Unmet Rehabilitation Needs after Traumatic Brain Injury across Europe: Results from the CENTER-TBI Study
Abstract
1. Introduction
- To assess the rehabilitation needs and provision of rehabilitation services for individuals exhibiting TBI-related impairments and disability across Europe during the first six months post-injury.
- To investigate whether sociodemographic, premorbid, and injury-related factors predict the probability of receiving rehabilitation services at three and six months after injury.
- There will be a high percentage of rehabilitation needs among individuals with TBI-related impairments and disabilities in the first six months following TBI.
- There will be an association between the probability of receiving rehabilitation and age, sex, injury severity, comorbidities, and geographical regions.
2. Methods
2.1. Study Design
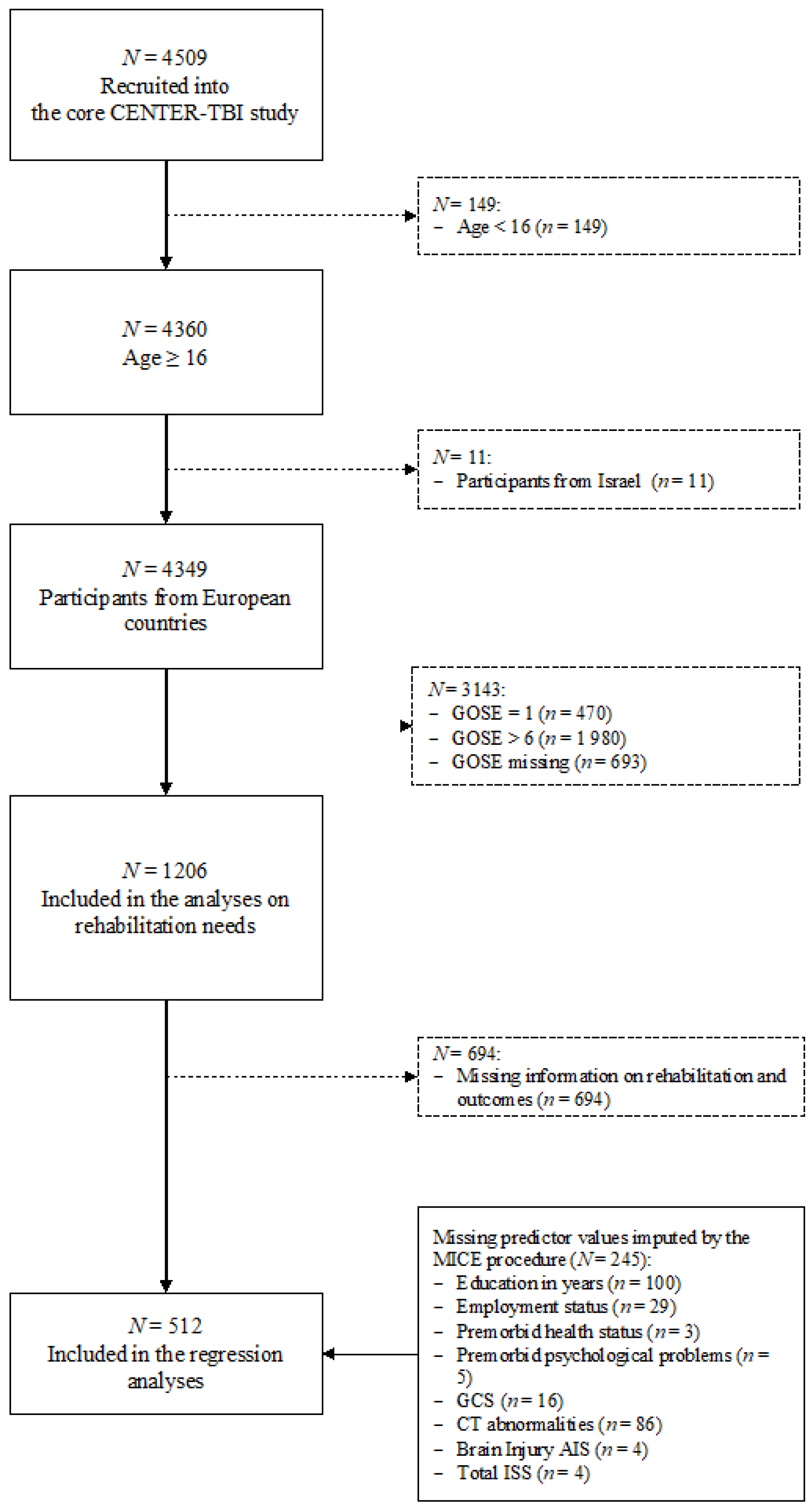
2.2. Participants
2.3. Instruments
2.3.1. Sociodemographic, Premorbid, and Injury-Related Data
2.3.2. Assessment of Rehabilitation Needs
- (i)
- Post-traumatic stress disorder (PTSD) was captured by the Posttraumatic Stress Disorder Checklist-5 (PCL-5) [35]. The PCL-5 measures 20 symptoms of PTSD based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [36] using a five-point Likert scale (from 0, ‘not at all’, to 4, ‘extreme’). The total score ranges from 0 to 80, with higher values indicating greater impairment. A cut-off value of 33 was applied to determine clinically relevant PTSD [37].
