Association between Visceral or Subcutaneous Fat Accumulation and B-Type Natriuretic Peptide among Japanese Subjects: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
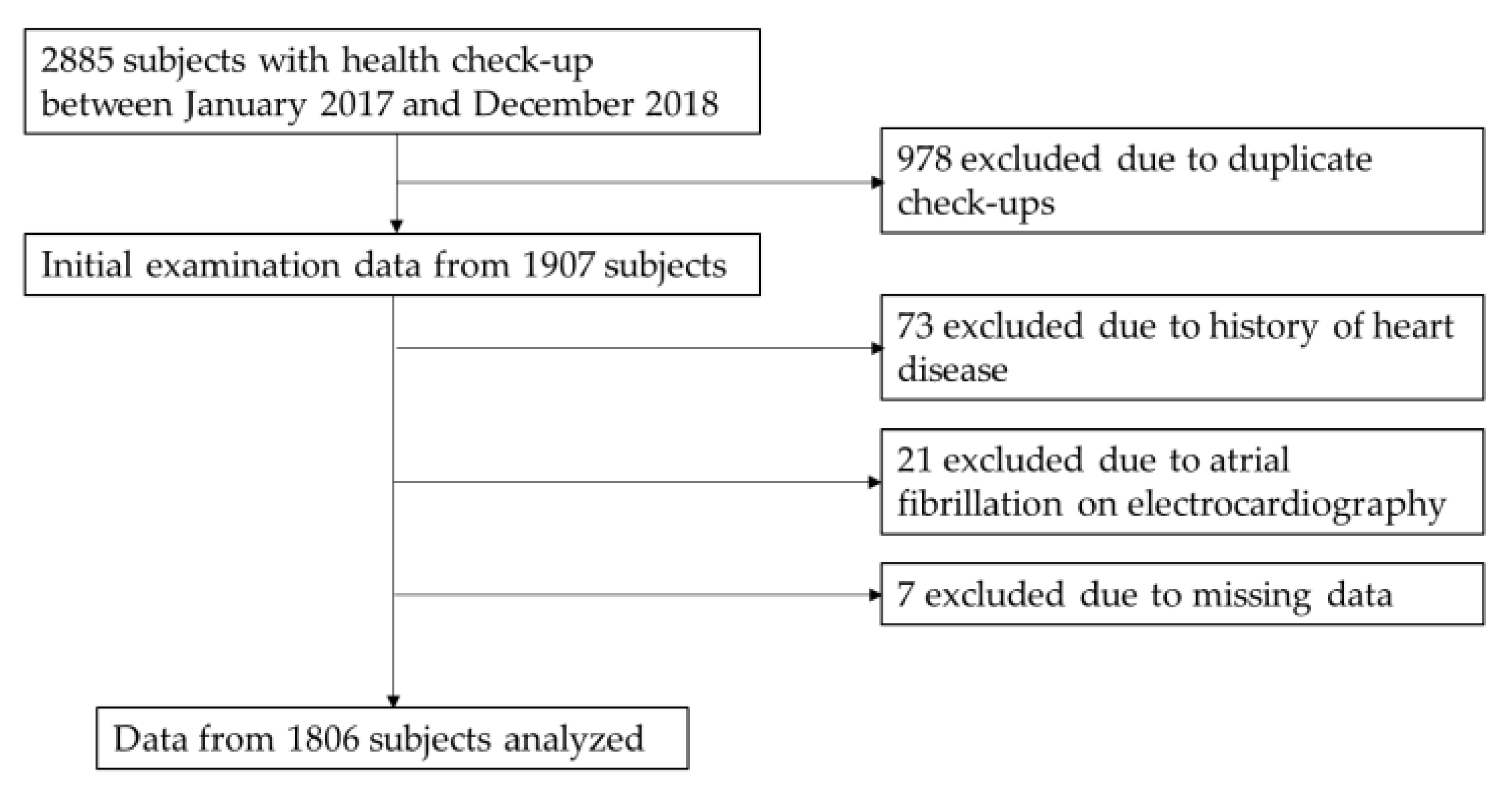
2.1. Study Participants
2.2. Data Handling
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniels, L.B.; Maisel, A.S. Natriuretic Peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, T.; Maeda, N.; Matsuoka, H. The role of natriuretic peptides in cardioprotection. Cardiovasc. Res. 2006, 69, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Isobe, M.; Ito, H.; Okumura, K.; Ono, M.; Kitakaze, M.; Kinugawa, K.; Kihara, Y.; Goto, Y.; Komuro, I.; et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure―digest version―. Circ. J. 2019, 83, 2084–2184. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uber, P.A.; Park, M.H.; Scott, R.L.; Ventura, H.O.; Harris, B.C.; Frohlich, E.D. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am. Coll. Cardiol. 2004, 43, 1590–1595. [Google Scholar] [CrossRef]
- Sugisawa, T.; Kishimoto, I.; Kokubo, Y.; Makino, H.; Miyamoto, Y.; Yoshimasa, Y. Association of plasma B-type natriuretic peptide levels with obesity in a general urban Japanese population: The Suita Study. Endocr. J. 2010, 57, 727–733. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Wilson, P.W.F.F.; Vasan, R.S. Impact of Obesity on Plasma Natriuretic Peptide Levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef]
- Krauser, D.G.; Lloyd-Jones, D.M.; Chae, C.U.; Cameron, R.; Anwaruddin, S.; Baggish, A.L.; Chen, A.; Tung, R.; Januzzi, J.L. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: A ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am. Heart J. 2005, 149, 744–750. [Google Scholar] [CrossRef]
- Oreopoulos, A.; Ezekowitz, J.A.; McAlister, F.A.; Kalantar-Zadeh, K.; Fonarow, G.C.; Norris, C.M.; Johnson, J.A.; Padwal, R.S. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin. Proc. 2010, 85, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K. Adiponectin and adiponectin receptors in obesity-linked insulin resistance. Novartis Found. Symp. 2007, 286, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H.; Yanai, H.; Kakei, M.; Noda, M.; Ezaki, O. The association between daily physical activity and plasma B-type natriuretic peptide in patients with glucose intolerance: A cross-sectional study. BMJ Open 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Khan, A.M.; Cheng, S.; Magnusson, M.; Larson, M.G.; Newton-Cheh, C.; McCabe, E.L.; Coviello, A.D.; Florez, J.C.; Fox, C.S.; Levy, D.; et al. Cardiac natriuretic peptides, obesity, and insulin resistance: Evidence from two community-based studies. J. Clin. Endocrinol. Metab. 2011, 96, 3242–3249. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Keyes, M.J.; Levy, D.; Benjamin, E.J.; Vasan, R.S. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 2007, 115, 1345–1353. [Google Scholar] [CrossRef]
