Common Genetic Variation in MC4R Does Not Affect Atherosclerotic Plaque Phenotypes and Cardiovascular Disease Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. General Population: CCHS and CGPS
2.3. General Population: UK Biobank
2.4. Population at Risk for Cardiovascular Disease: PROSPER
2.5. Atherosclerotic Plaque Phenotype: Athero-Express Study
3. Results
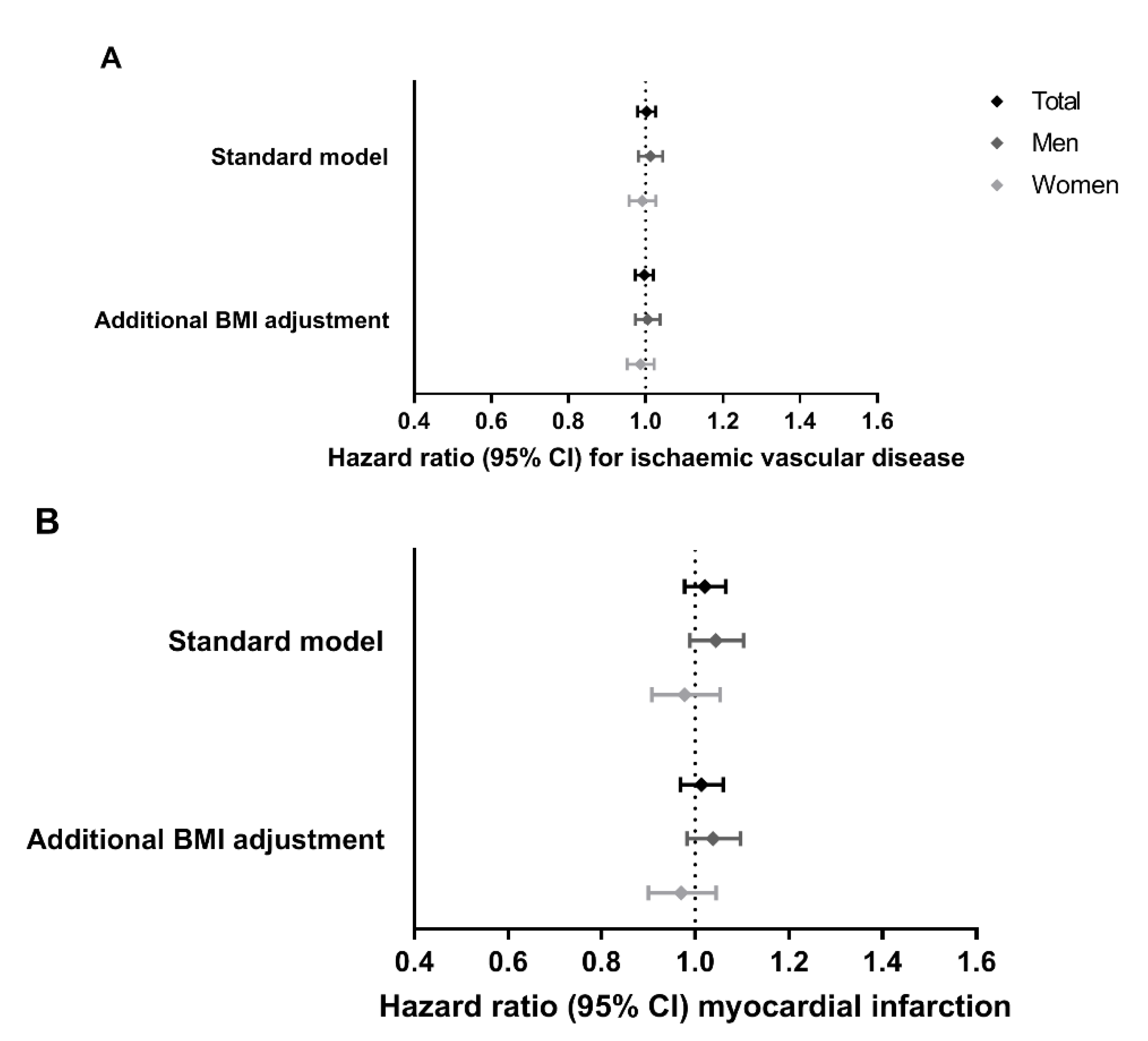
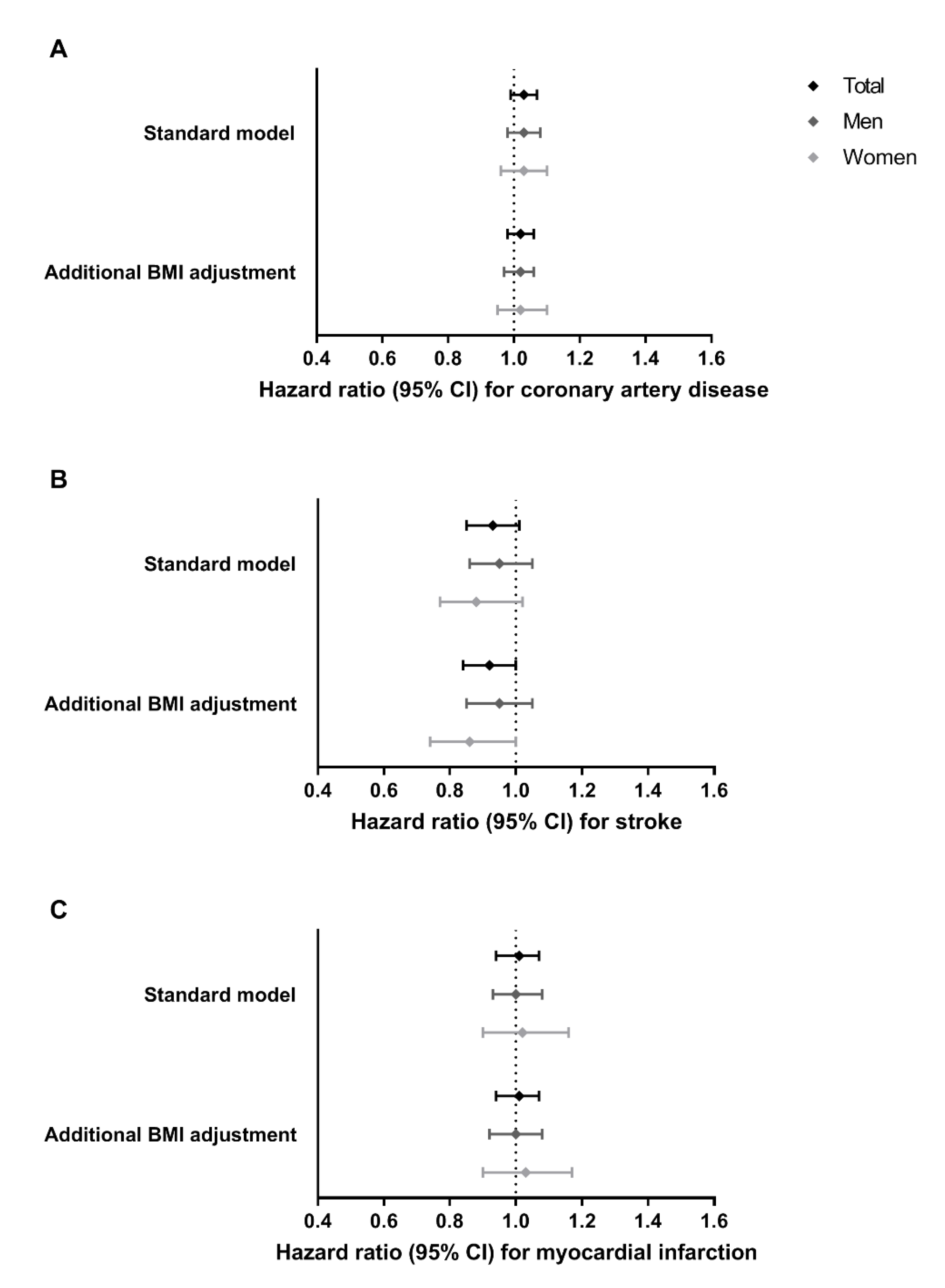
3.1. Associations of MC4R Genotype with Cardiovascular Disease Outcomes in the General Population
3.2. Associations of MC4R Genotype with Cardiovascular Disease Outcomes in an Aged Population
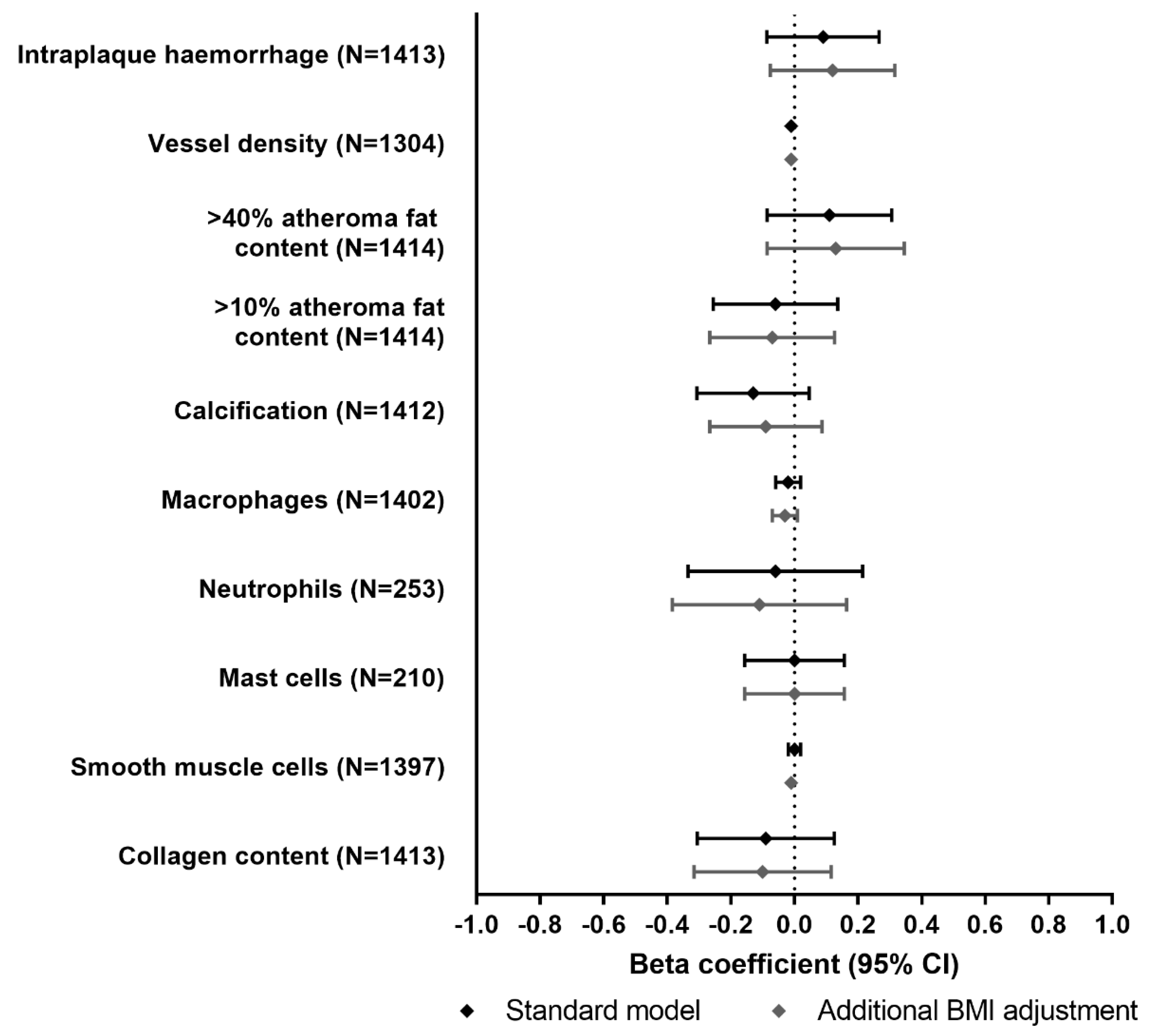
3.3. Associations of MC4R Genotype with Atherosclerotic Plaque Phenotype
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.S.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. New Engl. J. Med. 2003, 348, 1085–1095. [Google Scholar] [CrossRef]
- Collet, T.-H.; Dubern, B.; Mokrosinski, J.; Connors, H.; Keogh, J.M.; Mendes de Oliveira, E.; Henning, E.; Poitou-Bernert, C.; Oppert, J.-M.; Tounian, P.; et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 2017, 6, 1321–1329. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferre, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Molden, B.M.; Cooney, K.A.; West, K.; Van Der Ploeg, L.H.T.; Baldini, G. Temporal cAMP Signaling Selectivity by Natural and Synthetic MC4R Agonists. Mol. Endocrinol. 2015, 29, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, C.M.; Horvath, T.L. Mitochondrial dynamics in the central regulation of metabolism. Nat. Rev. Endocrinol. 2014, 10, 650–658. [Google Scholar] [CrossRef]
