Eribulin Efficacy on Brain Metastases in Heavily Pretreated Patients with Metastatic Breast Cancer
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patient Selection and Data Collection
2.2. Endpoints and Assessments
2.3. Statistical Analyses
3. Results
3.1. Patients
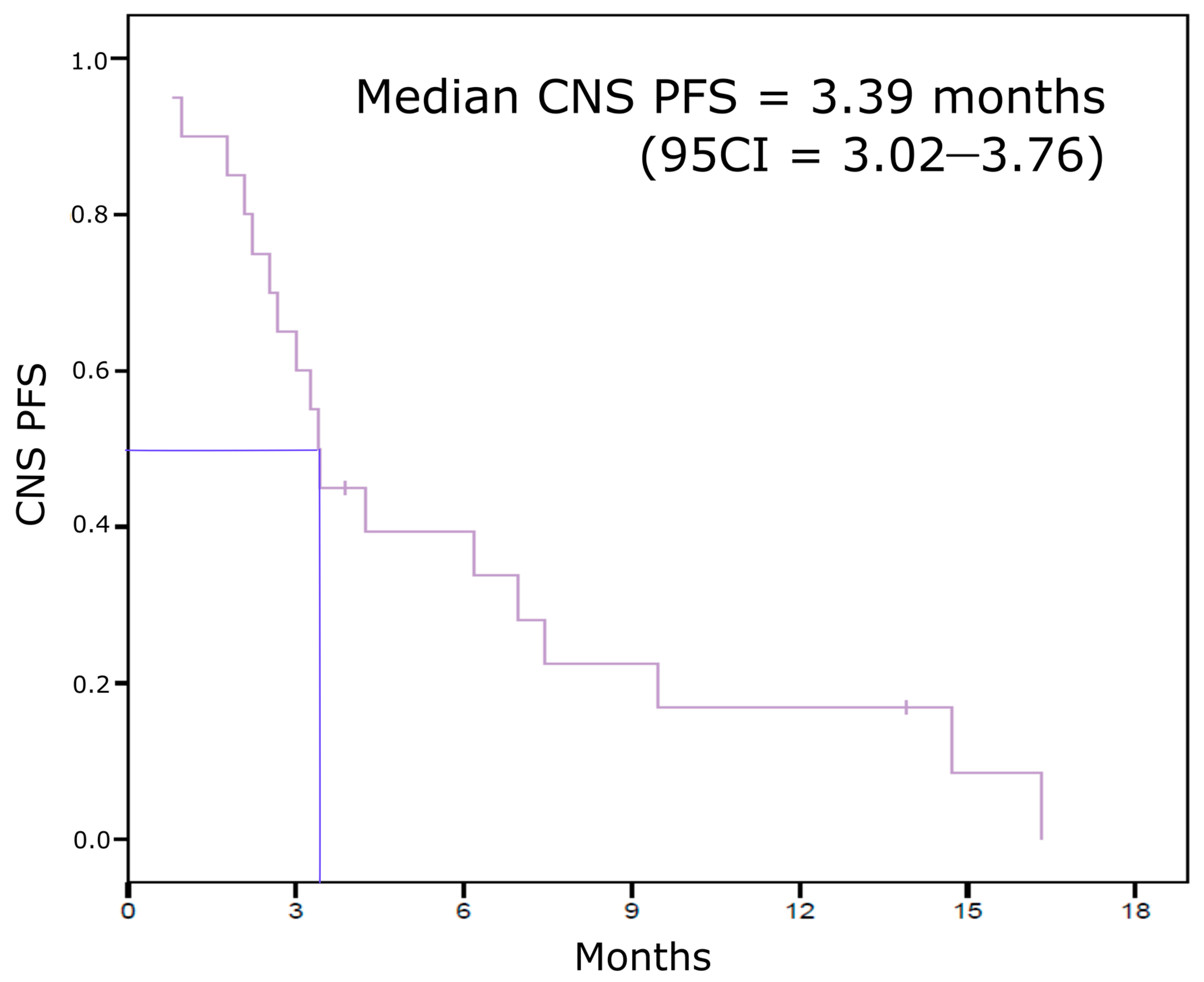
3.2. Eribulin Efficacy on BCBM
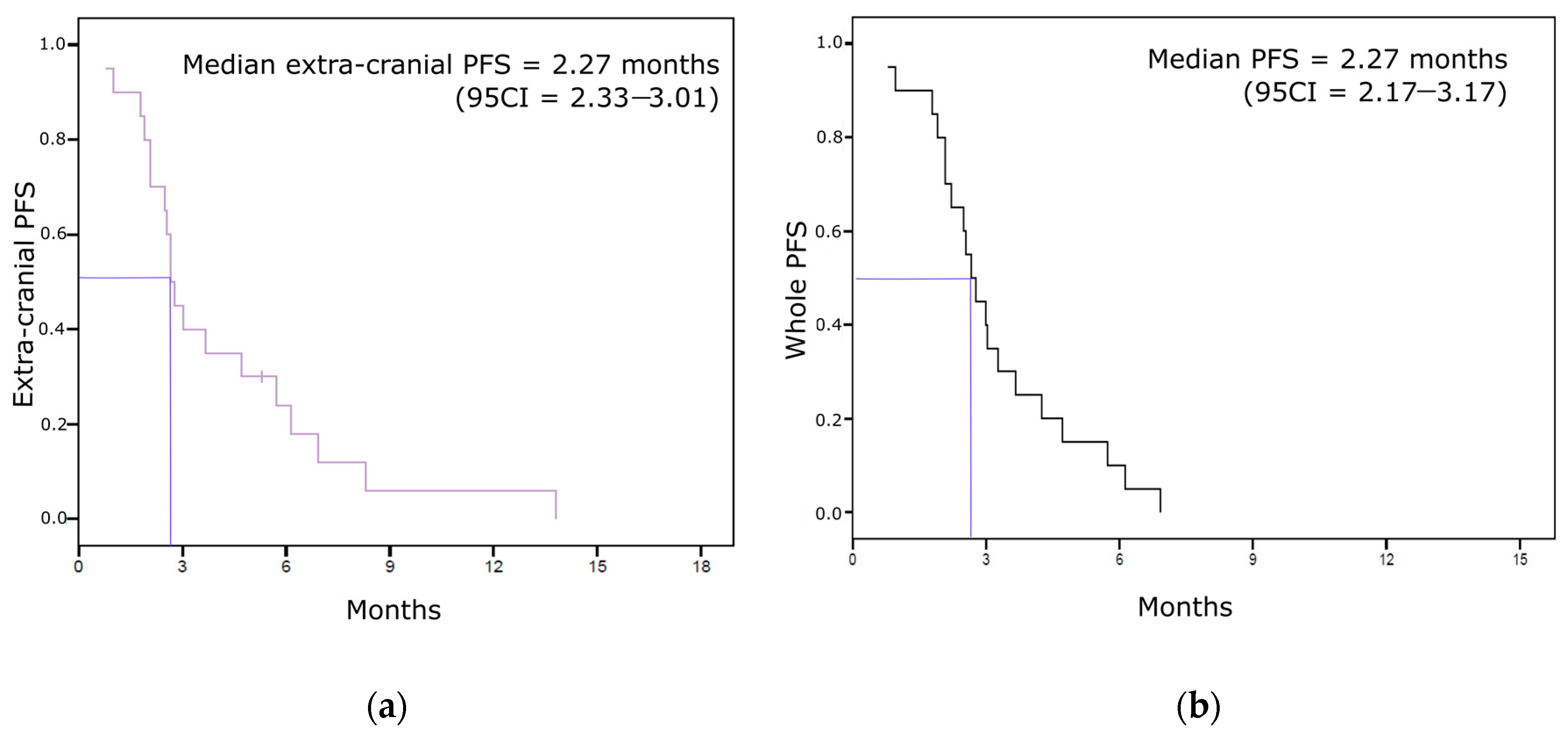
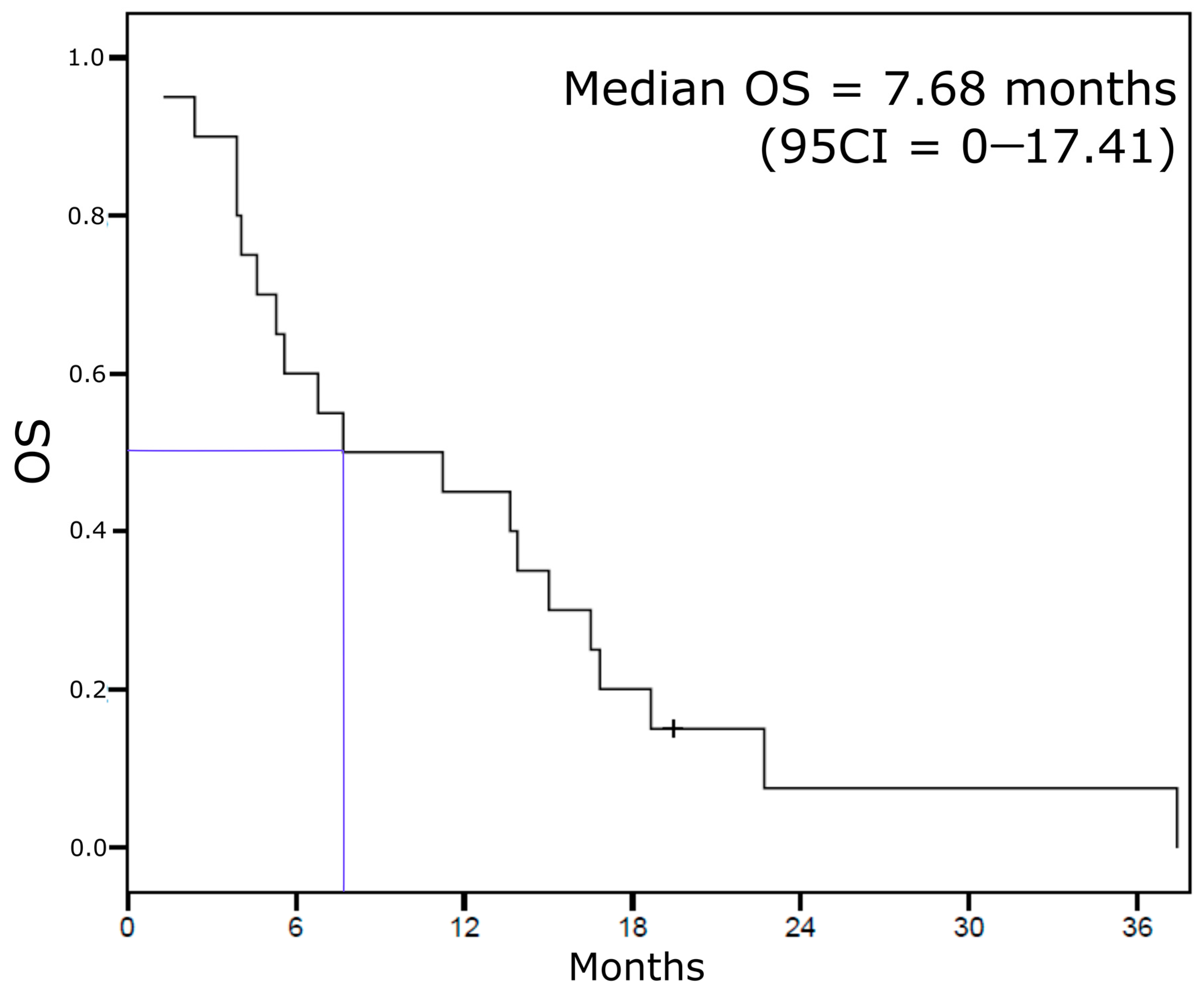
3.3. Other Survival Endpoints (Extra-Cranial Response, Extra-Cranial PFS, Whole PFS, and OS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Steeg, P.S.; Price, J.E.; Krishnamurthy, S.; Mani, S.A.; Reuben, J.; Cristofanilli, M.; Dontu, G.; Bidaut, L.; Valero, V.; et al. Breast Cancer Metastasis: Challenges and Opportunities. Cancer Res. 2009, 69, 4951–4953. [Google Scholar] [CrossRef] [Green Version]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy Versus Observation in Patients With One to Three Brain Metastases From Solid Tumors After Surgical Resection or Radiosurgery: Quality-of-Life Results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Ramakrishna, N.; Temin, S.; Chandarlapaty, S.; Crews, J.R.; Davidson, N.E.; Esteva, F.J.; Giordano, S.H.; Kirshner, J.J.; Krop, I.E.; Levinson, J.; et al. Recommendations on Disease Management for Patients With Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2804–2807. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, A. Eribulin: Rediscovering Tubulin as an Anticancer Target. Clin. Cancer Res. 2009, 15, 3903–3905. [Google Scholar] [CrossRef] [Green Version]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef] [Green Version]
- Cortes, J.; O’Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Diéras, V.; Delozier, T.; et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef]
- Kaufman, P.P.; Awada, A.; Twelves, C.C.; Yelle, L.L.; Perez, E.A.; Velikova, G.G.; Olivo, M.S.; He, Y.Y.; Dutcus, C.C.; Cortes, J. Phase III Open-Label Randomized Study of Eribulin Mesylate Versus Capecitabine in Patients With Locally Advanced or Metastatic Breast Cancer Previously Treated With an Anthracycline and a Taxane. J. Clin. Oncol. 2015, 33, 594–601. [Google Scholar] [CrossRef]
