Ferric Carboxymatose in Non-Hemodialysis CKD Patients: A Longitudinal Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Procedures
2.2. Efficacy Evaluation
2.3. Economic Evaluation
2.4. Statistical Analysis
3. Results
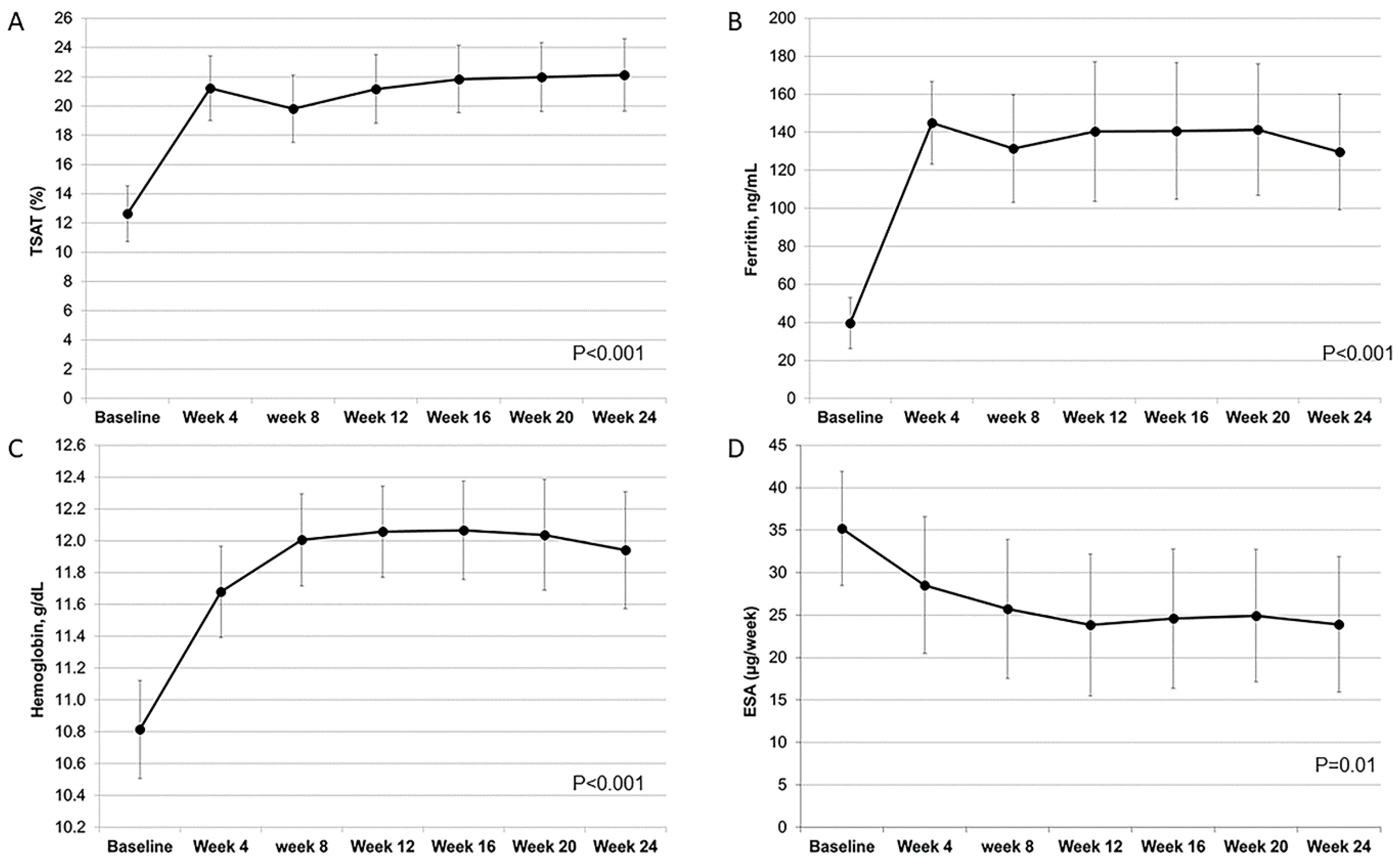
3.1. Efficacy of FCM
3.2. Cost Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minutolo, R.; Locatelli, F.; Gallieni, M.; Bonofiglio, R.; Fuiano, G.; Oldrizzi, L.; Conte, G.; De Nicola, L.; Mangione, F.; Esposito, P.; et al. Anaemia management in non-dialysis chronic kidney dis-ease (CKD) patients: A multicentre prospective study in renal clinics. Nephrol. Dial. Transplant. 2013, 28, 3035–3045. [Google Scholar] [CrossRef]
- Stack, A.G.; Alghali, A.; Li, X.; Ferguson, J.P.; Casserly, L.F.; Cronin, C.J.; Reddan, N.N.; Hussein, W.; Elsayed, M.E. Quality of care and practice patterns in anaemia management at specialist kidney clinics in Ireland: A national study. Clin. Kidney J. 2017, 11, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cases-Amenós, A.; Martínez-Castelao, A.; Fort-Ros, J.; Bonal-Bastons, J.; Ruiz, M.P.; Vallés-Prats, M.; Coll-Piera, E.; Galcerán-Gui, J.M. Prevalence of anaemia and its clinical man-agement in patients with stages 3-5 chronic kidney disease not on dialysis in Catalonia: MICENAS I study. Nefrologia 2014, 34, 189–198. [Google Scholar]
- Dmitrieva, O.; De Lusignan, S.; MacDougall, I.C.; Gallagher, H.; Tomson, C.; Harris, K.; DeSombre, T.; Goldsmith, D. Association of anaemia in primary care patients with chronic kidney disease: Cross sectional study of quality improvement in chronic kidney disease (QICKD) trial data. BMC Nephrol. 2013, 14, 24. [Google Scholar] [CrossRef]
- Iimori, S.; Naito, S.; Noda, Y.; Nishida, H.; Kihira, H.; Yui, N.; Okado, T.; Sasaki, S.; Uchida, S.; Rai, T. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: The CKD-ROUTE study. Nephrology 2015, 20, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, S. Etiological spectrum of anemia in non-dialysis-dependent chronic kidney disease: A sin-gle-center study from India. Saudi J. Kidney Dis. Transpl. 2019, 30, 932–942. [Google Scholar] [CrossRef]