- Farooqi, I.S.; O’Rahilly, S. Monogenic obesity in humans. Annu Rev. Med. 2005, 56, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Mokrosinski, J.; Mendes de Oliveira, E.; Li, C.; Sharp, S.J.; Luan, J.; Brouwers, B.; Ayinampudi, V.; Bowker, N.; Kerrison, N.; et al. Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity. Cell 2019, 177, 597–607. [Google Scholar] [CrossRef]
- Loos, R.J.; Lindgren, C.M.; Li, S.; Wheeler, E.; Zhao, J.H.; Prokopenko, I.; Inouye, M.; Freathy, R.M.; Attwood, A.P.; Beckmann, J.S.; et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 2008, 40, 768–775. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Lango Allen, H.; Lindgren, C.M.; Luan, J.; Magi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A. Commentary: Two-sample Mendelian randomization: Opportunities and challenges. Int J. Epidemiol. 2016, 45, 908–915. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Palmer, T.M.; Benn, M.; Zacho, J.; Tybjaerg-Hansen, A.; Davey Smith, G.; Timpson, N.J. The effect of elevated body mass index on ischemic heart disease risk: Causal estimates from a Mendelian randomisation approach. Plos Med. 2012, 9, e1001212. [Google Scholar] [CrossRef]
- Holmes, M.V.; Lange, L.A.; Palmer, T.; Lanktree, M.B.; North, K.E.; Almoguera, B.; Buxbaum, S.; Chandrupatla, H.R.; Elbers, C.C.; Guo, Y.; et al. Causal effects of body mass index on cardiometabolic traits and events: A Mendelian randomization analysis. Am. J. Hum. Genet. 2014, 94, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Dale, C.E.; Fatemifar, G.; Palmer, T.M.; White, J.; Prieto-Merino, D.; Zabaneh, D.; Engmann, J.E.L.; Shah, T.; Wong, A.; Warren, H.R.; et al. Causal Associations of Adiposity and Body Fat Distribution With Coronary Heart Disease, Stroke Subtypes, and Type 2 Diabetes Mellitus: A Mendelian Randomization Analysis. Circulation 2017, 135, 2373–2388. