The Relationships Between Serum DHEA-S and AMH Levels in Infertile Women: A Retrospective Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Biochemical Measurements
2.3. Statistical Analysis
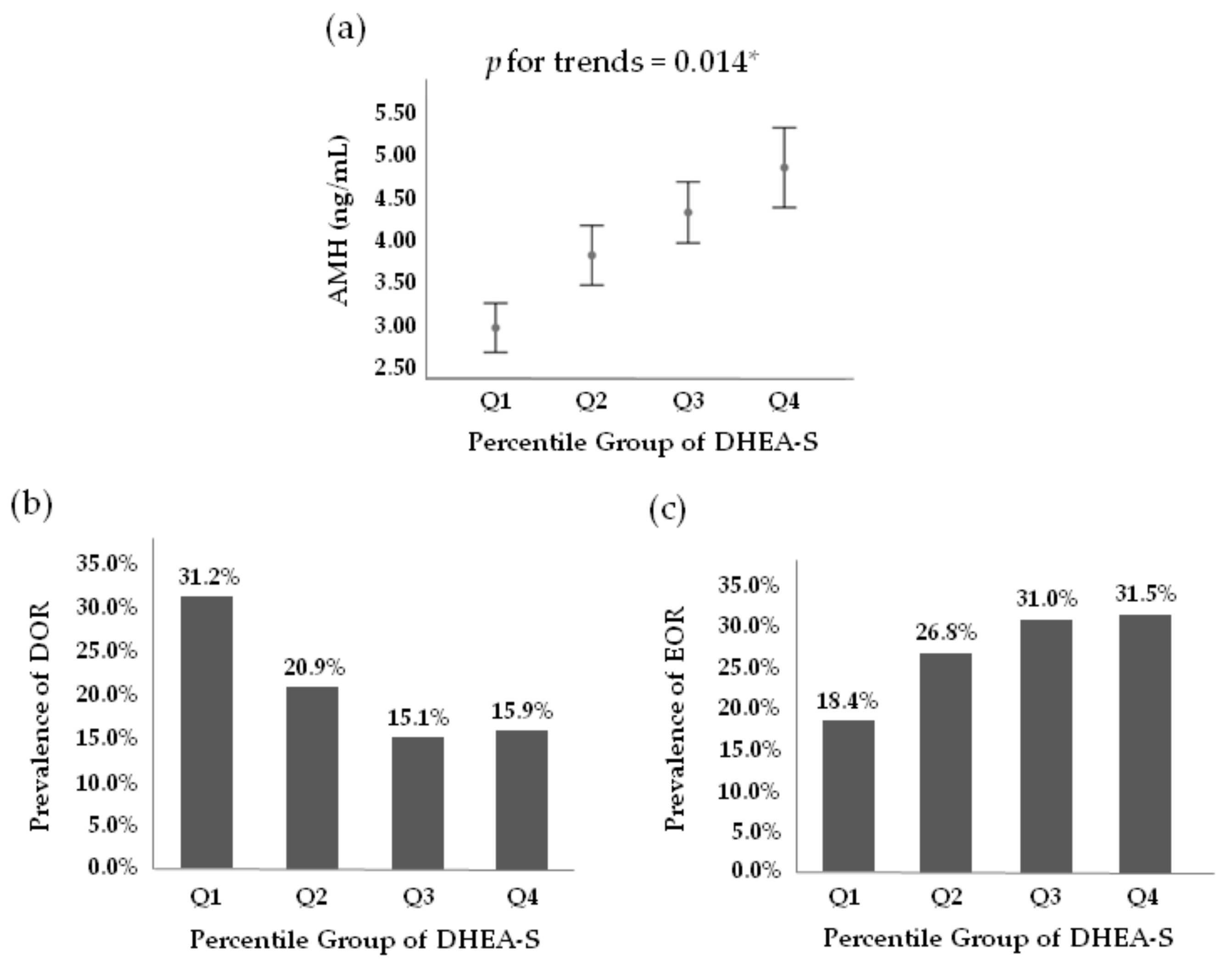
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.W.; La Marca, A.; Lambalk, C.; et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum. Reprod. Update 2014, 20, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.V.; Anderson, R.A.; Kelsey, T.W.; Christiansen, S.L.; Kristensen, S.G.; Jayaprakasan, K.; Raine-Fenning, N.; Campbell, B.K.; Yding Andersen, C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol. Hum. Reprod. 2013, 19, 519–527. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Mullerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, T.W.; Wright, P.; Nelson, S.M.; Anderson, R.A.; Wallace, W.H. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS ONE 2011, 6, e22024. [Google Scholar] [CrossRef] [PubMed]
- Lie Fong, S.; Visser, J.A.; Welt, C.K.; de Rijke, Y.B.; Eijkemans, M.J.; Broekmans, F.J.; Roes, E.M.; Peters, W.H.; Hokken-Koelega, A.C.; Fauser, B.C.; et al. Serum anti-müllerian hormone levels in healthy females: A nomogram ranging from infancy to adulthood. J. Clin. Endocrinol. Metab. 2012, 97, 4650–4655. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Herring, A.H.; Kesner, J.S.; Meadows, J.W.; Stanczyk, F.Z.; Hoberman, S.; Baird, D.D. Antimüllerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet. Gynecol. 2011, 117, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Broer, S.L.; Dólleman, M.; van Disseldorp, J.; Broeze, K.A.; Opmeer, B.C.; Bossuyt, P.M.; Eijkemans, M.J.; Mol, B.W.; Broekmans, F.J. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: An individual patient data meta-analysis. Fertil. Steril. 2013, 100, 420–429.e427. [Google Scholar] [CrossRef]
- Broer, S.L.; van Disseldorp, J.; Broeze, K.A.; Dolleman, M.; Opmeer, B.C.; Bossuyt, P.; Eijkemans, M.J.; Mol, B.W.; Broekmans, F.J. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: An individual patient data approach. Hum. Reprod. Update 2013, 19, 26–36. [Google Scholar] [CrossRef]
- Oh, S.R.; Choe, S.Y.; Cho, Y.J. Clinical application of serum anti-Mullerian hormone in women. Clin. Exp. Reprod. Med. 2019, 46, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Gidwani, B.; Dhongade, H.J. Pharmacological activities of dehydroepiandrosterone: A review. Steroids 2020, 153, 108507. [Google Scholar] [CrossRef]
- Hornsby, P.J. Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann. N. Y. Acad. Sci. 1995, 774, 29–46. [Google Scholar] [CrossRef]