- Thang, L.V.; Kien, N.T.; Van Hung, N.; Kien, T.Q.; Dung, N.H.; Huong, N.T.T.; Toan, N.D.; Toan, P.Q.; Vinh, H.T.; Nghia, V.X.; et al. Serum total iron-binding capacity and iron status in patients with non-dialysis-dependent chronic kidney disease: A cross-sectional study in Vietnam. Asia Pac. J. Clin. Nutr. 2020, 29, 48–54. [Google Scholar]
- National Clinical Guideline Centre. Anaemia management in chronic kidney disease: Update 2015. NICE guideline 8. National Institute for Health and Care Excellence (NICE). Updated 2015. Available online: http://www.nice.org.uk/guidance/ng8 (accessed on 18 August 2020).
- KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 2, 279–335.
- Locatelli, F.; Bárány, P.; Covic, A.; De Francisco, A.; Del Vecchio, L.; Goldsmith, D.; Hörl, W.; London, G.; Vanholder, R.; Van Biesen, W.; et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol. Dial. Transplant. 2013, 28, 1346–1359. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G. FIND-CKD: A randomized trial of intravenous ferric car-boxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014, 29, 2075–2084. [Google Scholar] [CrossRef]
- Liberti, M.E.; Garofalo, C.; Sagliocca, A.; Borrelli, S.; Conte, G.; De Nicola, L.; Minutolo, R. [Iron deficiency in ND-CKD: From diagnosis to treatment]. G. Ital. Nefrol. 2017, 34, 50–61. [Google Scholar]
- Funk, F.; Ryle, P.; Canclini, C.; Neiser, S.; Geisser, P. The new generation of intravenous iron: Chemistry, pharmacology, and toxicology of ferric carboxymaltose. Arzneimittelforschung 2010, 60, 345–353. [Google Scholar] [CrossRef]
- Jahn, M.R.; Andreasen, H.B.; Fütterer, S.; Nawroth, T.; Schünemann, V.; Kolb, U.; Hofmeister, W.; Muñoz, M.; Bock, K.; Meldal, M.; et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 2011, 78, 480–491. [Google Scholar] [CrossRef]
- European Medicines Agency—News and Events—New Recommendations to Manage Risk of Allergic Reactions with Intravenous Iron-Containing Medicines. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/06/news_detail_001833.jsp&mid=WC0b01ac058004d5c1 (accessed on 17 August 2020).