- Pivot, X.; Marmé, F.; Koenigsberg, R.; Guo, M.; Berrak, E.; Wolfer, A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann. Oncol. 2016, 27, 1525–1531. [Google Scholar] [CrossRef]
- Narayan, S.; Carlson, E.M.; Cheng, H.; Condon, K.; Du, H.; Eckley, S.; Hu, Y.; Jiang, Y.; Kumar, V.; Lewis, B.M.; et al. Novel second generation analogs of eribulin. Part III: Blood–brain barrier permeability and in vivo activity in a brain tumor model. Bioorg. Med. Chem. Lett. 2011, 21, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Ying, X.X. Brain Metastases from Breast Cancer and Response to Treatment with Eribulin: A Case Series. Breast Cancer Basic Clin. Res. 2015, 9, 19–24. [Google Scholar] [CrossRef]
- Matsuoka, H.; Tsurutani, J.; Tanizaki, J.; Iwasa, T.; Komoike, Y.; Koyama, A.; Nakagawa, K. Regression of brain metastases from breast cancer with eribulin: A case report. BMC Res. Notes 2013, 6, 541. [Google Scholar] [CrossRef] [Green Version]
- Catania, G.; Malaguti, P.; Gasparro, S.; Cognetti, F.; Vidiri, A.; Fabi, A. Activity of Eribulin Mesylate in Brain Metastasis from Breast Cancer: A Stone in a Pond? Oncology 2018, 94, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, F.T.S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297–e835. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, R.; Fromm, S.; Rudas, M.; Wenzel, C.; Harbauer, S.; Roessler, K.; Kitz, K.; Steger, G.G.; Weitmann, H.-D.; Poetter, R.; et al. Intensified local treatment and systemic therapy significantly increase survival in patients with brain metastases from advanced breast cancer—A retrospective analysis. Radiother. Oncol. 2006, 80, 313–317. [Google Scholar] [CrossRef]
