Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review
Abstract
1. Introduction
An Unmet Need
2. Recurrent Questions: Are Estroprogestins and Progestins Effective?
2.1. Biological Evidence: The Concept of Progesterone Resistance in Endometriosis
2.1.1. Progesterone Receptors and Resistance
2.1.2. Causes of Progesterone Resistance
Congenital
Inflammation and Oxidative Stress
Genetics and Epigenetics
Mesenchymal Progenitors
Phenotype of Endometriosis
2.2. Clinical Evidence: Estroprogestins and Progestogens
2.2.1. Estroprogestins: OCPs
Pros and Cons of OCPs
2.2.2. Progestins
2.2.3. Summary
3. Why Do We Need New Options?
- The side effects of estroprogestins are essentially related to the type of progestin used [67].
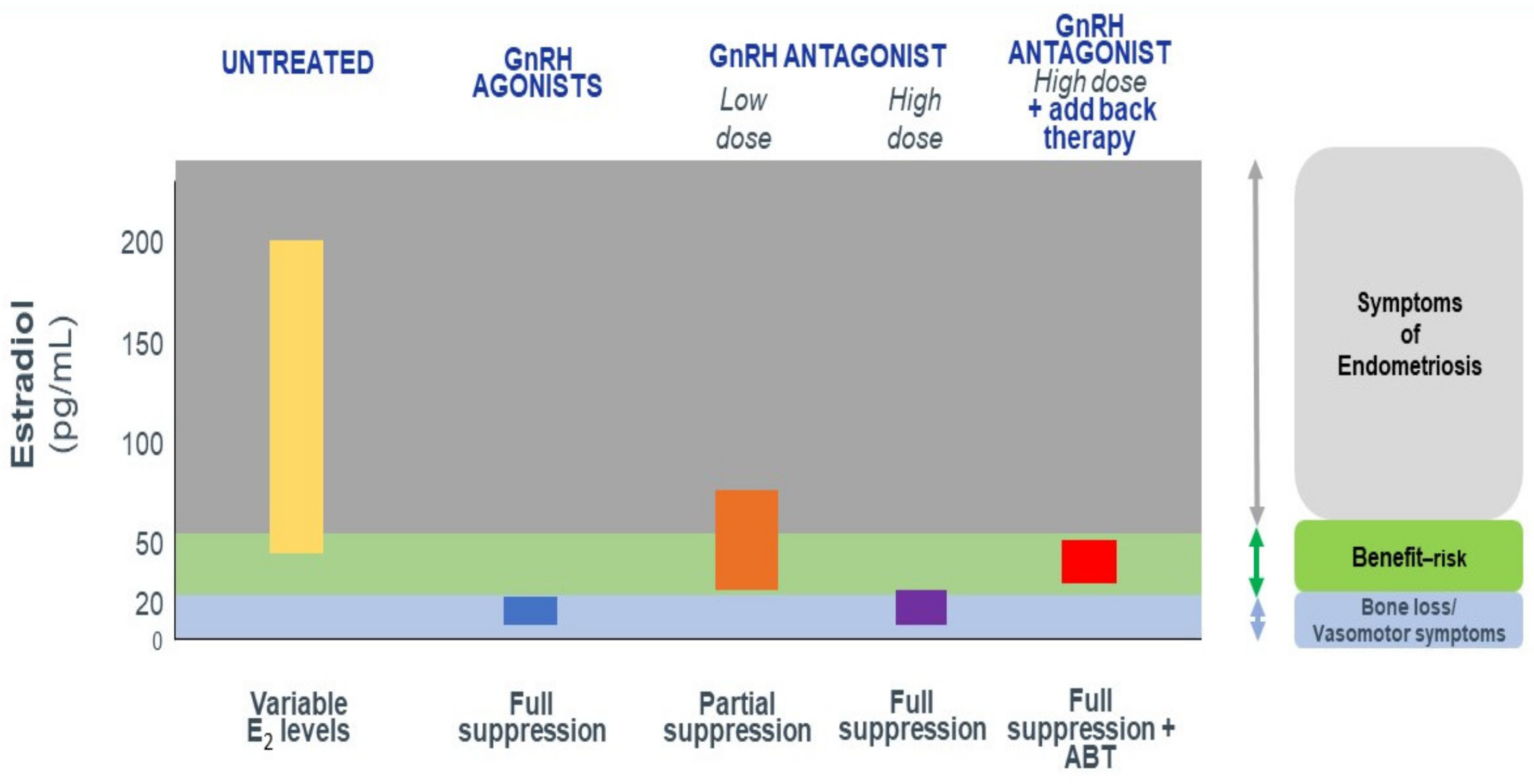
3.1. The Optimal Goal of Medical Therapy
How Do We Achieve Partial E2 Suppression?