- Minutolo, R.; Chiodini, P.; Cianciaruso, B.; Pota, A.; Bellizzi, V.; Avino, D.; Mascia, S.; Laurino, S.; Bertino, V.; Conte, G.; et al. Epoetin Therapy and Hemoglobin Level Variability in Nondialysis Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 552–559. [Google Scholar] [CrossRef]
- Rampton, D.; Folkersen, J.; Fishbane, S.; Hedenus, M.; Howaldt, S.; Locatelli, F.; Patni, S.; Szebeni, J.; Weiss, G. Hypersensitivity reactions to intravenous iron: Guidance for risk minimization and management. Haematologica 2014, 99, 1671–1676. [Google Scholar] [CrossRef]
- ISTAT. Reddito Netto: Regioni e Tipo di Comune. Available online: http://dati.istat.it/Index.aspx?QueryId=22919 (accessed on 18 August 2020).
- Minutolo, R.; Zamboli, P.; Chiodini, P.; Mascia, S.; Vitiello, S.; Stanzione, G.; Bertino, V.; Conte, G.; De Nicola, L. Conversion of Darbepoetin to Low Doses of CERA Maintains Hemoglobin Levels in Non-Dialysis Chronic Kidney Disease Patients. Blood Purif. 2010, 30, 186–194. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of Inflammation. New Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Auerbach, M.; Adamson, J.; Bircher, A.; Breymann, C.; Fishbane, S.; Gafter-Gvili, A.; Gasche, C.; Gilreath, J.; Grazzini, G.; Henry, D.; et al. On the safety of intravenous iron, evidence trumps conjecture. Haematologica 2015, 100, e214–e215. [Google Scholar] [CrossRef]
- Rognoni, C.; Venturini, S.; Meregaglia, M.; Marmifero, M.; Tarricone, R. Efficacy and safety of ferric car-boxymaltose and other formulations in iron-deficient patients: A systematic review and network me-ta-analysis of randomised controlled trials. Clin. Drug Investig. 2016, 36, 177–194. [Google Scholar] [CrossRef]
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The safety of intravenous iron preparations: Systematic review and meta-analysis. Mayo Clin. Proc. 2015, 90, 12–23. [Google Scholar] [CrossRef]
- Qunibi, W.Y.; Martinez, C.; Smith, M.; Benjamin, J.; Mangione, A.; Roger, S.D. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol. Dial. Transplant. 2010, 26, 1599–1607. [Google Scholar] [CrossRef]
- Onken, J.E.; Bregman, D.B.; Harrington, R.A.; Morris, D.; Buerkert, J.; Hamerski, D.; Iftikhar, H.; Mangoo-Karim, R.; Martin, E.R.; Martinez, C.O.; et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: The REPAIR-IDA trial. Nephrol. Dial. Transplant. 2013, 29, 833–842. [Google Scholar] [CrossRef]
- Toblli, J.E.; Di Gennaro, F. Switching Patients with Non-Dialysis Chronic Kidney Disease from Oral Iron to Intravenous Ferric Carboxymaltose: Effects on Erythropoiesis-Stimulating Agent Requirements, Costs, Hemoglobin and Iron Status. PLoS ONE 2015, 10, e0125528. [Google Scholar] [CrossRef]
- Minutolo, R.; Garofalo, C.; Chiodini, P.; Aucella, F.; Del Vecchio, L.; Locatelli, F.; Scaglione, F.; De Nicola, L. Types of erythropoiesis-stimulating agents and risk of end-stage kidney disease and death in patients with non-dialysis chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 267–274. [Google Scholar] [CrossRef]