- Sabatier, R.; Diéras, V.; Pivot, X.; Brain, E.; Roché, H.; Extra, J.-M.; Monneur, A.; Provansal, M.; Tarpin, C.; Bertucci, F.; et al. Safety Results and Analysis of Eribulin Efficacy according to Previous Microtubules-Inhibitors Sensitivity in the French Prospective Expanded Access Program for Heavily Pre-treated Metastatic Breast Cancer. Cancer Res. Treat. 2018, 50, 1226–1237. [Google Scholar] [CrossRef]
- Adamo, V.; Ricciardi, G.R.R.; Giuffrida, D.; Scandurra, G.; Russo, A.; Blasi, L.; Spadaro, P.; Iacono, C.; Parra, H.J.S.; Savarino, A.; et al. Eribulin mesylate use as third-line therapy in patients with metastatic breast cancer (VESPRY): A prospective, multicentre, observational study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919895755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witzel, I.; Laakmann, E.; Weide, R.; Neunhöffer, T.; Park-Simon, T.-J.; Schmidt, M.; Fasching, P.; Hesse, T.; Polasik, A.; Mohrmann, S.; et al. Treatment and outcomes of patients in the Brain Metastases in Breast Cancer Network Registry. Eur. J. Cancer 2018, 102, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Prestifilippo, A.; Grippaldi, D.; Blanco, G.; Memeo, L.; Puliafito, I.; Giuffrida, D. Eribulin efficacy based on type of metastatic site: A real-life study in heavily pretreated metastatic breast cancer. Future Oncol. 2017, 13, 5–10. [Google Scholar] [CrossRef]
- Ates, O.; Babacan, T.; Kertmen, N.; Sarici, F.; Cenoli, A.; Akin, S.; Karakas, Y.; Kilickap, S.; Ozisik, Y.; Sever, A.R.; et al. Efficacy and safety of eribulin monotherapy in patients with heavily pretreated metastatic breast cancer. J. Buon. Off. J. Balk. Union Oncol. 2016, 21, 375–381. [Google Scholar] [CrossRef]
- Arslan, C.; Dizdar, O.; Altundag, K. Chemotherapy and biological treatment options in breast cancer patients with brain metastasis: An update. Expert Opin. Pharmacother. 2014, 15, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.D.; Price, J.E.; Fujimaki, T.; Bucana, C.D.; Fidler, I.J. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am. J. Pathol. 1992, 141, 1115–1124. [Google Scholar] [PubMed]