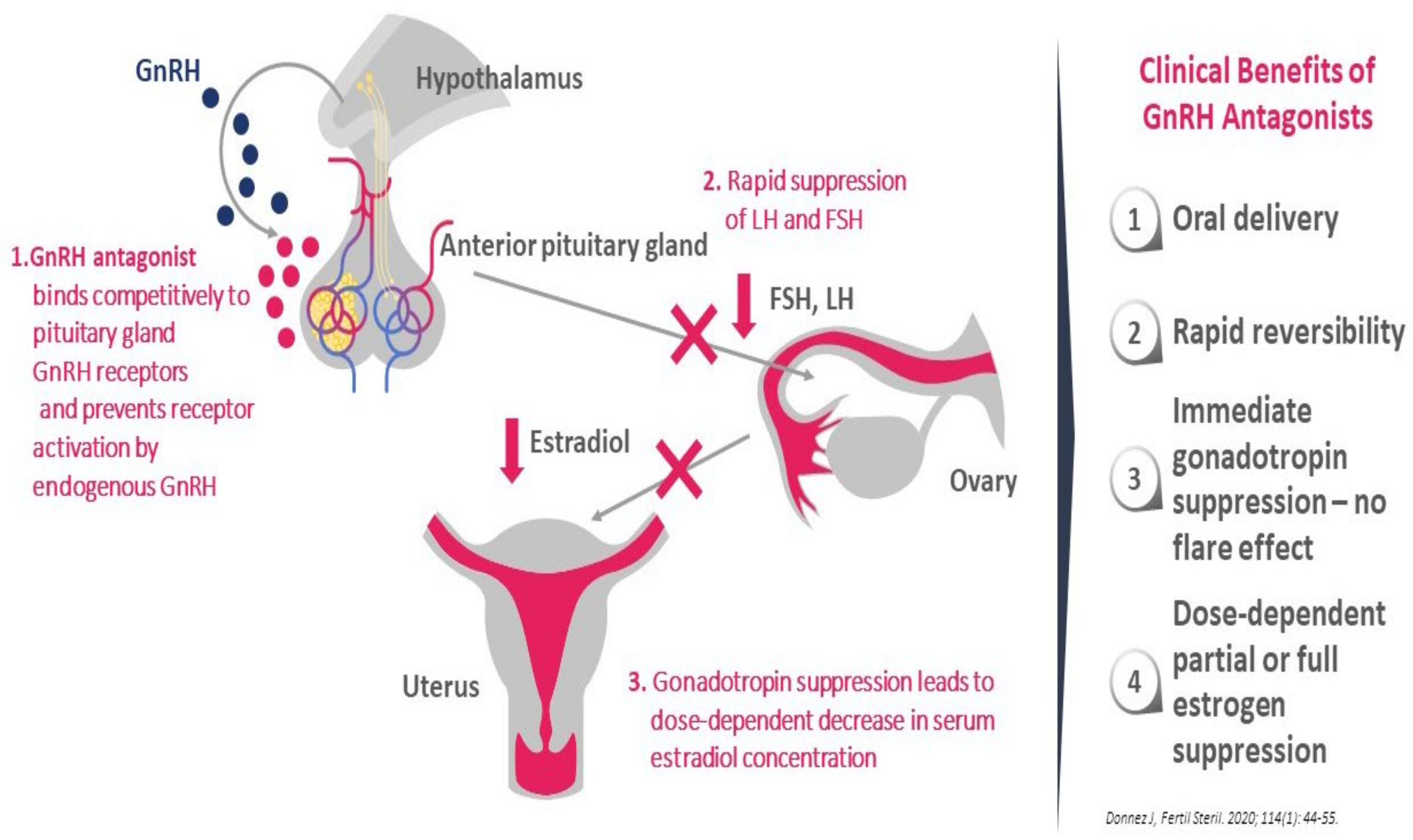
3.2. GnRH Antagonist: The Ideal New Option?
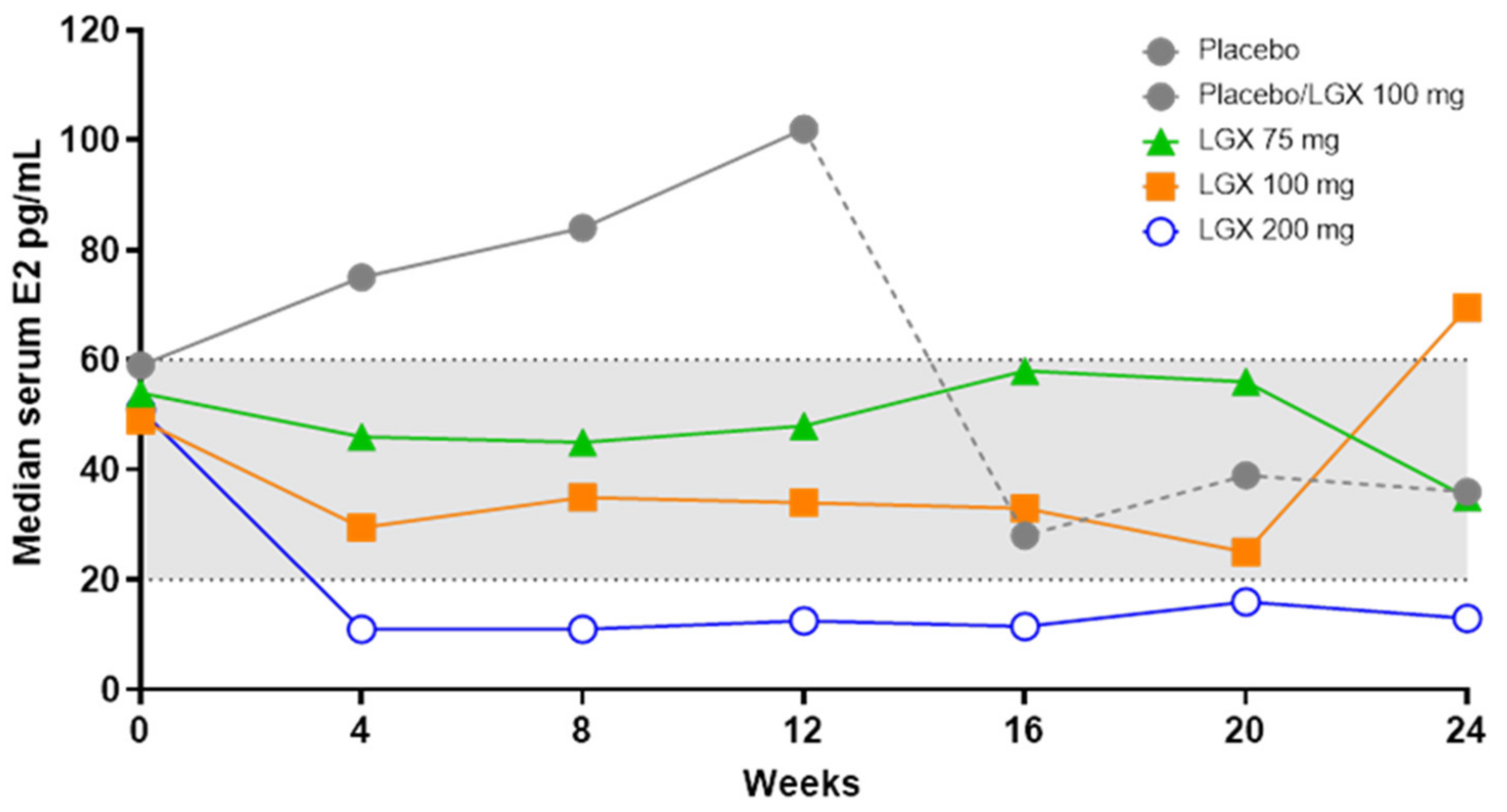
- They produce dose-dependent estrogen suppression, from partial suppression at lower doses to almost full suppression at higher doses (Figure 2).
- There is rapid reversibility and recovery of hormone secretion after stopping treatment.
- Striking a balance between efficacy and safety/tolerability may unlock the potential of this new class of drug, suggesting the possibility of individual tailoring according to symptoms and the wishes of the patient.