- Singh, H.; Reed, J.; Noble, S.; Cangiano, J.L.; Van Wyck, D.B.; United States Iron Sucrose (Venofer) Clinical Trials Group. Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythro-poiesis-stimulating agents for anemia: A randomized, controlled trial. Clin. J. Am. Soc. Nephrol. 2006, 1, 475–482. [Google Scholar] [CrossRef]
- Mitsopoulos, E.; Lysitska, A.; Pateinakis, P.; Lamprou, V.; Intzevidou, E.; Minasidis, I.; Katsaounou, C.; Kougioumtzidou, O.; Anagnostou, N.; Lemonidis, N.; et al. Efficacy and safety of a low monthly dose of intravenous iron sucrose in peritoneal dialysis patients. Int. Urol. Nephrol. 2020, 52, 387–392. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.-X. Intravenous iron sucrose in peritoneal dialysis patients with renal anemia. Perit. Dial. Int. 2008, 28, 149–154. [Google Scholar] [CrossRef]
- Portolés-Pérez, J.; Durá-Gúrpide, B.; Merino-Rivas, J.L.; Martín-Rodriguez, L.; Hevia-Ojanguren, C.; Bur-guera-Vion, V.; Yuste-Lozano, C.; Sánchez-García, L.; Rodriguez-Palomares, J.R.; Paraiso, V.; et al. Effectiveness and safety of ferric carboxymaltose therapy in peritoneal dialysis patients: An observational study. Clin. Kidney J. 2019, 14, 174–180. [Google Scholar] [CrossRef]
- Perlman, R.L.; Zhao, J.; Fuller, D.S.; Bieber, B.; Li, Y.; Pisoni, R.L.; Robinson, B.M.; Johnson, D.W.; Kawanishi, H.; Davies, S.J.; et al. International Anemia Prevalence and Management in Peritoneal Dialysis Patients. Perit. Dial. Int. 2019, 39, 539–546. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Gafter-Gvili, A.; Zingerman, B.; Levy-Drummer, R.S.; Levy, L.; Mor, E.; Gafter, U.; Rahamimov, R. Intravenous iron supplementation after kidney transplantation. Clin. Transplant. 2012, 26, 608–614. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Czira, M.; Ambrus, C.; Szeifert, L.; Szentkiralyi, A.; Bekő, G.; Rosivall, L.; Remport, A.; Novak, M.; Mucsi, I. Anemia Is Associated with Mortality in Kidney-Transplanted Patients?A Prospective Cohort Study. Arab. Archaeol. Epigr. 2007, 7, 818–824. [Google Scholar] [CrossRef]
- Heinze, G.; Kainz, A.; Hörl, W.H.; Oberbauer, R. Mortality in renal transplant recipients given erythropoietins to increase haemoglobin concentration: Cohort study. BMJ 2009, 339, b4018. [Google Scholar] [CrossRef]
- Kamar, N.; Rostaing, L.; Ignace, S.; Villar, E. Impact of post-transplant anemia on patient and graft survival rates after kidney transplantation: A meta-analysis. Clin. Transplant. 2011, 26, 461–469. [Google Scholar] [CrossRef]
- O’Lone, E.L.; Hodson, E.M.; Nistor, I.; Bolignano, D.; Webster, A.C.; Craig, J.C. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst. Rev. 2019, 2, CD007857. [Google Scholar] [CrossRef]
- Hougen, I.; Collister, D.; Bourrier, M.; Ferguson, T.; Hochheim, L.; Komenda, P.; Rigatto, C.; Tangri, N. Safety of intravenous iron in dialysis: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2018, 13, 457–467. [Google Scholar] [CrossRef]