- Halbirk, M.; Nørrelund, H.; Møller, N.; Schmitz, O.; Bøtker, H.E.; Wiggers, H. Short-term changes in circulating insulin and free fatty acids affect Nt-pro-BNP levels in heart failure patients. Int. J. Cardiol. 2010, 144, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.H.; Hansen, T.W.; Christensen, M.K.; Gustafsson, F.; Rasmussen, S.; Wachtell, K.; Borch-Johnsen, K.; Ibsen, H.; Jørgensen, T.; Hildebrandt, P. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension 2005, 46, 660–666. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Ho, J.E.; Gansevoort, R.T.; Voors, A.A.; van der Meer, P.; Bakker, S.J.L.L.; Heymans, S.; van Empel, V.; Schroen, B.; et al. Sex-specific associations of obesity and N-terminal pro-B-type natriuretic peptide levels in the general population. Eur. J. Heart Fail. 2018, 20, 1205–1214. [Google Scholar] [CrossRef]
- Koizumi, M.; Watanabe, H.; Kaneko, Y.; Iino, K.; Ishida, M.; Kosaka, T.; Motohashi, Y.; Ito, H. Impact of obesity on plasma B-type natriuretic peptide levels in Japanese community-based subjects. Heart Vessels 2012, 27, 287–294. [Google Scholar] [CrossRef]
- Cheng, S.; Fox, C.S.; Larson, M.G.; Massaro, J.M.; McCabe, E.L.; Khan, A.M.; Levy, D.; Hoffmann, U.; O’Donnell, C.J.; Miller, K.K.; et al. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am. J. Cardiol. 2011, 108, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Winders, B.R.; Ayers, C.R.; Das, S.R.; Chang, A.Y.; Berry, J.D.; Khera, A.; McGuire, D.K.; Vega, G.L.; de Lemos, J.A.; et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J. Am. Coll. Cardiol. 2013, 62, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, T.; Kishimoto, I.; Kokubo, Y.; Nagumo, A.; Makino, H.; Miyamoto, Y.; Yoshimasa, Y. Visceral fat is negatively associated with B-type natriuretic peptide levels in patients with advanced type 2 diabetes. Diabetes Res. Clin. Pract. 2010. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Definition and the diagnostic standard for metabolic syndrome. Nihon Naika Gakkai Zasshi 2005, 94, 794–809. [Google Scholar] [CrossRef]
- Oike, M.; Yokokawa, H.; Fukuda, H.; Haniu, T.; Oka, F.; Hisaoka, T.; Isonuma, H. Association between abdominal fat distribution and atherosclerotic changes in the carotid artery. Obes. Res. Clin. Pract. 2014, 8, e448–e458. [Google Scholar] [CrossRef] [PubMed]
- Belloc, N.B.; Breslow, L. Relationship of physical health status and health practices. Prev. Med. 1972, 1, 409–421. [Google Scholar] [CrossRef]