- Labrie, F.; Luu-The, V.; Labrie, C.; Simard, J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Front. Neuroendocrinol. 2001, 22, 185–212. [Google Scholar] [CrossRef]
- Longcope, C. Dehydroepiandrosterone metabolism. J. Endocrinol. 1996, 150, S125–S127. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Handelsman, D.J. Role of androgens in the ovary. Mol. Cell. Endocrinol. 2018, 465, 36–47. [Google Scholar] [CrossRef]
- Astapova, O.; Minor, B.M.N.; Hammes, S.R. Physiological and Pathological Androgen Actions in the Ovary. Endocrinology 2019, 160, 1166–1174. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Li, N.; Liu, J.; Tan, S.C.; Low, T.Y.; Ma, Z. A dose-response and meta-analysis of dehydroepiandrosterone (DHEA) supplementation on testosterone levels: Perinatal prediction of randomized clinical trials. Exp. Gerontol. 2020, 141, 111110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Niu, W.; Wang, Y.; Xu, J.; Bao, X.; Wang, L.; Du, L.; Sun, Y. Dehydroepiandrosterone treatment in women with poor ovarian response undergoing IVF or ICSI: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2016, 33, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Poseidon, G.; Alviggi, C.; Andersen, C.Y.; Buehler, K.; Conforti, A.; De Placido, G.; Esteves, S.C.; Fischer, R.; Galliano, D.; Polyzos, N.P.; et al. A new more detailed stratification of low responders to ovarian stimulation: From a poor ovarian response to a low prognosis concept. Fertil. Steril. 2016, 105, 1452–1453. [Google Scholar] [CrossRef]
- Lauritsen, M.P.; Bentzen, J.G.; Pinborg, A.; Loft, A.; Forman, J.L.; Thuesen, L.L.; Cohen, A.; Hougaard, D.M.; Nyboe Andersen, A. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum. Reprod. 2014, 29, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hong, L.; Nie, M.; Wang, Q.; Fang, Y.; Dai, Y.; Zhai, Y.; Wang, S.; Yin, C.; Yang, X. The effect of dehydroepiandrosterone supplementation on ovarian response is associated with androgen receptor in diminished ovarian reserve women. J. Ovarian Res. 2017, 10, 32. [Google Scholar] [CrossRef]
- Sen, A.; Prizant, H.; Light, A.; Biswas, A.; Hayes, E.; Lee, H.J.; Barad, D.; Gleicher, N.; Hammes, S.R. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3008–3013. [Google Scholar] [CrossRef]
- Laird, M.; Thomson, K.; Fenwick, M.; Mora, J.; Franks, S.; Hardy, K. Androgen Stimulates Growth of Mouse Preantral Follicles In Vitro: Interaction With Follicle-Stimulating Hormone and with Growth Factors of the TGFβ Superfamily. Endocrinology 2017, 158, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Marrocco, D.L.; Amato, F.; Ritter, L.J.; Norman, R.J.; Gilchrist, R.B.; Armstrong, D.T. Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol. Reprod. 