Predictors Associated with Outcomes of Epidural Blood Patch in Patients with Spontaneous Intracranial Hypotension
Abstract
1. Introduction
2. Materials and Methods
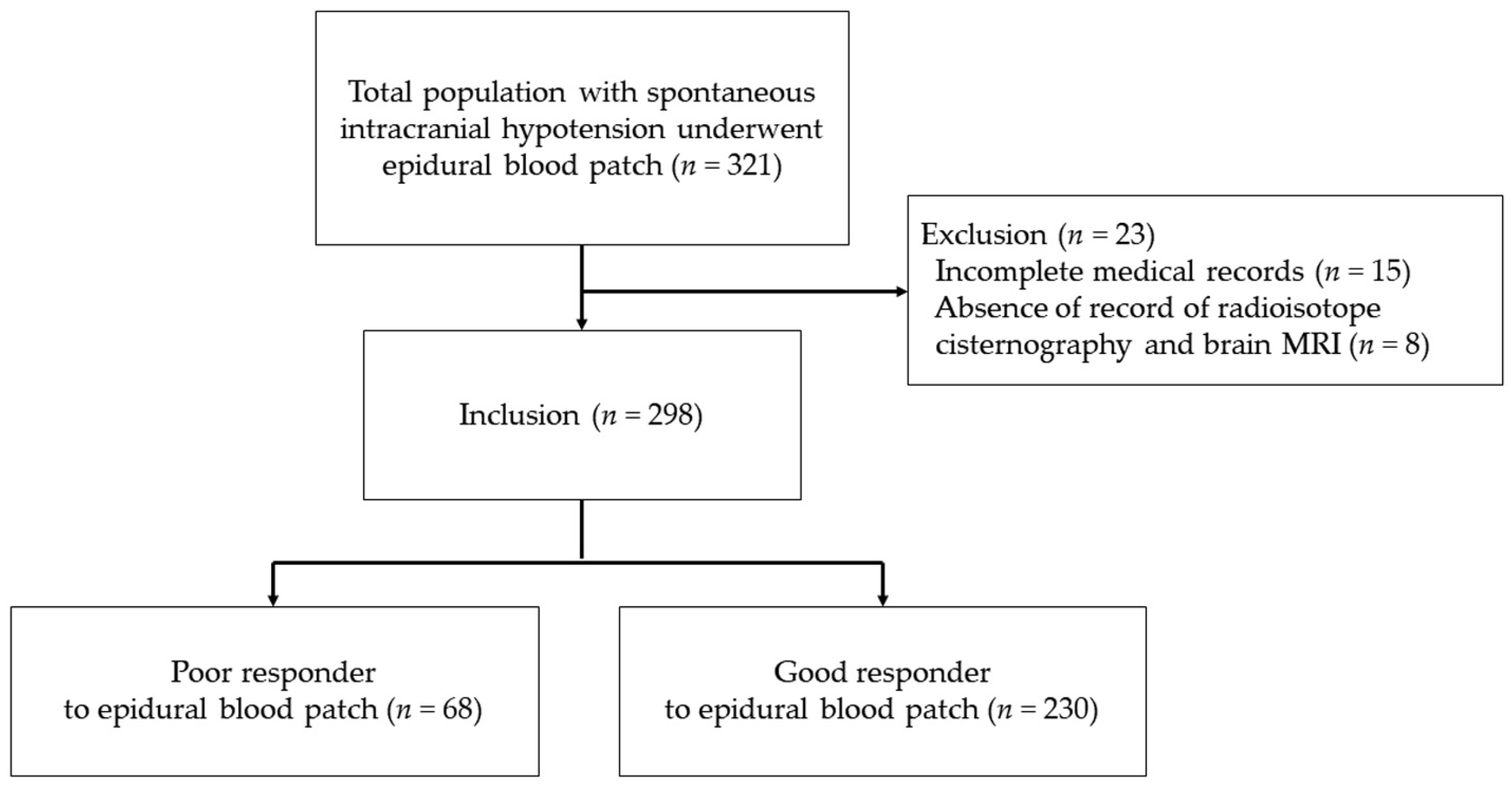
2.1. Patients
2.2. Demographic, Clinical Characteristics, and Neuroimaging
2.3. Epidural Blood Patch
2.4. Laboratory Data
2.5. Hospitalization Period
2.6. Treatment Response
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characterstics
3.2. Neuroimaging
3.3. Number, Volume, and Site of Epidural Blood Patch
3.4. Laboratory Data
3.5. Hospitalization Period
3.6. Univariate and Multivariate Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kranz, P.G.; Gray, L.; Malinzak, M.D.; Amrhein, T.J. Spontaneous Intracranial Hypotension: Pathogenesis, Diagnosis, and Treatment. Neuroimaging Clin. 2019, 29, 581–594. [Google Scholar] [CrossRef]
- Kranz, P.G.; Malinzak, M.D.; Amrhein, T.J.; Gray, L. Update on the Diagnosis and Treatment of Spontaneous Intracranial Hypotension. Curr. Pain Headache Rep. 2017, 21, 37. [Google Scholar] [CrossRef]
- Inamasu, J.; Moriya, S.; Shibata, J.; Kumai, T.; Hirose, Y. Spontaneous intracranial hypotension manifesting as a unilateral subdural hematoma with a marked midline shift. Case Rep. Neurol. 2015, 7, 71–77. [Google Scholar] [CrossRef]
- Amrhein, T.J.; Kranz, P.G. Spontaneous Intracranial Hypotension: Imaging in Diagnosis and Treatment. Radiol. Clin. N. Am. 2019, 57, 439–451. [Google Scholar] [CrossRef]
- Kranz, P.G.; Gray, L.; Amrhein, T.J. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache 2018, 58, 948–959. [Google Scholar] [CrossRef]
- Davidson, B.; Nassiri, F.; Mansouri, A.; Badhiwala, J.H.; Witiw, C.D.; Shamji, M.F.; Peng, P.W.; Farb, R.I.; Bernstein, M. Spontaneous Intracranial Hypotension: A Review and Introduction of an Algorithm for Management. World Neurosurg. 2017, 101, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hong, J.H. Epidural Blood Patches in a Patient with Multi-level Cerebrospinal Fluid Leakage That Was Induced by Spontaneous Intracranial Hypotension. Korean J. Pain 2010, 23, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sencakova, D.; Mokri, B.; McClelland, R.L. The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology 2001, 57, 1921–1923. [Google Scholar] [CrossRef]
- Berroir, S.; Loisel, B.; Ducros, A.; Boukobza, M.; Tzourio, C.; Valade, D.; Bousser, M.G. Early epidural blood patch in spontaneous intracranial hypotension. Neurology 2004, 63, 1950–1951. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, P.; Ailani, J. A Review of Spontaneous Intracranial Hypotension. Curr. Neurol. Neurosci. Rep. 2019, 19, 22. [Google Scholar] [CrossRef]