- Ershler, W.B.; Chen, K.; Reyes, E.B.; Dubois, R. Economic Burden of Patients with Anemia in Selected Diseases. Value Heal. 2005, 8, 629–638. [Google Scholar] [CrossRef]
- Nissenson, A.R.; Wade, S.; Goodnough, T.; Knight, K.; Dubois, R.W. Economic Burden of Anemia in an Insured Population. J. Manag. Care Pharm. 2005, 11, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Duh, M.S.; Buteau, S.; Bookhart, B.; Mody, S.H. Medical Costs of Untreated Anemia in Elderly Patients with Predialysis Chronic Kidney Disease. J. Am. Soc. Nephrol. 2006, 17, 3497–3502. [Google Scholar] [CrossRef]
- Maddux, F.W.; Shetty, S.; Del Aguila, M.A.; Nelson, M.A.; Murray, B.M. Effect of Erythropoiesis-Stimulating Agents on Healthcare Utilization, Costs, and Outcomes in Chronic Kidney Disease. Ann. Pharmacother. 2007, 41, 1761–1769. [Google Scholar] [CrossRef]
- De Nicola, L.; Donfrancesco, C.; Minutolo, R.; Noce, C.L.; Palmieri, L.; De Curtis, A.; Iacoviello, L.; Zoccali, C.; Gesualdo, L.; Conte, G.; et al. Prevalence and cardiovascular risk profile of chronic kidney disease in Italy: Results of the 2008–12 National Health Examination Survey. Nephrol. Dial. Transplant. 2014, 30, 806–814. [Google Scholar] [CrossRef]
- Minutolo, R.; De Nicola, L.; Mazzaglia, G.; Postorino, M.; Cricelli, C.; Mantovani, L.G.; Conte, G.; Cianciaruso, B. Detection and Awareness of Moderate to Advanced CKD by Primary Care Practitioners: A Cross-sectional Study from Italy. Am. J. Kidney Dis. 2008, 52, 444–453. [Google Scholar] [CrossRef]
- Auerbach, M.; Muñoz, M.; MacDougall, I.C. Intravenous iron: Out of sight, out of mind. Lancet Haematol. 2018, 5, e10–e12. [Google Scholar] [CrossRef]
- Rivera, R.F.; Guido, D.; Del Vecchio, L.; Corghi, E.; D’Amico, M.; Camerini, C.; Spotti, D.; Galassi, A.; Pozzi, C.; Cancarini, G.; et al. Impact of European medicines agency recommendations for hypersensitivity reactions on intravenous iron prescription in haemodialysis centres of the Lombardy region. J. Nephrol. 2015, 29, 673–681. [Google Scholar] [CrossRef]
| Resources | Unit Cost | Reference |
|---|---|---|
| Ferric gluconate 62.5 mg | €0.47 | Hospital Pharmacy |
| Ferric carboxymaltose 500 mg | €42.64 | Hospital Pharmacy |
| Consumables for IV iron infusion | €0.65 | Hospital Pharmacy |
| Darbepoetin alpha (1 µg) | €1.40 | Hospital Pharmacy |
| CERA (1 µg) | €1.49 | Hospital Pharmacy |
| Mean annual income (Campania) | €24,732 | http://dati.istat.it/Index.aspx?QueryId=22919 (accessed on 18 August 2020) |
| Personnel time for nurse (1 h) | €19.00 | https://www.contoannuale.mef.gov.it (accessed on 18 August 2020) |
| Personnel time for physician (1 h) | €36.00 | https://www.contoannuale.mef.gov.it (accessed on 18 August 2020) |
| Age (Years) | 57.6 ± 17.7 |
| Women, n (%) | 38 (64%) |
| Body mass index (kg/m2) | 26.3 ± 5.4 |
| Diabetes Mellitus, n (%) | 17 (29%) |
| History of cardiovascular disease, n (%) | 18 (31%) |
| eGFR, (mL/min per 1.73 m2) | 44.4 (35.4–53.5) |