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.B.; Frikke-Schmidt, R.; West, A.S.; Grande, P.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013, 34, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G. Remnant Cholesterol as a Causal Risk Factor for Ischemic Heart Disease. J. Am. Coll. Cardiol. 2013, 61, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Loss-of-Function Mutations in APOC3 and Risk of Ischemic Vascular Disease. New Engl. J. Med. 2014, 371, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Cobbe, S.M.; Bollen, E.L.; Buckley, B.M.; Ford, I.; Jukema, J.W.; Hyland, M.; Gaw, A.; et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am. J. Cardiol. 1999, 84, 1192–1197. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Trompet, S.; de Craen, A.J.; Postmus, I.; Ford, I.; Sattar, N.; Caslake, M.; Stott, D.J.; Buckley, B.M.; Sacks, F.; Devlin, J.J.; et al. Replication of LDL GWAs hits in PROSPER/PHASE as validation for future (pharmaco)genetic analyses. Bmc Med. Genet. 2011, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, B.A.; Velema, E.; Schoneveld, A.H.; de Vries, J.P.; de Bruin, P.; Seldenrijk, C.A.; de Kleijn, D.P.; Busser, E.; van der Graaf, Y.; Moll, F.; et al. Athero-express: Differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur J. Epidemiol. 2004, 19, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- van Lammeren, G.W.; Reichmann, B.L.; Moll, F.L.; Bots, M.L.; de Kleijn, D.P.; de Vries, J.P.; Pasterkamp, G.; de Borst, G.J. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke 2011, 42, 2550–2555. [Google Scholar] [CrossRef]