2005, 73, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Grynberg, M.; Pierre, A.; Arouche, N.; Massart, P.; Belville, C.; Hesters, L.; Frydman, R.; Catteau-Jonard, S.; Fanchin, R.; et al. FSH and its second messenger cAMP stimulate the transcription of human anti-Müllerian hormone in cultured granulosa cells. Mol. Endocrinol. 2011, 25, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.P.; Jin, M.; Rao, J.P.; Chen, J.; Wang, L.Q.; Huang, C.C.; Yang, S.Q.; Yao, Q.P.; Feng, L.; Shen, J.M.; et al. Role of anti-Mullerian hormone and testosterone in follicular growth: A cross-sectional study. BMC Endocr. Disord. 2020, 20, 101. [Google Scholar] [CrossRef]
- Pigny, P.; Merlen, E.; Robert, Y.; Cortet-Rudelli, C.; Decanter, C.; Jonard, S.; Dewailly, D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: Relationship to the ovarian follicle excess and to the follicular arrest. J. Clin. Endocrinol. Metab. 2003, 88, 5957–5962. [Google Scholar] [CrossRef]
- Xie, M.; Zhong, Y.; Xue, Q.; Wu, M.; Deng, X.; Santos, H.O.; Tan, S.C.; Kord-Varkaneh, H.; Jiao, P. Impact of dehydroepianrosterone (DHEA) supplementation on serum levels of insulin-like growth factor 1 (IGF-1): A dose-response meta-analysis of randomized controlled trials. Exp. Gerontol. 2020, 136, 110949. [Google Scholar] [CrossRef]
- Monte, A.P.O.; Barros, V.R.P.; Santos, J.M.; Menezes, V.G.; Cavalcante, A.Y.P.; Gouveia, B.B.; Bezerra, M.E.S.; Macedo, T.J.S.; Matos, M.H.T. Immunohistochemical localization of insulin-like growth factor-1 (IGF-1) in the sheep ovary and the synergistic effect of IGF-1 and FSH on follicular development in vitro and LH receptor immunostaining. Theriogenology 2019, 129, 61–69. [Google Scholar] [CrossRef]
- Baumgarten, S.C.; Convissar, S.M.; Fierro, M.A.; Winston, N.J.; Scoccia, B.; Stocco, C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells. J. Clin. Endocrinol. Metab. 2014, 99, 2995–3004. [Google Scholar] [CrossRef]
- Zhao, J.; Taverne, M.A.; Van Der Weijden, G.C.; Bevers, M.M.; Van Den Hurk, R. Insulin-like growth factor-I (IGF-I) stimulates the development of cultured rat pre-antral follicles. Mol. Reprod. Dev. 2001, 58, 287–296. [Google Scholar] [CrossRef]
- Magalhães-Padilha, D.M.; Fonseca, G.R.; Haag, K.T.; Wischral, A.; Gastal, M.O.; Jones, K.L.; Geisler-Lee, J.; Figueiredo, J.R.; Gastal, E.L. Long-term in vitro culture of ovarian cortical tissue in goats: Effects of FSH and IGF-I on preantral follicular development and FSH and IGF-I receptor mRNA expression. Cell Tissue Res. 2012, 350, 503–511. [Google Scholar] [CrossRef]