3.3. Elagolix
Clinical Efficacy
3.4. Linzagolix
Clinical Efficacy
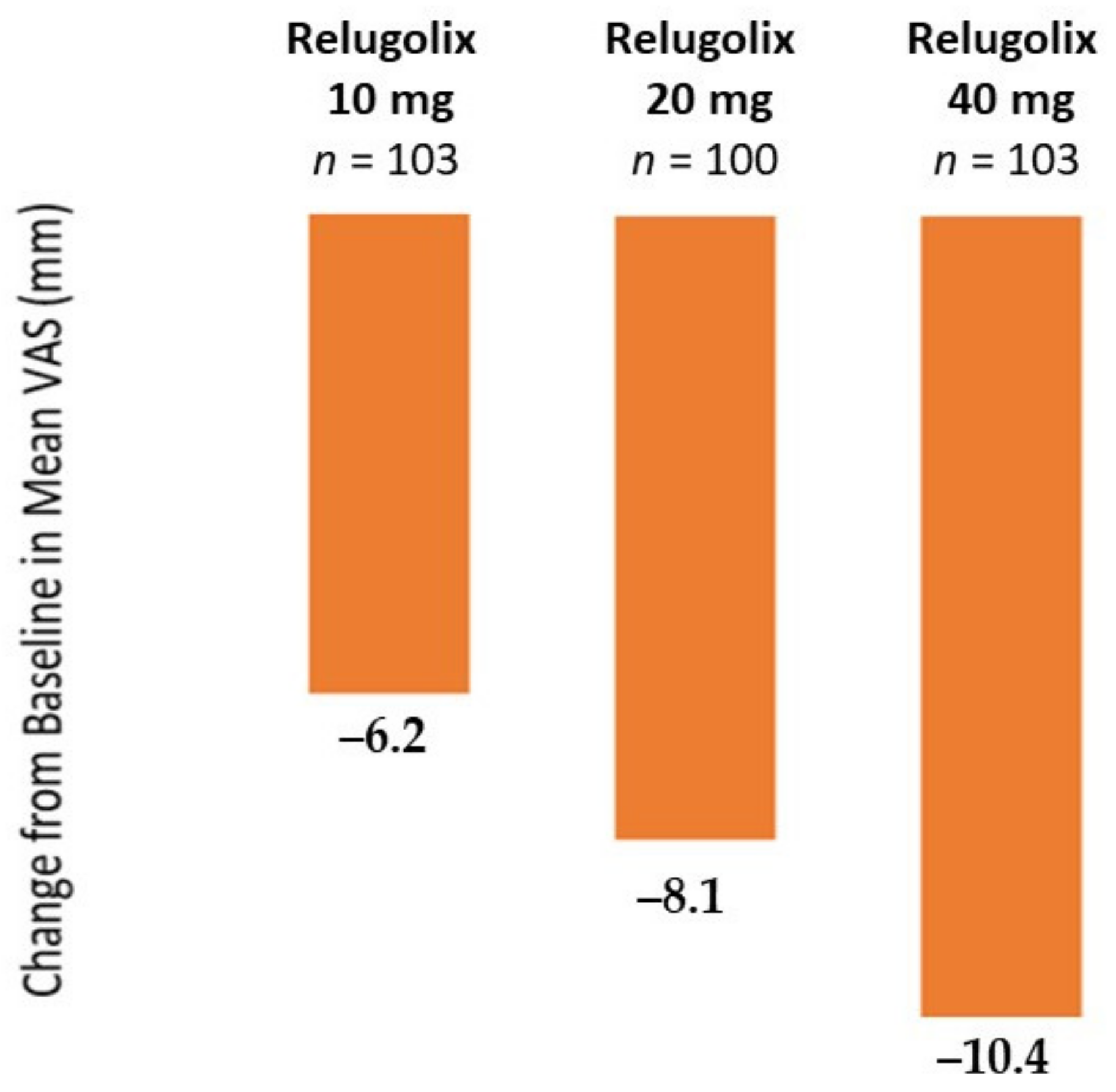
3.5. Relugolix
Clinical Efficacy
4. Discussion and Conclusion: A Combined Symptom-Oriented and Phenotype-Adapted Approach
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Donnez, J.; Chantraine, F.; Nisolle, M. The efficacy of medical and surgical treatment of endometriosis-associated infertility: Arguments in favour of a medico-surgical approach. Hum. Reprod. Update. 2002, 8, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharmacother. 2018, 19, 1109–1125. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Berlanda, N.; Barbara, G.; Somigliana, E.; Bosari, S. Estrogen-progestins and progestins for the management of endometriosis. Fertil. Steril. 2016, 106, 1552–1571.e2. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Frattaruolo, M.P.; Borghi, A.; Dridi, D.; Somigliana, E. Medical treatment of endometriosis-related pain. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 51, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endome-triosis: A committee opinion. Fertil. Steril. 2014, 101, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Management of endometriosis. ACOG practice bulletin no. 114. Obstet. Gynecol. 2010, 116, 223–236. [Google Scholar] [CrossRef]
- Leyland, N.; Casper, R.; Laberge, P.; Singh, S.S.; Society of Obstetricians and Gynaecologists of Canada. Endometriosis: Diagnosis and management. J. Obstet. Gynecol. Can. 2010, 32, S1–S32. [Google Scholar] [CrossRef]
- Surrey, E.S.; Soliman, A.M.; Johns, B.; Vora, J.B.; Taylor, H.S.; Agarwal, S.K. Real-World Characterization of Women with Diagnosed Endometriosis Initiating Therapy with Elagolix Using a US Claims Database. Clinicoecon Outcomes Res. 2020, 12, 473–479. [Google Scholar] [CrossRef]
- Soliman, A.M.; Coyne, K.S.; Zaiser, E.; Castelli-Haley, J.; Fuldeore, M.J. The burden of endometriosis symptoms on health-related quality of life in women in the United States: A cross-sectional study. J. Psychosom. Obstet. Gynecol. 2017, 38, 238–248. [Google Scholar] [CrossRef]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelley, C.; Winkel, C. The direct and indirect costs associated with endometriosis: A systematic literature review. Hum. Reprod. 2016, 31, 712–722. [Google Scholar] [CrossRef]
- Bulun, S.E.; Cheng, Y.H.; Pavone, M.E.; Yin, P.; Imir, G.; Utsunomiya, H.; Thung, S.; Xue, Q.; Marsh, E.E.; Tokunaga, H.; et al. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin. Reprod. Med. 2010, 28, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone receptor isoform a but not b is expressed in endometriosis1. J. Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef]
- Bulun, S.E.; Cheng, Y.-H.; Yin, P.; Imir, G.; Utsunomiya, H.; Attar, E.; Innes, J.; Kim, J.J. Progesterone resistance in endometriosis: Link to failure to metabolize estradiol. Mol. Cell. Endocrinol. 2006, 248, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen receptor b modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Pavone, M.E.; Reierstad, S.; Sun, H.; Milad, M.; Bulun, S.E.; Cheng, Y.-H. Altered retinoid uptake and action contributes to cell survival in endometriosis. J. Clin. Endocrinol. Metab. 2010, 95, E300–E309. [Google Scholar] [CrossRef] [PubMed]
- Pavone, M.E.; Dyson, M.; Reirstad, S.; Pearson, E.; Ishikawa, H.; Cheng, Y.H.; Bulun, S.E. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum. Reprod. 2011, 26, 2157–2164. [Google Scholar] [CrossRef]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Brosens, J.J.; Puttemans, P.; Benagiano, G.; Brosens, I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 2014, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Ober, W.B.; Bernstein, J. Observations on the endometrium and ovary in the newborn. Pediatrics 1955, 16, 445–460. [Google Scholar]
- Brosens, I.; Benagiano, G. The endometrium from the neonate to the adolescent. J. Matern. Neonatal Med. 2015, 29, 1195–1199. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; Viceconte, R.; Bulzomi, P.; D’Armiento, M.; D’Avino, A.; Baldi, A. Embryologic origin of endometriosis: Analysis of 101 human female fetuses. J. Cell. Physiol. 2011, 227, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Iron overload in the peritoneal cavity of women with pelvic endome-triosis. Fertil. Steril. 2002, 78, 712–718. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Dolmans, M.-M.; Donnez, J. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil. Steril. 2002, 77, 561–570. [Google Scholar] [CrossRef]
- Lousse, J.-C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflam-matory disease. Front. Biosci. Elite Ed. 2012, 4, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.-M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Lousse, J.-C.; Defrère, S.; Van Langendonckt, A.; Gras, J.; González-Ramos, R.; Colette, S.; Donnez, J. Iron storage is significantly increased in peritoneal macrophages of endometriosis patients and correlates with iron overload in peritoneal fluid. Fertil. Steril. 2009, 91, 1668–1675. [Google Scholar] [CrossRef]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Updat. 2014, 21, 155–173. [Google Scholar] [CrossRef]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Simpson, J.L.; Bischoff, F.Z.; Kamat, A.; Buster, J.E.; A Carson, S. Genetics of endometriosis. Obstet. Gynecol. Clin. North Am. 2003, 30, 21–40. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genet-ic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Zubrzycki, M.; Perdas, E.; Zubrzycka, M. Genetic, epigenetic, and steroidogenic modulation mechanisms in en-dometriosis. J. Clin. Med. 2020, 9, 130938. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.S.; Nunez, M.; Lai, T.; Tosevska, A.; Morselli, M.; Amneus, M.; Zakhour, M.; Moatamed, N.A.; Pellegrini, M.; Memarzadeh, S. Expression of stromal progesterone receptor and differential methylation patterns in the endometrium may correlate with response to progesterone therapy in endometrial complex atypical hyperplasia. Reprod. Sci. 2020, 27, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.-W. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005, 193, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H.; Taylor, H.S. Molecular mechanisms of treatment resistance in endometriosis: The role of progesterone–hox gene interactions. Semin. Reprod. Med. 2010, 28, 69–74. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Ding, T.; Osteen, K.G. Dioxin and endometrial progesterone resistance. Semin. Reprod. Med. 2010, 28, 59–68. [Google Scholar] [CrossRef]