- Yokokawa, H.; Goto, A.; Sanada, H.; Watanabe, T.; Felder, R.A.; Jose, P.A.; Yasumura, S. Achievement status toward goal blood pressure levels and healthy lifestyles among Japanese hypertensive patients; cross-sectional survey results from fukushima research of hypertension (FRESH). Intern. Med. 2011, 50, 1149–1156. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Dessì-Fulgheri, P.; Paci, V.M.; Espinosa, E.; Rappelli, A. Expression of natriuretic peptide receptors in human adipose and other tissues. J. Endocrinol. Investig. 1996, 19, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Lafontan, M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, 358–368. [Google Scholar] [CrossRef]
- Gruden, G.; Landi, A.; Bruno, G. Natriuretic peptides, heart, and adipose tissue: New findings and future developments for diabetes research. Diabetes Care 2014, 37, 2899–2908. [Google Scholar] [CrossRef]
- Sengenès, C.; Zakaroff-Girard, A.; Moulin, A.; Berlan, M.; Bouloumié, A.; Lafontan, M.; Galitzky, J. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, 257–265. [Google Scholar] [CrossRef]
- Lafontan, M.; Moro, C.; Berlan, M.; Crampes, F.; Sengenes, C.; Galitzky, J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 2008, 19, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Gentili, A.; Frangione, M.R.; Albini, E.; Vacca, C.; Ricci, M.A.; De Vuono, S.; Boni, M.; Rondelli, F.; Rotelli, L.; Lupattelli, G.; et al. Modulation of natriuretic peptide receptors in human adipose tissue: Molecular mechanisms behind the “natriuretic handicap” in morbidly obese patients. Transl. Res. 2017, 186, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, O.; Gögebakan, Ö.; Klöting, N.; Sparwasser, A.; Weickert, M.O.; Haddad, I.; Nikiforova, V.J.; Bergmann, A.; Kruse, M.; Seltmann, A.C.; et al. Insulin up-regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: A missing link between CVD risk and obesity? J. Clin. Endocrinol. Metab. 2012, 97, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Enzi, G.; Gasparo, M.; Biondetti, P.R.; Fiore, D.; Semisa, M.; Zurlo, F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am. J. Clin. Nutr. 1986, 44, 739–746. [Google Scholar] [CrossRef]

| Variables | All Subjects | Men | Women |
|---|---|---|---|
| n | 1806 | 981 | 825 |
| Age (years), mean (SD) | 60.4 (12.4) | 60.5 (12.1) | 60.4 (12.8) |
| Sex (male), n (%) | 981 (54.3) | − | − |
| Height (cm), mean (SD) | 163.6 (9.0) | 169.5 (6.4) | 156.6 (6.1) |
| Body weight (kg), mean (SD) | 62.9 (13.2) | 70.4 (11.0) | 53.9 (9.5) |
| Body mass index (kg/m2), mean (SD) | 23.3 (3.6) | 24.5 (3.3) | 22.0 (3.5) |
| Waist circumference (cm), mean (SD) | 84.6 (9.7) | 87.2 (8.8) | 81.4 (9.9) |
| Visceral fat area (cm2), mean (SD) | 81.8 (47.5) | 97.8 (47.8) | 62.8 (39.4) |
| logVFA, mean (SD) | 1.82 (0.33) | 1.92 (0.28) | 1.69 (0.34) |
| Subcutaneous fat area (cm2), mean (SD) | 134.1 (69.5) | 123.6 (57.5) | 146.6 (79.7) |
| logSFA, mean (SD) | 2.06 (0.29) | 2.03 (0.27) | 2.08 (0.31) |
| BNP (pg/mL), mean (SD) | 20.2 (20.9) | 18.2 (20.7) | 22.6 (20.9) |