- Bezerra, M.É.S.; Barberino, R.S.; Menezes, V.G.; Gouveia, B.B.; Macedo, T.J.S.; Santos, J.M.S.; Monte, A.P.O.; Barros, V.R.P.; Matos, M.H.T. Insulin-like growth factor-1 (IGF-1) promotes primordial follicle growth and reduces DNA fragmentation through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signalling pathway. Reprod. Fertil. Dev. 2018, 30, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, L.; Vidali, A.; Lindheim, S.R.; Sauer, M.V. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-binding protein-1 and -3 vary as a function of ovarian reserve and ovarian stimulation. J. Assist. Reprod. Genet. 1998, 15, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod. Biomed. Online 2010, 21, 360–365. [Google Scholar] [CrossRef]
- Yilmaz, N.; Uygur, D.; Inal, H.; Gorkem, U.; Cicek, N.; Mollamahmutoglu, L. Dehydroepiandrosterone supplementation improves predictive markers for diminished ovarian reserve: Serum AMH, inhibin B and antral follicle count. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 257–260. [Google Scholar] [CrossRef]
- Singh, N.; Zangmo, R.; Kumar, S.; Roy, K.K.; Sharma, J.B.; Malhotra, N.; Vanamail, P. A prospective study on role of dehydroepiandrosterone (DHEA) on improving the ovarian reserve markers in infertile patients with poor ovarian reserve. Gynecol. Endocrinol. 2013, 29, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Zangmo, R.; Singh, N.; Kumar, S.; Vanamail, P.; Tiwari, A. Role of dehydroepiandrosterone in improving oocyte and embryo quality in IVF cycles. Reprod. Biomed. Online 2014, 28, 743–747. [Google Scholar] [CrossRef][Green Version]
- Agarwal, R.; Shruthi, R.; Radhakrishnan, G.; Singh, A. Evaluation of Dehydroepiandrosterone Supplementation on Diminished Ovarian Reserve: A Randomized, Double-Blinded, Placebo-Controlled Study. J. Obstet. Gynaecol. India 2017, 67, 137–142. [Google Scholar] [CrossRef]
- Yeung, T.W.; Li, R.H.; Lee, V.C.; Ho, P.C.; Ng, E.H. A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 2013, 98, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.W.; Chai, J.; Li, R.H.; Lee, V.C.; Ho, P.C.; Ng, E.H. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertil. Steril. 2014, 102, 108–115.e101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Shu, J.; Guo, J.; Chang, H.M.; Leung, P.C.K.; Sheng, J.Z.; Huang, H. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: A systematic review and network meta-analysis. Hum. Reprod. Update 2020, 26, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.A.; El Shourbagy, S.; St. John, J.C. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril. 2006, 85, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.D.; Hsieh, Y.Y.; Hsieh, J.N.; Chang, C.C.; Yang, C.Y.; Yang, J.G.; Cheng, W.L.; Tsai, F.J.; Liu, C.S. Mitochondria DNA deletion and copy numbers of cumulus cells associated with in vitro fertilization outcomes. J. Reprod. Med. 2010, 55, 491–497. [Google Scholar]