- Wu, J.W.; Hseu, S.S.; Fuh, J.L.; Lirng, J.F.; Wang, Y.F.; Chen, W.T.; Chen, S.P.; Wang, S.J. Factors predicting response to the first epidural blood patch in spontaneous intracranial hypotension. Brain 2017, 140, 344–352. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Hashizume, K.; Watanabe, K.; Inoue, S.; Furuya, H. Fluoroscopically guided epidural blood patch in patients with postdural puncture headache after spinal and epidural anesthesia. J. Anesth. 2011, 25, 450–453. [Google Scholar] [CrossRef]
- Levi, V.; Di Laurenzio, N.E.; Franzini, A.; Tramacere, I.; Erbetta, A.; Chiapparini, L.; D’Amico, D.; Franzini, A.; Messina, G. Lumbar epidural blood patch: Effectiveness on orthostatic headache and MRI predictive factors in 101 consecutive patients affected by spontaneous intracranial hypotension. J. Neurosurg. 2020, 132, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Karm, M.H.; Choi, J.H.; Kim, D.; Park, J.Y.; Yun, H.J.; Suh, J.H. Predictors of the Treatment Response of Spontaneous Intracranial Hypotension to an Epidural Blood Patch. Medicine 2016, 95, e3578. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J. The international classification of headache disorders. 2nd edition (ICHD-II). Rev. Neurol 2005, 161, 689–691. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Pannullo, S.C.; Reich, J.B.; Krol, G.; Deck, M.D.; Posner, J.B. MRI changes in intracranial hypotension. Neurology 1993, 43, 919–926. [Google Scholar] [CrossRef]
- Schievink, W.I. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA 2006, 295, 2286–2296. [Google Scholar] [CrossRef]
- Fishman, R.A.; Dillon, W.P. Dural enhancement and cerebral displacement secondary to intracranial hypotension. Neurology 1993, 43, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.M.; McLean, L.A.; Heilbrun, M.E.; Salzman, K.L. Intracranial Hypotension: Improved MRI Detection with Diagnostic Intracranial Angles. Am. J. Roentgenol. 2013, 200, 400–407. [Google Scholar] [CrossRef]
- Savoiardo, M.; Minati, L.; Farina, L.; De Simone, T.; Aquino, D.; Mea, E.; Filippini, G.; Bussone, G.; Chiapparini, L. Spontaneous intracranial hypotension with deep brain swelling. Brain 2007, 130, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. Spontaneous intracranial hypotension. Curr. Neurol. Neurosci. Rep. 2001, 1, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Davies, M.A.; Sharpe, R.; Cordato, D.; Schwartz, R. Epidural Blood Patch as a Diagnostic and Therapeutic Intervention in Spontaneous Intracranial Hypotension: A Novel Approach to Management. World Neurosurg. 2020, 137, e242–e250. [Google Scholar] [CrossRef]
- Duffy, P.J.; Crosby, E.T. The epidural blood patch. Resolving the controversies. Can. J. Anaesth. 1999, 46, 878–886. [Google Scholar] [CrossRef] [PubMed]