- Tóth, K.; Vaughan, M.M.; Peress, N.S.; Slocum, H.K.; Rustum, Y.M. MDR1 P-glycoprotein is expressed by endothelial cells of newly formed capillaries in human gliomas but is not expressed in the neovasculature of other primary tumors. Am. J. Pathol. 1996, 149, 853–858. [Google Scholar]
- Bart, J.; Nagengast, W.B.; Coppes, R.P.; Wegman, T.D.; Van Der Graaf, W.T.A.; Groen, H.J.M.; Vaalburg, W.; De Vries, E.G.E.; Hendrikse, N.H. Irradiation of rat brain reduces P-glycoprotein expression and function. Br. J. Cancer 2007, 97, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, D.-X.; Zheng, R.; Tang, J.; Li, J.-X.; Hu, Y.-H. Influence of radiation on the blood-brain barrier and optimum time of chemotherapy. Int. J. Radiat. Oncol. 1990, 19, 1507–1510. [Google Scholar] [CrossRef]
- Byun, K.-D.; Ahn, S.G.; Baik, H.J.; Lee, A.; Bae, K.B.; An, M.S.; Kim, K.H.; Shin, J.H.; Park, H.K.; Cho, H.; et al. Eribulin Mesylate Combined with Local Treatment for Brain Metastasis from Breast Cancer: Two Case Reports. J. Breast Cancer 2016, 19, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.U.; Jeon, C.W.; Choi, J.H. Long-term survival with eribulin monotherapy after whole brain radiation therapy in a patient with brain metastasis from breast cancer. Asian J. Surg. 2020, 43, 1008–1009. [Google Scholar] [CrossRef]
- Darlix, A.; Griguolo, G.; Thezenas, S.; Kantelhardt, E.; Thomssen, C.; Dieci, M.V. Hormone receptors status: A strong determinant of the kinetics of brain metastases occurrence compared with HER2 status in breast cancer. J. Neurooncol. 2018, 138, 369–382. [Google Scholar] [CrossRef]
- Darlix, A.; Louvel, G.; Fraisse, J.; Jacot, W.; Brain, E.; Debled, M.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; Delaloge, S.; et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br. J. Cancer 2019, 121, 991–1000. [Google Scholar] [CrossRef]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Warren, L.E.; Bellon, J.R.; Punglia, R.S.; Claus, E.B.; Lee, E.Q.; Wen, P.Y.; Haas-Kogan, D.A.; et al. Brain Metastases in Newly Diagnosed Breast Cancer. JAMA Oncol. 2017, 3, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Romieu, G.; Campone, M.; Diéras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.-Y.; Gonçalves, A.; et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef]