- Lin, L.T.; Wang, P.H.; Wen, Z.H.; Li, C.J.; Chen, S.N.; Tsai, E.M.; Cheng, J.T.; Tsui, K.H. The Application of Dehydroepiandrosterone on Improving Mitochondrial Function and Reducing Apoptosis of Cumulus Cells in Poor Ovarian Responders. Int. J. Med. Sci. 2017, 14, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Wang, P.H.; Lin, L.T.; Li, C.J. DHEA protects mitochondria against dual modes of apoptosis and necroptosis in human granulosa HO23 cells. Reproduction 2017, 154, 101–110. [Google Scholar] [CrossRef]
- Li, C.J.; Chen, S.N.; Lin, L.T.; Chern, C.U.; Wang, P.H.; Wen, Z.H.; Tsui, K.H. Dehydroepiandrosterone Ameliorates Abnormal Mitochondrial Dynamics and Mitophagy of Cumulus Cells in Poor Ovarian Responders. J. Clin. Med. 2018, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Ramer, I.; Kanninen, T.T.; Sisti, G.; Witkin, S.S.; Spandorfer, S.D. Association of in vitro fertilization outcome with circulating insulin-like growth factor components prior to cycle initiation. Am. J. Obstet. Gynecol. 2015, 213, 356.e1–356.e6. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Minis, E.; Irani, M.; Kreines, F.; Witkin, S.S.; Spandorfer, S.D. Insulin-like growth factor-1 and soluble FMS-like tyrosine kinase-1 prospectively predict cancelled IVF cycles. J. Assist. Reprod. Genet. 2019, 36, 2485–2491. [Google Scholar] [CrossRef]
- Wang, T.H.; Chang, C.L.; Wu, H.M.; Chiu, Y.M.; Chen, C.K.; Wang, H.S. Insulin-like growth factor-II (IGF-II), IGF-binding protein-3 (IGFBP-3), and IGFBP-4 in follicular fluid are associated with oocyte maturation and embryo development. Fertil. Steril. 2006, 86, 1392–1401. [Google Scholar] [CrossRef]

| All Population (n = 2155) | Age < 35 Years (n = 972) | Age ≥ 35 Years (n = 1183) | |
|---|---|---|---|
| Age (years) | 35.1 ± 4.7 | 30.9 ± 2.7 | 38.6 ± 2.8 |
| Body height (cm) | 160.5 ± 5.8 | 160.8 ± 5.9 | 160.3 ± 5.7 |
| Body weight (kg) | 57.8 ± 10.5 | 56.8 ± 10.7 | 58.7 ± 10.4 |
| BMI (kg/m2) | 22.4 ± 3.8 | 21.9 ± 3.9 | 22.8 ± 3.8 |
| TSH (μIU/mL) | 1.7 ± 1.4 | 1.7 ± 1.7 | 1.7 ± 1.1 |
| Prolactin (ng/mL) | 15.5 ± 15.2 | 15.2 ± 12.0 | 15.7 ± 17.4 |
| 25(OH)vitamin D (ng/mL) | 21.7 ± 6.9 | 21.0 ± 6.2 | 22.2 ± 7.3 |
| FSH (mIU/mL) | 5.4 ± 4.0 | 4.9 ± 3.0 | 5.8 ± 4.6 |
| Testosterone (ng/mL) | 0.35 ± 0.45 | 0.38 ± 0.48 | 0.32 ± 0.43 |
| DHEA-S (μg/dL) | 240.7 ± 113.6 | 262.7 ± 107.6 | 222.5 ± 115.3 |
| AMH (ng/mL) | 4.0 ± 4.0 | 5.2 ± 4.5 | 2.9 ± 3.1 |
| Variables | All Women | Age < 35 Years | Age ≥ 35 Years | |||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| DHEA-S (μg/dL) | 0.103 | <0.001 | 0.113 | 0.004 | 0.091 | 0.009 |
| Age (years) | −0.336 | <0.001 | −0.133 | 0.001 | −0.334 | <0.001 |