- So, Y.; Park, J.M.; Lee, P.M.; Kim, C.L.; Lee, C.; Kim, J.H. Epidural Blood Patch for the Treatment of Spontaneous and Iatrogenic Orthostatic Headache. Pain Physician 2016, 19, E1115–E1122. [Google Scholar] [PubMed]
- Nowaczewska, M.; Kaźmierczak, H. Cerebral Blood Flow in Low Intracranial Pressure Headaches—What is Known? Brain Sci. 2019, 10, 2. [Google Scholar] [CrossRef]
- Bezov, D.; Ashina, S.; Lipton, R. Post-dural puncture headache: Part II—Prevention, management, and prognosis. Headache 2010, 50, 1482–1498. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, E.; Trimboli, M.; Rubino, F. Spontaneous intracranial hypotension: Review and expert opinion. Acta Neurol. Belg. 2020, 120, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Farb, R.I.; Nicholson, P.J.; Peng, P.W.; Massicotte, E.M.; Lay, C.; Krings, T.; terBrugge, K.G. Spontaneous Intracranial Hypotension: A Systematic Imaging Approach for CSF Leak Localization and Management Based on MRI and Digital Subtraction Myelography. AJNR Am. J. Neuroradiol. 2019, 40, 745–753. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Mărginean, M.O. Early Inflammatory Status Related to Pediatric Obesity. Front. Pediatr. 2019, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş, M.; Yaylali, Y.T.; Kaya, Y.; Ozdemir, M.; Ozkan, I.; Aladağ, N. Neutrophil-to-lymphocyte ratio may predict subclinical atherosclerosis in patients with psoriasis. Echocardiography 2014, 31, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, P.; Sinha, P.; Gupta, B.; Firmal, P.; Rajaram, S. Neutrophil-to-lymphocyte ratio and platelet indices in pre-eclampsia. Int. J. Gynaecol. Obstet. 2019, 144, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Erre, G.L.; Paliogiannis, P.; Castagna, F.; Mangoni, A.A.; Carru, C.; Passiu, G.; Zinellu, A. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur. J. Clin. Invest. 2019, 49, e13037. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38, S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chung, D.W. Inflammation, von Willebrand factor, and ADAMTS13. Blood 2018, 132, 141–147. [Google Scholar] [CrossRef]
- Mokri, B. Headaches caused by decreased intracranial pressure: Diagnosis and management. Curr. Opin. Neurol. 2003, 16, 319–326. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Ahrar, A.; Jadhav, V.; Wallery, S.S. Epidural Injection of Platelet Rich Plasma for Postlumbar Puncture Headaches. J. Neurosurg. Anesthesiol. 2018, 30, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, B.; Acar, M.; Emmez, G.; Akcali, D.; Tokgoz, N. Epidural patch with autologous platelet rich plasma: A novel approach. J. Anesth. 2017, 31, 907–910. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Jiang, Z.; Ming, L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: A systematic review and meta-analysis. Int. Angiol. 2018, 37, 4–11. [Google Scholar] [CrossRef]