| logBNP, mean (SD) | 1.16 (0.34) | 1.10 (0.35) | 1.24 (0.31) |
| Pulse rate (beats/min), mean (SD) | 70.3 (10.7) | 68.6 (10.2) | 72.3 (10.9) |
| Serum creatinine (mg/dL), mean (SD) | 0.69 (0.18) | 0.80 (0.16) | 0.57 (0.10) |
| High-sensitivity C-reactive peptide (mg/dL), mean (SD) | 0.12 (0.34) | 0.12 (0.28) | 0.11 (0.41) |
| Left ventricular hypertrophy (yes), n (%) | 41 (2.3) | 32 (3.3) | 9 (1.1) |
| Hypertension-related factors | |||
| Systolic blood pressure (mmHg), mean (SD) | 120.5 (15.2) | 123.1 (14.3) | 117.5 (15.6) |
| Diastolic blood pressure (mmHg), mean (SD) | 72.9 (10.8) | 74.8 (10.6) | 70.6 (10.5) |
| Antihypertensive drug use (yes), n (%) | 231 (12.8) | 149 (15.2) | 82 (9.9) |
| Hypertension (yes), n (%) | 706 (39.1) | 438 (44.6) | 268 (32.5) |
| Lipid-related items | |||
| High-density lipoprotein cholesterol (mg/dL), mean (SD) | 61.2 (16.1) | 55.4 (14.1) | 68.0 (15.6) |
| Low-density lipoprotein cholesterol (mg/dL), mean (SD) | 116.0 (28.9) | 113.7 (28.8) | 118.7 (28.9) |
| Triglycerides (mg/dL), mean (SD) | 111.4 (74.4) | 127.9 (85.1) | 91.8 (52.9) |
| Antidyslipidemic drug use (yes), n (%) | 111 (6.1) | 64 (6.5) | 47 (5.7) |
| Metabolic lipid disorder (yes), n (%) | 727 (40.3) | 434 (44.2) | 293 (35.5) |
| Diabetes-related items | |||
| Fasting plasma glucose (mg/dL), mean (SD) | 101.0 (17.4) | 105.2 (18.9) | 96.1 (14.1) |
| Antidiabetic drug use (yes), n (%) | 69 (3.8) | 52 (5.3) | 17 (2.1) |
| Metabolic glucose disorder (yes), n (%) | 348 (19.3) | 252 (25.7) | 96 (11.6) |
| Immunoreactive insulin (μU/mL), mean (SD) | 8.14 (5.62) | 8.75 (5.60) | 7.42 (5.56) |
| HOMA-IR, mean (SD) | 2.09 (1.61) | 2.33 (1.69) | 1.81 (1.47) |
| Lifestyle characteristics | |||
| Alcohol consumption (not daily), n (%) | 1507 (83.4) | 760 (77.5) | 747 (90.5) |
| Exercise frequency (≥2 times/week), n (%) | 296 (16.4) | 161 (16.4) | 135 (16.4) |
| Smoking behavior (not current smoker), n (%) | 932 (51.6) | 455 (46.4) | 477 (57.8) |
| Sleep hours (6–9 h), n (%) | 825 (45.7) | 433 (44.1) | 392 (47.5) |
| Breakfast every morning (yes), n (%) | 862 (47.7) | 450 (45.9) | 412 (49.9) |
| Snack between meals (no), n (%) | 712 (39.4) | 375 (38.2) | 337 (40.8) |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Models | β-Coefficient (95% CI) | SE | p | β-Coefficient (95% CI) | SE | p | β-Coefficient (95% CI) | SE | p |
| Include SFA | |||||||||
| logSFA | −0.14 (−0.19, −0.09) | 0.02 | <0.001 | −0.11(−0.17, −0.04) | 0.03 | 0.002 | −0.10 (−0.17, −0.04) | 0.03 | 0.002 |
| Include VFA | |||||||||
| logVFA | −0.16 (−0.21, −0.12) | 0.02 | <0.001 | −0.16(−0.22, −0.10) | 0.03 | <0.001 | −0.14 (−0.2, −0.09) | 0.03 | <0.001 |
| Include SFA and VFA | |||||||||
| logSFA | −0.04 (−0.11, −0.03) | 0.03 | 0.28 | −0.03 (−0.1, −0.04) | 0.04 | 0.44 | −0.04 (−0.11, −0.04) | 0.04 | 0.34 |