- Heilier, J.-F.; Donnez, J.; Lison, D. Organochlorines and endometriosis: A mini-review. Chemosphere 2008, 71, 203–210. [Google Scholar] [CrossRef]
- Heilier, J.-F.; Ha, A.T.; Lison, M.; Donnez, J.; Tonglet, R.; Nackers, F. Increased serum polychlorobiphenyl levels in Belgian women with adenomyotic nodules of the rectovaginal septum. Fertil. Steril. 2004, 81, 456–458. [Google Scholar] [CrossRef]
- Barragan, F.; Irwin, J.C.; Balayan, S.; Erikson, D.W.; Chen, J.C.; Houshdaran, S.; Piltonen, T.T.; Spitzer, T.L.B.; Joseph, A.G.; Rabbanet, T.; et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol. Reprod. 2016, 94, 118. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar]
- Donnez, J.; Nisolle, M.; Casanas-Roux, F.; Brion, P.; DaFerreira, N. Stereometric evaluation of peritoneal endometriosis and endometriotic nodules of the rectovaginal septum. Hum. Reprod. 1996, 11, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Nisolle, M.; Smoes, P.; Gillet, N.; Beguin, S.; Casanas-Roux, F. Peritoneal endometriosis and “endometriotic” nodules of the rectovaginal septum are two different entities. Fertil. Steril. 1996, 66, 362–368. [Google Scholar] [CrossRef]
- Donnez, J.; Smoes, P.; Gillerot, S.; Casanas-Roux, F.; Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum. Reprod. 1998, 13, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Masuzaki, H.; Fujishita, A.; Kitajima, M.; Sekine, I.; Ishimaru, T. Higher activity by opaque endometriotic lesions than nonopaque lesions. Acta Obstet. Gynecol. Scand. 2004, 83, 375–382. [Google Scholar] [CrossRef]
- Hogg, C.; Horne, A.W.; Greaves, E. Endometriosis-associated macrophages: Origin, phenotype, and function. Front. Endocrinol. 2020, 11, 7. [Google Scholar] [CrossRef]
- Redwine, D.B. Was Sampson wrong? Fertil. Steril. 2002, 78, 686–693. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Somigliana, E. Role of medical therapy in the management of deep rectovaginal endometriosis. Fertil. Steril. 2017, 108, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Donnez, J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil. Steril. 2012, 98, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Donnez, J. Deep endometriosis: The place of laparoscopic shaving. Best Pr. Res. Clin. Obstet. Gynaecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Nisolle, M.; Casanas-Roux, F.; Bassil, S.; Anaf, V. Surgery: Rectovaginal septum, endometriosis or adenomyosis: Laparoscopic management in a series of 231 patients. Hum. Reprod. 1995, 10, 630–635. [Google Scholar] [CrossRef]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum. Reprod. Updat. 2020, 26, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Barra, F.; Grandi, G.; Tantari, M.; Scala, C.; Facchinetti, F.; Ferrero, S. A comprehensive review of hormonal and biological therapies for endometriosis: Latest developments. Expert Opin. Biol. Ther. 2019, 19, 343–360. [Google Scholar] [CrossRef]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone receptor status predicts response to progestin therapy in endome-triosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Facchin, F.; Buggio, L.; Barbara, G.; Berlanda, N.; Frattaruolo, M.P.; Somigliana, E. Management of endometriosis: Toward value-based, cost-effective, affordable care. J. Obstet. Gynaecol. Can. 2018, 40, 726–749.e10. [Google Scholar] [CrossRef]
- Vercellini, P.; Donati, A.; Ottolini, F.; Frassineti, A.; Fiorini, J.; Nebuloni, V.; Frattaruolo, M.P.; Roberto, A.; Mosconi, P.; Somigliana, E. A stepped-care approach to symptomatic endometriosis management: A participatory research initiative. Fertil. Steril. 2018, 109, 1086–1096. [Google Scholar] [CrossRef]
- Vercellini, P.; Frattaruolo, M.P.; Buggio, L. Toward minimally disruptive management of symptomatic endometriosis: Reducing low-value care and the burden of treatment. Expert Rev. Pharmacoecon. Outcomes Res. 2017, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P. Are combined hormonal contraceptives the neglected treatment for symptomatic endometriosis? Fertil. Steril. 2018, 110, 61–62. [Google Scholar] [CrossRef]
- Harada, T.; Momoeda, M.; Taketani, Y.; Hoshiai, H.; Terakawa, N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: A placebo-controlled, double-blind, randomized trial. Fertil. Steril. 2008, 90, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Use of combined oral contraceptives and risk of venous thromboembolism: Nested case-control studies using the QResearch and CPRD databases. BMJ 2015, 350, h2135. [Google Scholar] [CrossRef]
- Manzoli, L.; De Vito, C.; Marzuillo, C.; Boccia, A.; Villari, P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012, 35, 191–205. [Google Scholar] [CrossRef]
- Lidegaard, O.; Nielsen, L.H.; Skovlund, C.W.; Løkkegaard, E. Venous thrombosis in users of non-oral hormonal contraception: Follow-up study, Denmark 2001–2010. BMJ 2012, 344, e2990. [Google Scholar] [CrossRef] [PubMed]
- Lidegaard, O.; Nielsen, L.H.; Skovlund, C.W.; Skjeldestad, F.E.; Løkkegaard, E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–2009. BMJ 2011, 343, d6423. [Google Scholar] [CrossRef]