- Nasrazadani, A.; Brufsky, A. Neratinib: The emergence of a new player in the management of HER2+ breast cancer brain metastasis. Future Oncol. 2020, 16, 247–254. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, M.; Zhang, J.; Wang, B.; Tao, Z.; Du, Y.; Zhang, S.; Cao, J.; Wang, L.; Hu, X. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res. Treat. 2020, 52, 1059–1066. [Google Scholar] [CrossRef]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Mohammad, A.S.; Saralkar, P.; Sprowls, S.A.; Vickers, S.D.; John, D.; Tallman, R.M.; Lucke-Wold, B.P.; Jarrell, K.E.; Pinti, M.; et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol. Res. 2018, 132, 47–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
| n = 20 | ||
|---|---|---|
| Age at inclusion, years (median, min-max) | 56.4 (29.7–74.3) | |
| Performance status at eribulin initiation | 1 | 15 (75) |
| ≥2 | 5 (25) | |
| Previous brain metastases treatments | None | 5 (25) |
| Whole brain radiotherapy | 8 (40) | |
| Stereotactic surgery | 5 (25) | |
| Brain neurosurgery | 4 (20) | |
| Carcinomatous meningitis | 2 (10) | |
| Priori systemic lines of treatment for MBC (median, min-max) | 4 (1–8) | |
| HR status | Positive | 10 (50) |
| Negative | 10 (50) | |
| HER2 status | Positive | 5 (25) |
| Negative | 15 (75) | |
| Triple negative phenotype | 7 (35) | |
| Number of lines post-eribulin (median, min-max) | 1 (0–5) |
| CNS PFS Cox Univariate Analysis (n = 20) | Hazard Ratio (95CI) | p-Value | |
|---|---|---|---|
| Age at inclusion, years | 0.98 (0.95–1.02) | 0.30 | |
| ECOG PS | 1 vs. ≥2 | 0.47 (0.14–1.60) | 0.23 |
| Pathological subtype | 0.03 | ||
| HR+ vs. TN | 0.23 (0.07–0.80) | 0.02 | |
| HER2+ vs. TN | 1.38 (0.37–5.01) | 0.63 | |