- Suárez-Cuenca, J.A.; Ruíz-Hernández, A.S.; Mendoza-Castañeda, A.A.; Domínguez-Pérez, G.A.; Hernández-Patricio, A.; Vera-Gómez, E.; De la Peña-Sosa, G.; Banderas-Lares, D.Z.; Montoya-Ramírez, J.; Blas-Azotla, R.; et al. Neutrophil-to-lymphocyte ratio and its relation with pro-inflammatory mediators, visceral adiposity and carotid intima-media thickness in population with obesity. Eur. J. Clin. Invest. 2019, 49, e13085. [Google Scholar] [CrossRef]
- Al-Halawani, M.; Naik, S.; Chan, M.; Kreinin, I.; Meiers, J.; Kryger, M. Neutrophil-to-lymphocyte ratio decreases in obstructive sleep apnea treated with mandibular advancement devices. Sleep Breath 2018, 22, 989–995. [Google Scholar] [CrossRef]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef]
- Hamer, J.; Alberti, E.; Hoyer, S.; Wiedemann, K. Influence of systemic and cerebral vascular factors on the cerebrospinal fluid pulse waves. J. Neurosurg. 1977, 46, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kroin, J.S.; Nagalla, S.K.; Buvanendran, A.; McCarthy, R.J.; Tuman, K.J.; Ivankovich, A.D. The mechanisms of intracranial pressure modulation by epidural blood and other injectates in a postdural puncture rat model. Anesth. Analg. 2002, 95, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Platelets in inflammation and infection. Platelets 2015, 26, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Patients (n = 298) | Poor Responders (n = 68) | Good Responders (n = 230) | p-Value |
|---|---|---|---|---|
| Age, years | 38 (33–46) | 38 (34–46) | 38 (33–46) | 0.952 |
| Sex | ||||
| Male/Female, n (%) | 108 (36.2%)/190 (63.8%) | 27 (39.7%)/41 (60.3%) | 81 (35.2%)/149 (64.8%) | 0.499 |
| Height, cm | 164.6 ± 22.3 | 165.4 ± 8.9 | 164.4 ± 8.2 | 0.365 |
| Weight, kg | 58.0 (52.3–68.0) | 58.5 (52.1–69.0) | 58.0 (52.4–67.9) | 0.816 |
| Body mass index, kg/m2 | 22.3 ± 2.9 | 21.9 ± 2.8 | 22.4 ± 2.9 | 0.270 |
| Underlying disease | ||||
| Diabetes mellitus, n (%) Hypertension, n (%) Coronary arterial disease, n (%) Cerebrovascular accident, n (%) Herniated intervertebral disc, n (%) | 12 (4.0%) 15 (5.0%) 6 (2.0%) 1 (0.3%) 10 (3.4%) | 1 (1.5%) 0 (0.0%) 3 (4.4%) 0 (0.0%) 2 (2.9%) | 11 (4.8%) 15 (6.5%) 3 (10.3%) 1 (0.4%) 8 (3.5%) | 0.310 0.027 0.130 >0.999 >0.999 |
| History of headache | ||||
| Migraine, n (%) Tension headach, n (%) Cluster headache, n (%) | 11 (3.7%) 3 (1.0%) 0 (0.0%) | 5 (7.4%) 0 (0.0%) 0 (0.0%) | 6 (2.6%) 3 (1.3%) 0 (0.0%) | 0.613 |
| Associated symptoms | ||||
| Nausea, n (%) Vomiting, n (%) Photophobia, n (%) Hearing impairment, n (%) Tinnitus, n (%) Vertigo, n (%) Diplopia, n (%) | 166 (55.7%) 100 (33.6%) 2 (0.7%) 4 (1.3%) 65 (21.8%) 1 (0.3%) 1 (0.3%) | 42 (61.8%) 29 (42.6%) 1 (1.5%) 2 (2.9%) 8 (11.8%) 0 (0.0%) 0 (0.0%) | 124 (53.9%) 71 (30.9%) 1 (0.4%) 2 (0.9%) 24 (10.4%) 1 (0.4%) 1 (0.4%) | 0.194 0.056 0.400 0.220 0.723 >0.999 >0.999 |