- van den Borne, P.; van der Laan, S.W.; Bovens, S.M.; Koole, D.; Kowala, M.C.; Michael, L.F.; Schoneveld, A.H.; van de Weg, S.M.; Velema, E.; de Vries, J.P.; et al. Leukotriene B4 levels in human atherosclerotic plaques and abdominal aortic aneurysms. PLoS ONE 2014, 9, e86522. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; van der Velden, D.; Quax, P.H.; de Borst, G.J.; de Vries, J.P.; Moll, F.L.; Kuiper, J.; Toes, R.E.; de Jager, S.C.; de Kleijn, D.P.; et al. Circulating immunoglobulins are not associated with intraplaque mast cell number and other vulnerable plaque characteristics in patients with carotid artery stenosis. PLoS ONE 2014, 9, e88984. [Google Scholar] [CrossRef]
- Nikpay, M.; Goel, A.; Won, H.H.; Hall, L.M.; Willenborg, C.; Kanoni, S.; Saleheen, D.; Kyriakou, T.; Nelson, C.P.; Hopewell, J.C.; et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar] [CrossRef]
- Rovella, V.; Anemona, L.; Cardellini, M.; Scimeca, M.; Saggini, A.; Santeusanio, G.; Bonanno, E.; Montanaro, M.; Legramante, I.M.; Ippoliti, A.; et al. The role of obesity in carotid plaque instability: Interaction with age, gender, and cardiovascular risk factors. Cardiovasc. Diabetol. 2018, 17, 46. [Google Scholar] [CrossRef] [PubMed]

| Non-Carriers | Heterozygotes | Homozygotes | |
|---|---|---|---|
| Number of participants, N (%) | 60,080 (57%) | 39,426 (37%) | 6512 (6%) |
| Women, N (%) | 33,005 (55%) | 21,750 (55%) | 3626 (56%) |
| Age (years) | 58 (48–67) | 58 (48–67) | 58 (48–68) |
| Body mass index (kg/m2) | 25 (23–28) | 26 (23–29) | 26 (23–29) |
