (0.92–7.23); 0.071 |
| Previous major amputation | 3.33 (1.54–7.21); 0.002 | 1.32 (0.18–9.65); 0.785 |
| All-Cause Mortality | CV Mortality | |
|---|---|---|
| Variable | Hazard Ratio (95% CI); p-Value | Hazard Ratio (95% CI); p-Value |
| Gender (female) | 0.72 (0.42;1.22); 0.224 | 0.51 (0.22;1.14); 0.102 |
| Age (years) | 1.04 (1.02;1.06); 0.001 | 1.04 (1.01;1.08); 0.014 |
| DM duration (years) | 1.00 (0.98;1.03); 0.826 | 1.01 (0.98;1.04); 0.659 |
| Hypertension | 0.90 (0.44;1.83); 0.776 | 0.63 (0.26;1.55); 0.313 |
| Dyslipidaemia | 0.81 (0.46;1.41); 0.451 | 1.63 (0.62;4.28); 0.320 |
| Smoker | 1.13 (0.40;3.20); 0.813 | 0.57 (0.08;4.28); 0.585 |
| CHD | 1.29 (0.76;2.19); 0.339 | 1.59 (0.76;3.34); 0.217 |
| CVD | 1.58 (0.89;2.80); 0.119 | 1.71 (0.76;3.88); 0.197 |
| Arrhythmia | 1.56 (0.71;3.43); 0.272 | 2.32 (0.88;6.08); 0.088 |
| HF | 1.41 (0.64;3.12); 0.399 | 2.06 (0.78;5.46); 0.144 |
| PAD | 1.70 (0.90;3.20); 0.101 | 1.44 (0.55;3.79); 0.456 |
| Diabetic retinopathy | 6.51 (1.57;27.0); 0.010 | *; 0.998 |
| Diabetic neuropathy | *; 0.997 | *; 0.998 |
| Previous FU | 1.52 (0.85;2.73); 0.159 | 1.23 (0.50;3.01); 0.658 |
| Current FU | 1.19 (0.64;2.20); 0.575 | 1.37 (0.58;3.20); 0.470 |
| Previous minor amputation | 1.86 (0.84;4.12); 0.128 | 1.55 (0.47;5.14); 0.477 |
| Previous major amputation | 2.03 (0.86;4.78); 0.105 | 0.68 (0.09;5.05); 0.706 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dòria, M.; Betriu, À.; Belart, M.; Rosado, V.; Hernández, M.; Sarro, F.; Real, J.; Castelblanco, E.; Pacheco, L.R.; Fernández, E.; et al. High Incidence of Adverse Outcomes in Haemodialysis Patients with Diabetes with or without Diabetic Foot Syndrome: A 5-Year Observational Study in Lleida, Spain. J. Clin. Med. 2021, 10, 1368. https://doi.org/10.3390/jcm10071368
Dòria M, Betriu À, Belart M, Rosado V, Hernández M, Sarro F, Real J, Castelblanco E, Pacheco LR, Fernández E, et al. High Incidence of Adverse Outcomes in Haemodialysis Patients with Diabetes with or without Diabetic Foot Syndrome: A 5-Year Observational Study in Lleida, Spain. Journal of Clinical Medicine. 2021; 10(7):1368. https://doi.org/10.3390/jcm10071368
Chicago/Turabian StyleDòria, Montserrat, Àngels Betriu, Montserrat Belart, Verónica Rosado, Marta Hernández, Felipe Sarro, Jordi Real, Esmeralda Castelblanco, Linda Roxana Pacheco, Elvira Fernández, and et al. 2021. "High Incidence of Adverse Outcomes in Haemodialysis Patients with Diabetes with or without Diabetic Foot Syndrome: A 5-Year Observational Study in Lleida, Spain" Journal of Clinical Medicine 10, no. 7: 1368. https://doi.org/10.3390/jcm10071368
APA StyleDòria, M., Betriu, À., Belart, M., Rosado, V., Hernández, M., Sarro, F., Real, J., Castelblanco, E., Pacheco, L. R., Fernández, E., Franch-Nadal, J., Gratacòs, M., & Mauricio, D. (2021). High Incidence of Adverse Outcomes in Haemodialysis Patients with Diabetes with or without Diabetic Foot Syndrome: A 5-Year Observational Study in Lleida, Spain. Journal of Clinical Medicine, 10(7), 1368. https://doi.org/10.3390/jcm10071368