| Duration of headache, days | 10.0 (9.0–30.0) | 15.0 (9.0–30.0) | 10.0 (9.0–30.0) | 0.579 |
| Headache, numeric rating scale | 7.0 (5.0–9.0) | 7.0 (4.0–9.0) | 7.0 (5.0–8.0) | 0.790 |
| Variables | Total Patients (n = 298) | Poor Responders (n = 68) | Good Responders (n = 230) | p-Value |
|---|---|---|---|---|
| Brain MRI signs | ||||
| Pachymeningeal enhancement, n (%) Engorgement of venous structures, n (%) Brain sagging, n (%) Pituitary hyperemia, n (%) Midline shift, n (%) Midbrain–pons angle, degrees Vein of Galen–Straight sinus angle, degrees | 161 (54.0%) 101 (33.9%) 40 (13.4%) 32 (10.7%) 5 (1.7%) 55.3 ± 10.0 63.4 (46.6–74.1) | 35 (51.5%) 29 (42.6%) 7 (10.3%) 7 (10.3%) 0 (0.0%) 55.0 ± 8.8 64.1 (43.0–74.6) | 126 (54.8%) 72 (31.3%) 33 (14.3%) 25 (10.9%) 5 (2.2%) 55.3 ± 10.4 63.1 (47.5–73.5) | 0.542 0.095 0.367 0.864 0.592 0.800 0.934 |
| Cisternography | ||||
| Level of cerebrospinal fluid leakage | ||||
| Cervical, n (%) | 132 (44.3%) | 29 (42.6%) | 103 (44.8%) | 0.755 |
| Thoracic, n (%) | 153 (51.3%) | 37 (54.4%) | 116 (50.4%) | 0.564 |
| Lumbar, n (%) | 64 (21.5%) | 19 (27.9%) | 45 (19.6%) | 0.140 |
| Undetermined, n (%) | 45 (15.1%) | 6 (8.8%) | 39 (17.0%) | 0.100 |
| Multiple leakage, n (%) | 160 (53.7%) | 43 (63.2%) | 117 (50.9%) | 0.159 |
| Cerebrospinal opening pressure, mmHg | 4.8 (0.0–8.0) | 4.5 (0.0–7.5) | 5.0 (0.0–8.2) | 0.580 |
| Early bladder activity, n (%) | 59 (19.8%) | 17 (25.0%) | 42 (18.3%) | 0.369 |
| Variables | Total Patients (n = 298) | Poor Responders (n = 68) | Good Responders (n = 230) | p-Value |
|---|---|---|---|---|
| Number of epidural blood patches, times | 2 (1–2) | 3 (3–4) | 1 (1–2) | <0.001 |
| First epidural blood patch volume, mL | 15.0 (12.0–15.0) | 15.0 (12.0–17.8) | 15.0 (12.0–15.0) | 0.717 |
| First epidural blood patch sites | ||||
| Cervical, n (%) Thoracic, n (%) Lumbar, n (%) | 130 (43.6%) 138 (46.3%) 30 (10.1%) | 21 (30.9%) 40 (58.8%) 7 (10.3%) | 109 (47.4%) 98 (42.6%) 23 (10.0%) | 0.062 |
| Variables | Total Patients (n = 298) | Poor Responders (n = 68) | Good Responders (n = 230) | p-Value |
|---|---|---|---|---|
| Hemoglobin, g/dL | 13.6 ± 1.6 | 13.8 ± 1.3 | 13.6 ± 1.7 | 0.430 |
| Hematocrit, % | 40.3 ± 4.0 | 40.6 ± 3.6 | 40.2 ± 4.2 | 0.386 |
| Platelet, 103/μL | 241.2 ± 55.6 | 238.8 ± 48.8 | 241.9 ± 57.5 | 0.694 |
| Prothrombin time, INR | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.99 ± 0.1 | <0.001 |
| Platelet distribution width, % | 11.3 (10.6–12.5) | 11.1 (10.2–12.0) | 11.4 (10.7–12.7) | 0.046 |
| Activated PTT, sec | 27.5 (26.0–29.1) | 27.7 (26.2–29.0) | 27.4 (26.0–29.1) | 0.429 |