| BMI (kg/m2) | 0.047 | 0.059 | 0.058 | 0.131 | 0.035 | 0.314 |
| FSH (mIU/mL) | −0.070 | 0.005 | −0.076 | 0.045 | −0.076 | 0.027 |
| Prolactin (ng/mL) | −0.034 | 0.172 | −0.061 | 0.112 | −0.022 | 0.521 |
| Quartile of Serum DHEA-S | |||||
|---|---|---|---|---|---|
| Q1 (n = 502) | Q2 (n = 523) | Q3 (n = 523) | Q4 (n = 513) | p-Value for Trends | |
| DHEA-S (μg/dL) | ≤168.05 | 168.05~221.0 | 221.0~292.95 | ≥292.95 | |
| Age (years) | 36.8 ± 4.5 * | 35.8 ± 4.5 * | 34.4 ± 4.5 * | 33.4 ± 4.7 | <0.001 |
| Body height (cm) | 160.6 ± 5.5 | 160.8 ± 5.5 * | 160.3 ± 5.7 | 159.8 ± 5.4 | 0.018 |
| Body weight (kg) | 57.4 ± 10.4 | 57.7 ± 10.3 | 57.3 ± 10.0 | 57.9 ± 10.9 | 0.669 |
| BMI (kg/m2) | 22.3 ± 3.9 | 22.3 ± 3.8 | 22.3 ± 3.8 | 22.6 ± 4.1 | 0.161 |
| TSH (μIU/mL) | 1.7 ± 1.5 | 1.6± 1.0 | 1.6 ± 1.0 | 1.7 ± 1.9 | 0.931 |
| Prolactin (ng/mL) | 14.5 ± 13.4 | 15.0 ± 11.7 | 15.8 ± 10.9 | 16.5 ± 22.1 | 0.025 |
| Vitamin D (ng/mL) | 21.6 ± 7.3 | 21.4 ± 6.9 | 21.7 ± 6.5 | 22.3 ± 6.9 | 0.192 |
| FSH (mIU/mL) | 5.7 ± 4.2 * | 5.7 ± 4.8 * | 5.0 ± 3.2 | 5.0 ± 2.7 | <0.001 |
| Testosterone (ng/mL) | 0.23 ± 0.10 * | 0.28 ± 0.14 * | 0.32 ± 0.13 * | 0.37 ± 0.15 | <0.001 |
| AMH (ng/mL) | 2.9 ± 2.9 * | 3.8 ± 3.7 * | 4.3 ± 3.8 | 4.8 ± 5.0 | <0.001 |
| Age (Years) | Mean | Percentile | n | ||||
|---|---|---|---|---|---|---|---|
| 5th | 10th | Median | 90th | 95th | |||
| 20~25 | 291.7 | 117.7 | 157.3 | 272.3 | 470.1 | 541.5 | 38 |
| 26~30 | 280.2 | 139.9 | 158.8 | 264.5 | 412.6 | 476.0 | 310 |
| 31~35 | 248.1 | 109.0 | 134.8 | 234.2 | 370.4 | 423.5 | 753 |
| 36~40 | 226.7 | 94.6 | 115.1 | 206.1 | 354.0 | 432.2 | 682 |
| 41~46 | 204.1 | 80.8 | 104.6 | 181.4 | 314.6 | 367.3 | 278 |
| All | 240.7 | 102.8 | 125.1 | 221.0 | 370.4 | 436.8 | 2061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.-T.; Tsui, K.-H. The Relationships Between Serum DHEA-S and AMH Levels in Infertile Women: A Retrospective Cross-Sectional Study. J. Clin. Med. 2021, 10, 1211. https://doi.org/10.3390/jcm10061211
Lin L-T, Tsui K-H. The Relationships Between Serum DHEA-S and AMH Levels in Infertile Women: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(6):1211. https://doi.org/10.3390/jcm10061211
Chicago/Turabian StyleLin, Li-Te, and Kuan-Hao Tsui. 2021. "The Relationships Between Serum DHEA-S and AMH Levels in Infertile Women: A Retrospective Cross-Sectional Study" Journal of Clinical Medicine 10, no. 6: 1211. https://doi.org/10.3390/jcm10061211
APA StyleLin, L.-T., & Tsui, K.-H. (2021). The Relationships Between Serum DHEA-S and AMH Levels in Infertile Women: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine, 10(6), 1211. https://doi.org/10.3390/jcm10061211