| Hypertension, N (%) | 35,708 (59%) | 23,552 (60%) | 3894 (60%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blauw, L.L.; Noordam, R.; van der Laan, S.W.; Trompet, S.; Kooijman, S.; van Heemst, D.; Jukema, J.W.; van Setten, J.; de Borst, G.J.; Tybjærg-Hansen, A.; et al. Common Genetic Variation in MC4R Does Not Affect Atherosclerotic Plaque Phenotypes and Cardiovascular Disease Outcomes. J. Clin. Med. 2021, 10, 932. https://doi.org/10.3390/jcm10050932
Blauw LL, Noordam R, van der Laan SW, Trompet S, Kooijman S, van Heemst D, Jukema JW, van Setten J, de Borst GJ, Tybjærg-Hansen A, et al. Common Genetic Variation in MC4R Does Not Affect Atherosclerotic Plaque Phenotypes and Cardiovascular Disease Outcomes. Journal of Clinical Medicine. 2021; 10(5):932. https://doi.org/10.3390/jcm10050932
Chicago/Turabian StyleBlauw, Lisanne L., Raymond Noordam, Sander W. van der Laan, Stella Trompet, Sander Kooijman, Diana van Heemst, Johan Wouter Jukema, Jessica van Setten, Gert J. de Borst, Anne Tybjærg-Hansen, and et al. 2021. "Common Genetic Variation in MC4R Does Not Affect Atherosclerotic Plaque Phenotypes and Cardiovascular Disease Outcomes" Journal of Clinical Medicine 10, no. 5: 932. https://doi.org/10.3390/jcm10050932
APA StyleBlauw, L. L., Noordam, R., van der Laan, S. W., Trompet, S., Kooijman, S., van Heemst, D., Jukema, J. W., van Setten, J., de Borst, G. J., Tybjærg-Hansen, A., Pasterkamp, G., Berbée, J. F. P., & Rensen, P. C. N. (2021). Common Genetic Variation in MC4R Does Not Affect Atherosclerotic Plaque Phenotypes and Cardiovascular Disease Outcomes. Journal of Clinical Medicine, 10(5), 932. https://doi.org/10.3390/jcm10050932