- Casper, R.F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 2017, 107, 533–536. [Google Scholar] [CrossRef]
- Brion, F.; Le Page, Y.; Piccini, B.; Cardoso, O.; Tong, S.-K.; Chung, B.-C.; Kah, O. Screening estrogenic activities of chemicals or mixtures in vivo using transgenic (cyp19a1b-gfp) zebrafish embryos. PLoS ONE 2012, 7, e36069. [Google Scholar] [CrossRef]
- Speroff, L.; Symons, J.; Kempfert, N.; Rowan, J. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (femhrt??) on the frequency and intensity of vasomotor symptoms. Menopause 2000, 7, 383–390. [Google Scholar] [CrossRef]
- Bernuit, D.; Ebert, A.D.; Halis, G.; Strothmann, A.; Gerlinger, C.; Geppert, K.; Faustmann, T. Female perspectives on endometriosis: Findings from the uterine bleeding and pain women’s research study. J. Endometr. 2011, 3, 73–85. [Google Scholar] [CrossRef]
- Chapron, C.; Souza, C.; Borghese, B.; Lafay-Pillet, M.-C.; Santulli, P.; Bijaoui, G.; Goffinet, F.; De Ziegler, D. Oral contraceptives and endometriosis: The past use of oral contraceptives for treating severe primary dysmenorrhea is associated with endometriosis, especially deep infiltrating endometriosis. Hum. Reprod. 2011, 26, 2028–2035. [Google Scholar] [CrossRef]
- Vercellini, P.; Eskenazi, B.; Consonni, D.; Somigliana, E.; Parazzini, F.; Abbiati, A.; Fedele, L. Oral contraceptives and risk of endo-metriosis: A systematic review and meta-analysis. Hum. Reprod. Update 2011, 17, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Farquhar, C. Endometriosis: An overview of cochrane reviews. Cochrane Database Syst. Rev. 2014, 2014, CD009590. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.T.; Schlaff, W.; Gordon, K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: A systematic review of the evidence. Fertil. Steril. 2018, 110, 137–152.e1. [Google Scholar] [CrossRef]
- Buggio, L.; Somigliana, E.; Barbara, G.; Frattaruolo, M.P.; Vercellini, P. Oral and depot progestin therapy for endometriosis: Towards a personalisez medicine. Expert Opin. Pharmacother. 2017, 18, 1569–1581. [Google Scholar] [CrossRef]
- Donnez, J.; Lemaire-Rubbers, M.; Karaman, Y.; Nisolle-Pochet, M.; Casanas-Roux, F. Combined (hormonal and microsurgical) therapy in infertile women with endometriosis. Fertil. Steril. 1987, 48, 239–242. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Gillet, N.; Smets, M.; Bassil, S.; Casanas-Roux, F. Large ovarian endometriomas. Hum. Reprod. 1996, 11, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Nisolle-Pochet, M.; Casanas-Roux, F.; Donnez, J. Histologic study of ovarian endometriosis after hormonal therapy. Fertil. Steril. 1988, 49, 423–426. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Clerckx, F.; Casanas, F. Evaluation of preoperative use of danazol, gestrinone, lynestrenol, buserelin spray and buserelin implant, in the treatment of endometriosis associated infertility. Prog. Clin. Boil. Res. 1990, 323, 427–442. [Google Scholar]
- Donnez, J.; Nisolle-Pochet, M.; Casanas-Roux, F. Endometriosis-associated infertility: Evaluation of preoperative use of danazol, gestrinone, and buserelin. Int. J. Fertil. 1990, 35, 297–301. [Google Scholar]
- Mabrouk, M.; Paradisi, R.; Arena, A.; Del Forno, S.; Matteucci, C.; Zannoni, L.; Caprara, G.; Seracchioli, R. Short-term histopathological effects of dienogest therapy on ovarian endometriomas: In vivo, nonrandomized, controlled trial. Gynecol. Endocrinol. 2017, 34, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Bracco, B.; Mosconi, P.; Roberto, A.; Alberico, D.; Dhouha, D.; Somigliana, E. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: A before and after study. Fertil. Steril. 2016, 105, 734.e3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Taylor, R.N.; Taylor, H.S. Partial suppression of estradiol: A new strategy in endometriosis management? Fertil. Steril. 2017, 107, 568–570. [Google Scholar] [CrossRef]
- Ferrero, S.; Maggiore, U.L.R.; Scala, C.; Di Luca, M.; Venturini, P.L.; Remorgida, V. Changes in the size of rectovaginal endometriotic nodules infiltrating the rectum during hormonal therapies. Arch. Gynecol. Obstet. 2012, 287, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Razzi, S.; Luisi, S.; Ferretti, C.; Calonaci, F.; Gabbanini, M.; Mazzini, M.; Petraglia, F. Use of a progestogen only preparation containing desogestrel in the treatment of recurrent pelvic pain after conservative surgery for endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 135, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Leonardo-Pinto, J.P.; Benetti-Pinto, C.L.; Cursino, K.; Yela, D.A. Dienogest and deep infiltrating endometriosis: The remission of symptoms is not related to endometriosis nodule remission. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 211, 108–111. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Hormone therapy for intramural myoma-related infertility from ulipristal acetate to GnRH an-tagonist: A review. Reprod. Biomed. Online 2020, 41, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.-M. Fibroids and medical therapy: Bridging the gap from selective progesterone receptor modulators to gonadotropin-releasing hormone antagonist. Fertil. Steril. 2020, 114, 739–741. [Google Scholar] [CrossRef]
- Barbieri, R.L. Hormone treatment of endometriosis: The estrogen threshold hypothesis. Am. J. Obstet. Gynecol. 1992, 166, 740–745. [Google Scholar] [CrossRef]
- Chwalisz, K.; Surrey, E.; Stanczyk, F.Z. The hormonal profile of norethindrone acetate: Rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod. Sci. 2012, 19, 563–571. [Google Scholar] [CrossRef] [PubMed]
- European Society of Human Reproduction and Embryology ESHRE Guideline for the Diagnosis and Treatment of Endome-Triosis. 2010. Available online: http://guidelines.endometriosis.org/index.html (accessed on 1 October 2020).