| White blood cell, cells/mm3 Neutrophil, % Lymphocyte, % Monocyte, % Eosinophil, % Basophil, % | 6600 (5400–8100) 61.8 ± 12.1 29.6 ± 10.3 6.2 (4.9–7.4) 1.4 (0.6–2.4) 0.3 (0.2–0.5) | 6500 (5100–8000) 57.8 ± 13.2 33.4 ± 10.7 6.6 (5.1–7.6) 1.4 (0.6–2.5) 0.3 (0.2–0.5) | 6600 (5500–8200) 63.0 ± 11.5 28.5 ± 9.9 6.1 (7.4–4.9) 1.4 (0.6–2.4) 0.37 (0.2–0.5) | 0.357 0.002 0.001 0.237 0.923 0.904 |
| Absolute neutrophil count, cells/μL | 3925 (3000–5552) | 3820 (2735–5262) | 4020 (3040–5632) | 0.266 |
| Erythrocyte sedimentation rate, mm/hr | 9.0 (4.0–18.0) | 7.5 (3.3–15.8) | 10.0 (4.0–18.0) | 0.209 |
| C-reactive protein, mg/L | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 0.215 |
| Neutrophil-to-lymphocyte ratio | 2.1 (1.5–3.2) | 1.8 (1.2–2.5) | 2.2 (1.6–3.5) | 0.002 |
| Platelet-to-lymphocyte ratio | 129.2 (100.5–158.5) | 117.0 (83.4–145.4) | 131.3 (104.0–160.9) | 0.010 |
| Platelet-to-neutrophil ratio | 60.8 (43.3–81.5) | 68.1 (44.4–87.6) | 58.2 (43.1–77.6) | 0.072 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Hypertension | 0.000 | 0.000–NC | 0.998 | |||
| Neutrophil-to-lymphocyte ratio | 0.705 | 0.556–0.894 | 0.004 | 0.720 | 0.565–0.917 | 0.008 |
| Platelet-to-lymphocyte ratio | 0.992 | 0.986–0.998 | 0.015 | 0.996 | 0.989–1.004 | 0.370 |
| Epidural blood patch site | ||||||
| Cervical | 1 | 1 | ||||
| Thoracic | 0.633 | 0.241–1.664 | 0.354 | 0.479 | 0.174–1.323 | 0.156 |
| Lumbar | 1.341 | 0.533–3.474 | 0.533 | 1.052 | 0.401–2.761 | 0.918 |
| Brain engorgement of venous structures | 1.611 | 0.919–2.826 | 0.096 | 1.679 | 0.931–3.028 | 0.085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Ro, Y.-J.; Leem, J.-G.; Shin, J.-W.; Oh, Y.; Choi, S.-S. Predictors Associated with Outcomes of Epidural Blood Patch in Patients with Spontaneous Intracranial Hypotension. J. Clin. Med. 2021, 10, 922. https://doi.org/10.3390/jcm10050922
Park J-Y, Ro Y-J, Leem J-G, Shin J-W, Oh Y, Choi S-S. Predictors Associated with Outcomes of Epidural Blood Patch in Patients with Spontaneous Intracranial Hypotension. Journal of Clinical Medicine. 2021; 10(5):922. https://doi.org/10.3390/jcm10050922
Chicago/Turabian StylePark, Jun-Young, Young-Jin Ro, Jeong-Gil Leem, Jin-Woo Shin, Yul Oh, and Seong-Soo Choi. 2021. "Predictors Associated with Outcomes of Epidural Blood Patch in Patients with Spontaneous Intracranial Hypotension" Journal of Clinical Medicine 10, no. 5: 922. https://doi.org/10.3390/jcm10050922
APA StylePark, J.-Y., Ro, Y.-J., Leem, J.-G., Shin, J.-W., Oh, Y., & Choi, S.-S. (2021). Predictors Associated with Outcomes of Epidural Blood Patch in Patients with Spontaneous Intracranial Hypotension. Journal of Clinical Medicine, 10(5), 922. https://doi.org/10.3390/jcm10050922