| Number of prior lines for MBC | 0.83 (0.59–1.18) | 0.30 | |
| Previous CNS treatment | Yes vs. No | 0.62 (0.21–1.80) | 0.38 |
| CNS therapies | WBRT vs. (SRS + neurosurgery) | 1.10 (0.35–3.51) | 0.87 |
| OS Cox Univariate Analysis (n = 20) | Hazard Ratio (95CI) | p-Value | |
|---|---|---|---|
| Age at inclusion, years | 0.99 (0.96–1.03) | 0.62 | |
| PS | 1 vs. ≥2 | 0.15 (0.04–0.58) | 0.006 |
| Pathological subtype | 0.27 | ||
| HR+ vs. TN | 0.49 (0.16–1.53) | 0.22 | |
| HER2+ vs. TN | 1.24 (0.37–4.10) | 0.73 | |
| Number of prior lines for MBC | 0.95 (0.69–1.31) | 0.76 | |
| Previous CNS treatment | Yes vs. No | 0.62 (0.21–1.80) | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatier, R.; Martin, J.; Vicier, C.; Guérin, M.; Monneur, A.; Provansal, M.; Tassy, L.; Tarpin, C.; Extra, J.-M.; Viret, F.; et al. Eribulin Efficacy on Brain Metastases in Heavily Pretreated Patients with Metastatic Breast Cancer. J. Clin. Med. 2021, 10, 1272. https://doi.org/10.3390/jcm10061272
Sabatier R, Martin J, Vicier C, Guérin M, Monneur A, Provansal M, Tassy L, Tarpin C, Extra J-M, Viret F, et al. Eribulin Efficacy on Brain Metastases in Heavily Pretreated Patients with Metastatic Breast Cancer. Journal of Clinical Medicine. 2021; 10(6):1272. https://doi.org/10.3390/jcm10061272
Chicago/Turabian StyleSabatier, Renaud, Johan Martin, Cécile Vicier, Mathilde Guérin, Audrey Monneur, Magali Provansal, Louis Tassy, Carole Tarpin, Jean-Marc Extra, Frédéric Viret, and et al. 2021. "Eribulin Efficacy on Brain Metastases in Heavily Pretreated Patients with Metastatic Breast Cancer" Journal of Clinical Medicine 10, no. 6: 1272. https://doi.org/10.3390/jcm10061272
APA StyleSabatier, R., Martin, J., Vicier, C., Guérin, M., Monneur, A., Provansal, M., Tassy, L., Tarpin, C., Extra, J.-M., Viret, F., & Goncalves, A. (2021). Eribulin Efficacy on Brain Metastases in Heavily Pretreated Patients with Metastatic Breast Cancer. Journal of Clinical Medicine, 10(6), 1272. https://doi.org/10.3390/jcm10061272