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, CD008475. [Google Scholar] [CrossRef]
- Dragoman, M.V.; Jatlaoui, T.; Nanda, K.; Curtis, K.M.; Gaffield, M.E. Research gaps identified during the 2014 update of the WHO medical eligibility criteria for contraceptive use and selected practice recommendations for contraceptive use. Contracept 2016, 94, 195–201. [Google Scholar] [CrossRef][Green Version]
- Donnez, J.; Pirard, C.; Smets, M.; Jadoul, P.; Squifflet, J. Surgical management of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 329–348. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Clerckx, F.; Casanas-Roux, F.; Saussoy, P.; Gillerot, S. Advanced endoscopic techniques used in dysfunctional bleeding, fibroids and endometriosis, and the role of gonadotrophin-releasing hormone agonist treatment. Br. J. Obstet. Gynaecol. 1994, 101, 2–9. [Google Scholar] [CrossRef]
- Ng, J.; Chwalisz, K.; Carter, D.C.; Klein, C.E. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J. Clin. Endocrinol. Metab. 2017, 102, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.; Dmowski, W.P.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: Effects on bone mineral density. Reprod. Sci. 2014, 21, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Carr, B.; Dmowski, W.P.; Koltun, W.; O’Brien, C.; Jiang, P.; Burke, J.; Jimenez, R.; Garner, E.; Chwalisz, K. Elagolix treatment for endometriosis-associated pain: Results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod. Sci. 2014, 21, 363–371. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Surrey, E.; Taylor, H.S.; Giudice, L.; Lessey, B.A.; Abrao, M.S.; Archer, D.F.; Diamond, M.P.; Johnson, N.P.; Watts, N.B.; Gallagher, J.C.; et al. Long-term outcomes of elagolix in women with endometriosis: Results from two extension studies. Obstet. Gynecol. 2018, 132, 147–160. [Google Scholar] [CrossRef]
- Osuga, Y.; Seki, Y.; Tanimoto, M.; Kusumoto, T.; Kudou, K.; Terakawa, N. Relugolix, an oral gonadotropin-releasing hormone receptor antagonist, reduces endometriosis-associated pain in a dose-response manner: A randomized, double-blind, place-bo-controlled study. Fertil. Steril. 2021, 115, 397–405. [Google Scholar] [CrossRef]
- Donnez, J.; Taylor, H.S.; Taylor, R.N.; Akin, M.D.; Tatarchuk, T.F.; Wilk, K.; Gotteland, J.P.; Lecomte, V.; Bestel, E. Treatment of endome-triosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone-antagonist: A randomized clinical trial. Fertil. Steril. 2020, 114, 44–55. [Google Scholar] [CrossRef]
- Pohl, O.; Marchand, L.; Bell, D.; Gotteland, J.-P. Effects of combined GnRH receptor antagonist linzagolix and hormonal add-back therapy on vaginal bleeding—Delayed add-back onset does not improve bleeding pattern. Reprod. Sci. 2020, 27, 988–995. [Google Scholar] [CrossRef]
- Taylor, H.S.; Dun, E.C.; Chwalisz, K. Clinical evaluation of the oral gonadotropin-releasing hormone-antagonist elagolix for the management of endometriosis-associated pain. Pain Manag. 2019, 9, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Donnez, J. Gonadotropin-releasing hormone antagonist (linzagolix): A new therapy for uterine adenomyosis. Fertil. Steril. 2020, 114, 640–645. [Google Scholar] [CrossRef]