| logVFA | −0.14 (−0.2, −0.07) | 0.03 | <0.001 | −0.15 (−0.21, −0.08) | 0.03 | <0.001 | −0.13 (−0.2, −0.06) | 0.03 | <0.001 |
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Models | β-Coefficient (95% CI) | SE | p | β-Coefficient (95% CI) | SE | p | β-Coefficient (95% CI) | SE | p | |
| Men | Include SFA | |||||||||
| logSFA | −0.07 (−0.15, −0.00) | 0.04 | 0.04 | −0.06 (−0.16, −0.04) | 0.05 | 0.21 | −0.05 (−0.15, −0.04) | 0.05 | 0.27 | |
| Include VFA | ||||||||||
| logVFA | −0.11 (−0.18, −0.04) | 0.03 | 0.001 | −0.11 (−0.19, −0.03) | 0.04 | 0.01 | −0.10 (−0.18, −0.02) | 0.04 | 0.019 | |
| Include SFA and VFA | ||||||||||
| logSFA | 0.02 (−0.08, −0.12) | 0.05 | 0.71 | 0.00 (−0.11, −0.11) | 0.06 | 0.97 | 0.00 (−0.11, −0.11) | 0.06 | 0.99 | |
| logVFA | −0.12 (−0.22, −0.03) | 0.05 | 0.012 | −0.11 (−0.21, −0.01) | 0.05 | 0.025 | −0.10 (−0.19, −0.00) | 0.05 | 0.039 | |
| Women | Include SFA | |||||||||
| logSFA | −0.18 (−0.24, −0.12) | 0.03 | <0.001 | −0.14 (−0.22, −0.05) | 0.05 | 0.003 | −0.14 (−0.22, −0.05) | 0.04 | 0.002 | |
| Include VFA | ||||||||||
| logVFA | −0.19 (−0.25, −0.13) | 0.03 | <0.001 | −0.17 (−0.26, −0.09) | 0.04 | <0.001 | −0.16 (−0.24, −0.08) | 0.04 | <0.001 | |
| Include SFA and VFA | ||||||||||
| logSFA | −0.09 (−0.18, −0.00) | 0.05 | 0.055 | −0.06 (−0.16, −0.04) | 0.05 | 0.24 | −0.07 (−0.17, −0.03) | 0.05 | 0.15 | |
| logVFA | −0.13 (−0.21, −0.04) | 0.04 | 0.004 | −0.15 (−0.24, −0.06) | 0.05 | 0.002 | −0.13 (−0.22, −0.03) | 0.05 | 0.008 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Yokokawa, H.; Fukuda, H.; Saita, M.; Miyagami, T.; Takahashi, Y.; Hisaoka, T.; Naito, T. Association between Visceral or Subcutaneous Fat Accumulation and B-Type Natriuretic Peptide among Japanese Subjects: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 1315. https://doi.org/10.3390/jcm10061315
Hayashi Y, Yokokawa H, Fukuda H, Saita M, Miyagami T, Takahashi Y, Hisaoka T, Naito T. Association between Visceral or Subcutaneous Fat Accumulation and B-Type Natriuretic Peptide among Japanese Subjects: A Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(6):1315. https://doi.org/10.3390/jcm10061315
Chicago/Turabian StyleHayashi, Yoshinori, Hirohide Yokokawa, Hiroshi Fukuda, Mizue Saita, Taiju Miyagami, Yuichi Takahashi, Teruhiko Hisaoka, and Toshio Naito. 2021. "Association between Visceral or Subcutaneous Fat Accumulation and B-Type Natriuretic Peptide among Japanese Subjects: A Cross-Sectional Study" Journal of Clinical Medicine 10, no. 6: 1315. https://doi.org/10.3390/jcm10061315
APA StyleHayashi, Y., Yokokawa, H., Fukuda, H., Saita, M., Miyagami, T., Takahashi, Y., Hisaoka, T., & Naito, T. (2021). Association between Visceral or Subcutaneous Fat Accumulation and B-Type Natriuretic Peptide among Japanese Subjects: A Cross-Sectional Study. Journal of Clinical Medicine, 10(6), 1315. https://doi.org/10.3390/jcm10061315