| Proteinuria, (g/day) | 0.75 (0.28–1.21) |
| Phosphorus (mg/dL) | 3.8 ± 1.1 |
| C-Reactive Protein (mg/dL) | 0.57 (0.37–0.77) |
| Hemoglobin (g/dL) | 10.8 ± 1.2 |
| Hb < 11.0 g/dL | 32 (54%) |
| TSAT (%) | 12.7 (10.7–14.6) |
| TSAT < 20% n (%) | 50 (85%) |
| Ferritin (ng/mL) | 40 (26–53) |
| Ferritin < 100 ng/mL n (%) | 56 (95%) |
| Darbepoetin alpha (%) | 15 (25%) |
| Dose (µg/week) | 42 ± 17 |
| C.E.R.A. (%) | 9 (15%) |
| Dose (µg/month) | 93 ± 25 |
| Baseline | Evaluation Period | p | |
|---|---|---|---|
| TSAT ≥20% (%) | 15.3 | 62.7 | <0.001 |
| Ferritin ≥100 ng/mL (%) | 6.8 | 59.3 | <0.001 |
| Hemoglobin ≥11 g/dL (%) | 45.7 | 83.1 | <0.001 |
| TSAT (%) | Ferritin (ng/mL) | Hemoglobin (g/dL) | |
|---|---|---|---|
| Overall (n = 59) | 9.5 (5.8–13.2) | 104 (40–168) | 1.16 (0.55–1.77) |
| Clinical setting | |||
| KTR (n = 12) | 8.0 (3.2–12.7) | 64 (4–125) | 1.47 (0.71–2.23) |
| ND-CKD (n = 40) | 10.3 (7.6–12.9) | 114 (84–144) | 1.16 (0.74–1.57) |
| PD (n = 7) | 7.2 (−2.4–16.8) | 55 (8–103) | 1.03 (−0.37–2.43) |
| p value | 0.835 | 0.179 | 0.787 |
| ESA use | |||
| No (n = 35) | 9.5 (6.8–12.2) | 92 (65–120) | 1.45 (1.08–1.83) |
| Yes (n = 24) | 9.4 (5.5–13.3) | 103 (57–149) | 0.84 (0.21–1.47) |
| p value | 0.749 | 0.703 | 0.246 |
| Ferric Carboxymaltose | Ferric Gluconate | Difference | |
|---|---|---|---|
| Drug | €72.27 | €3.19 | €69.08 |
| Infusion material | €1.10 | €4.41 | −€3.31 |
| Personnel costs | €20.90 | €126.55 | −€105.65 |
| Transportation | €12.84 | €51.34 | −€38.51 |
| Loss of productivity | €70.06 | €280.22 | −€210.17 |
| TOTAL | €177.17 | €465.71 | −€288.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minutolo, R.; Berto, P.; Liberti, M.E.; Peruzzu, N.; Borrelli, S.; Netti, A.; Garofalo, C.; Conte, G.; De Nicola, L.; Del Vecchio, L.; et al. Ferric Carboxymatose in Non-Hemodialysis CKD Patients: A Longitudinal Cohort Study. J. Clin. Med. 2021, 10, 1322. https://doi.org/10.3390/jcm10061322
Minutolo R, Berto P, Liberti ME, Peruzzu N, Borrelli S, Netti A, Garofalo C, Conte G, De Nicola L, Del Vecchio L, et al. Ferric Carboxymatose in Non-Hemodialysis CKD Patients: A Longitudinal Cohort Study. Journal of Clinical Medicine. 2021; 10(6):1322. https://doi.org/10.3390/jcm10061322
Chicago/Turabian StyleMinutolo, Roberto, Patrizia Berto, Maria Elena Liberti, Nicola Peruzzu, Silvio Borrelli, Antonella Netti, Carlo Garofalo, Giuseppe Conte, Luca De Nicola, Lucia Del Vecchio, and et al. 2021. "Ferric Carboxymatose in Non-Hemodialysis CKD Patients: A Longitudinal Cohort Study" Journal of Clinical Medicine 10, no. 6: 1322. https://doi.org/10.3390/jcm10061322
APA StyleMinutolo, R., Berto, P., Liberti, M. E., Peruzzu, N., Borrelli, S., Netti, A., Garofalo, C., Conte, G., De Nicola, L., Del Vecchio, L., & Locatelli, F. (2021). Ferric Carboxymatose in Non-Hemodialysis CKD Patients: A Longitudinal Cohort Study. Journal of Clinical Medicine, 10(6), 1322. https://doi.org/10.3390/jcm10061322