- Lamb, Y.N. Elagolix: First global approval. Drugs 2018, 78, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Barra, F.; Scala, C.; Ferrero, S. Elagolix sodium for the treatment of women with moderate to severe endometriosis-associated pain. Drugs Today 2019, 55, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywinski, R.M.; Soliman, A.M.; Chen, J.; Snabes, M.C.; Coyne, K.S.; Surrey, E.S.; Taylor, H.S. Achieving clinically meaningful response in endometriosis pain symptoms is associated with improvements in health-related quality of life and work productivity: Analysis of 2 phase III clinical trials. Am. J. Obstet. Gynecol. 2020, 222, 592.e1–592.e10. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Soliman, A.M.; Johns, B.; Pokrzywinski, R.M.; Snabes, M.; Coyne, K.S. Health-related quality of life improvements in patients with endometriosis treated with elagolix. Obstet. Gynecol. 2020, 136, 501–509. [Google Scholar] [CrossRef]
- Taylor, R.N.; Bestel, E.; Gotteland, J.P.; Lecomte, V.; Rachel Dubouloz, P.D.; Terrill, P.; Humberstone, A.; Loumaye, E. Long term treatment of endometriosis associated pain (EAP) with linzagolix: Efficacy and safety after 12 months of treatment. Fertil. Steril. 2019, 112, e323. [Google Scholar] [CrossRef]
- Bestel, E.; Gotteland, J.P.; Donnez, J.; Taylor, R.N.; Garner, E.I. Quality of life results after 52 weeks of treatment with linzagolix for endometriosis-associated pain. Obstet. Gynecol. 2020, 135, 26S–27S. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M.; Fellah, L. What if deep endometriotic nodules and uterine adenomyosis were actually two forms of the same disease? Fertil. Steril. 2019, 111, 454–456. [Google Scholar] [CrossRef]
- Borini, A.; Coticchio, G. Gonadotropin-releasing hormone antagonist linzagolix: Possible treatment for assisted reproduction patients presenting with adenomyosis and endometriosis? Fertil. Steril. 2020, 114, 517–518. [Google Scholar] [CrossRef]
- Markham, A. Relugolix: First global approval. Drugs 2019, 79, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Biberoglu, K.; Behrman, S. Dosage aspects of danazol therapy in endometriosis: Short-term and long-term effectiveness. Am. J. Obstet. Gynecol. 1981, 139, 645–654. [Google Scholar] [CrossRef]
- As-Sanie, S.; Becker, C.M.; Johnson, N.; Lessey, B.A.; Abrao, M.S.; Brown, E.L.; Wilk, K.; Ferreira, J.C.A.; Mathur, V.; Li, Y.; et al. Efficacy and safety of relugolix combination therapy in women with endometriosis-associated pain: Phase 3 randomized, double-blind, placebo-controlled study (spirit 2). Fertil. Steril. 2020, 114, e77. [Google Scholar] [CrossRef]
- Zandvliet, A.S.; Ouerdani, A.; Lee, T.Y.; Migoya, E.M.; Ferreira, J.C.A.; de Greef, R. Simulated long-term effects of relugolix combination therapy on bone mineral density at the lumbar spine as predicted by a validated semi-mechanistic exposure-response model. Fertil. Steril. 2020, 114, e350. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. American Society for Reproductive Medicine position statement on uterus transplantation: A committee opinion. Fertil. Steril. 2018, 110, 605–610. [Google Scholar] [CrossRef]
- Vercellini, P.; Vigano, P.; Barbara, G.; Buggio, L.; Somigliana, E.; Mangiagalli, L. Endometriosis Study, G. elagolix for endo-metriosis: All that glitters is not gold. Hum. Reprod. 2019, 34, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Roman, H. Choosing the right surgical technique for deep endometriosis: Shaving, disc excision, or bowel resection? Fertil. Steril. 2017, 108, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Surrey, E.; Bonafede, M.; Nelson, J.K.; Castelli-Haley, J. Real-world evaluation of direct and indirect economic burden among endometriosis patients in the United States. Adv. Ther. 2018, 35, 408–423. [Google Scholar] [CrossRef]
- Wang, S.-T.; Johnson, S.J.; Mitchell, D.; Soliman, A.M.; Vora, J.B.; Agarwal, S.K. Cost–effectiveness of elagolix versus leuprolide acetate for treating moderate-to-severe endometriosis pain in the USA. J. Comp. Eff. Res. 2019, 8, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Leyland, N.; Estes, S.J.; Lessey, B.A.; Advincula, A.P.; Taylor, H.S. A clinician’s guide to the treatment of endometriosis with elagolix. J. Womens Heal. 2020. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Valbrun, T.G.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef]
| Elagolix | Linzagolix | Relugolix | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessments | 150 mg Elaris-I | 200 mg Elaris-I | 150 mg Elaris-II | 200 mg Elaris-II | 75 mg | 100 mg | 200 mg | 40 mg CT Spirit-1 | 40 mg CT Spirit-2 |
| Pelvic Pain (OPP) | - | - | - | - | 70.8 | 66.7 | 77.3 | - | - |
| Dysmenorrhea (% responders) | 42.1 | 75.3 | 46.2 | 76.9 | 58.3 | 82.1 | 84.1 | 75.5 | 75.2 |
| NMPP (% responders) | 45.7 | 62.1 | 51.6 | 62.2 | 72.9 | 64.1 | 72.7 | 58.5 | 66 |
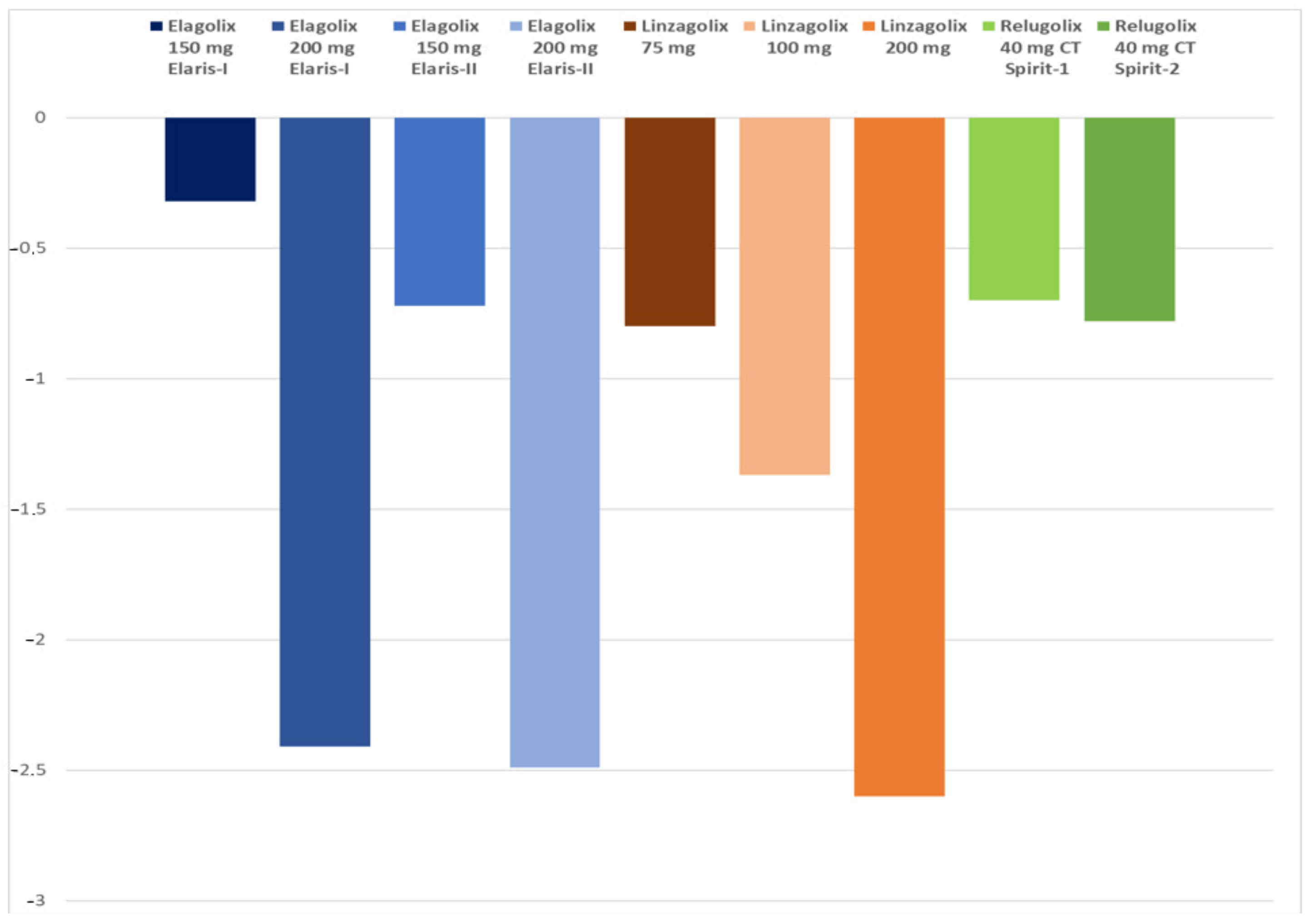
| BMD loss lumbar spine (%) | −0.32 | −2.41 | −0.72 | −2.49 | −0.80 | −1.37 | −2.60 | −0.70 | −0.78 |
| Hot flushes % | 23.7 | 42.3 | 22.6 | 47.6 | 19.0 | 28.8 | 45.6 | 10.4 | 13.6 |
| Elagolix | Linzagolix | Relugolix | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessments | 150 mg Elaris-III | 200 mg Elaris-III | 150 mg Elaris-IV | 200 mg Elaris-IV | 75 mg | 100 mg | 200 mg | 40 mg CT Spirit-1 | 40 mg CT Spirit-2 |
| Pelvic Pain (OPP) | - | - | - | - | 69.2 | 53.8 | 82.4 | - | - |
| Dysmenorrhea (% responders) | 52.1 | 78.1 | 50.8 | 75.9 | 69.2 | 69.2 | 64.7 | - | - |
| NMPP (% responders) | 67.8 | 69.1 | 66.4 | 67.2 | 69.2 | 53.8 | 76.5 | - | - |
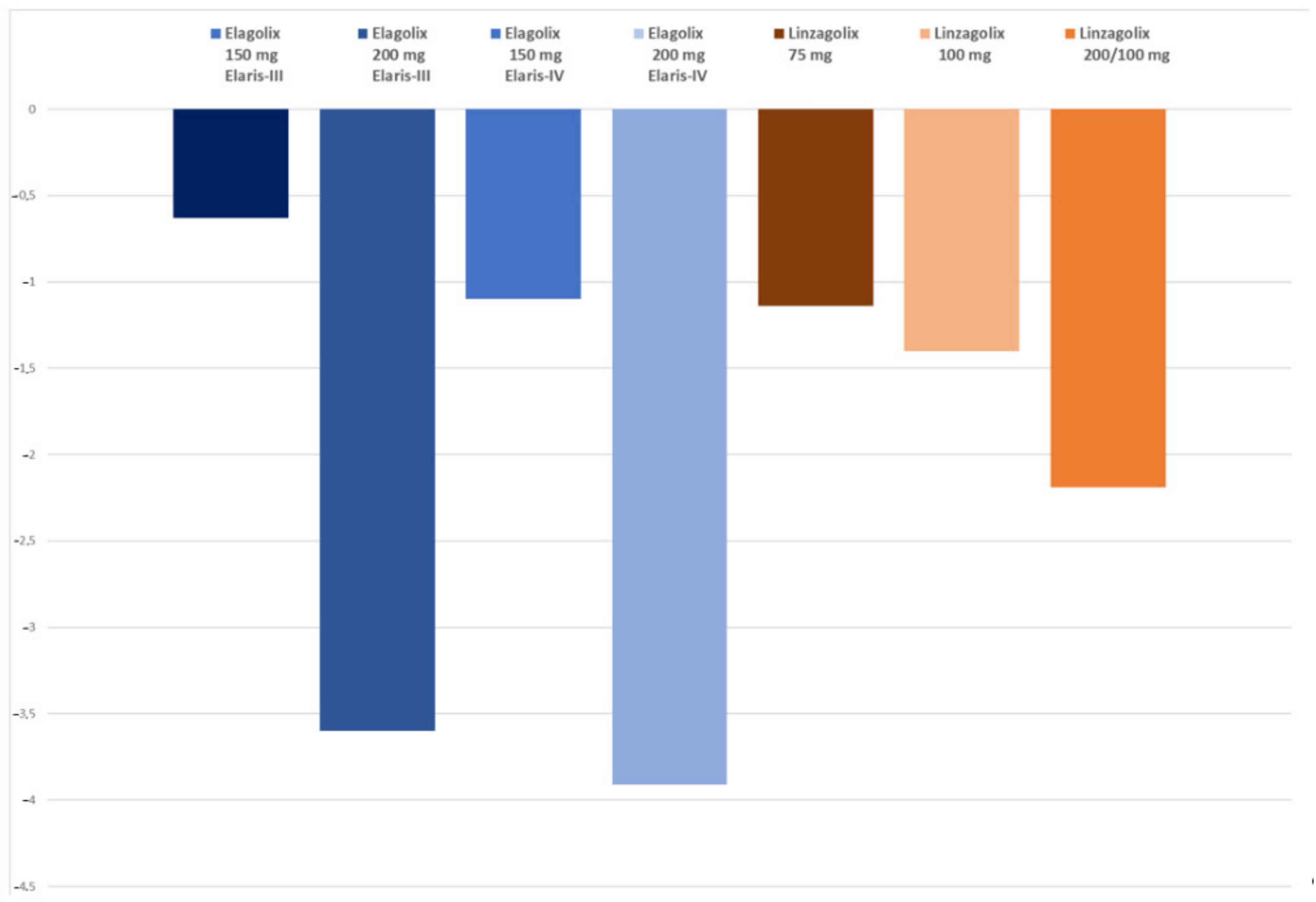
| BMD loss lumbar spine (%) | −0.63 | −3.60 | −1.10 | −3.91 | −1.14 | −1.40 | −2.19 | - | - |
| Hot flushes % | 44 | 72 | 36 | 77 | 22 | 27 | 60 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnez, J.; Dolmans, M.-M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. https://doi.org/10.3390/jcm10051085
Donnez J, Dolmans M-M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. Journal of Clinical Medicine. 2021; 10(5):1085. https://doi.org/10.3390/jcm10051085
Chicago/Turabian StyleDonnez, Jacques, and Marie-Madeleine Dolmans. 2021. "Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review" Journal of Clinical Medicine 10, no. 5: 1085. https://doi.org/10.3390/jcm10051085
APA StyleDonnez, J., & Dolmans, M.-M. (2021). Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. Journal of Clinical Medicine, 10(5), 1085. https://doi.org/10.3390/jcm10051085