- (ii)
- Depression was assessed with the Patient Health Questionnaire (PHQ-9) [38]. The PHQ-9 assesses symptoms of depression using nine items and a four-point Likert scale (from 0 ‘not at all’ to 3 ‘nearly every day’). The PHQ-9 total score ranges from 0 to 27, with higher values indicating greater impairment. A cut-off value of 10 was applied to determine clinically relevant depression [38,39].
- (iii)
- Anxiety was assessed with the self-reported Generalized Anxiety Disorder seven-item scale (GAD-7) [40]. The GAD-7 uses seven items and a four-point Likert scale (from 0 ‘not at all’ to 3 ‘nearly every day’). The total score ranges from 0 to 21. Higher values indicate greater impairment, with a cut-off value of 10 indicating impairment [40].
2.3.3. Professional Help and Rehabilitation Services
2.4. Statistical Analyses
2.4.1. Unmet Rehabilitation Needs
2.4.2. Prediction of Probability of Receiving Rehabilitation
3. Results
3.1. Sample Characteristics
3.2. Rehabilitation Needs
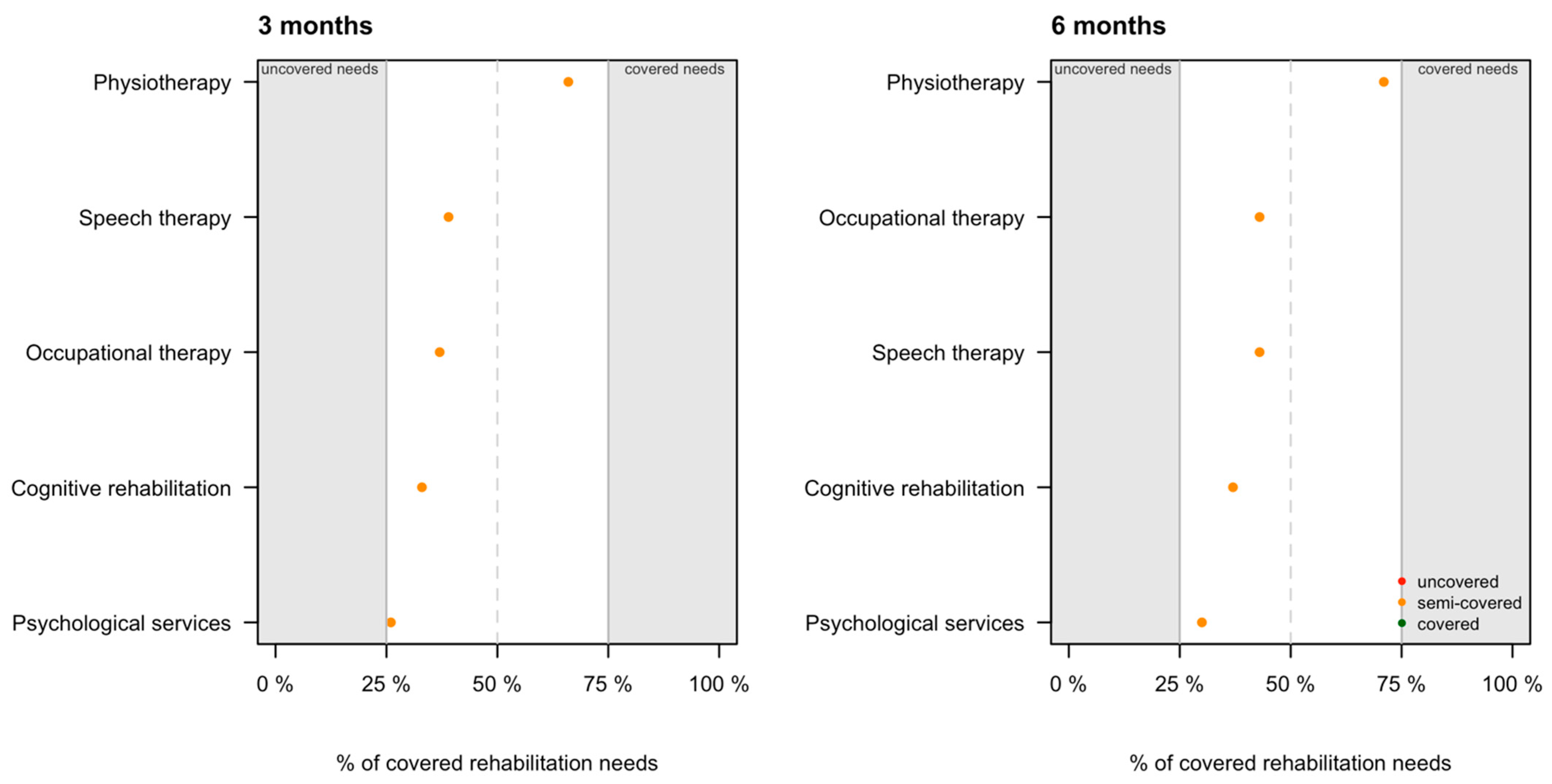
3.3. Coverage of Rehabilitation Needs
3.4. Prediction of the Probability of Receiving Rehabilitation
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Rehabilitation | 3 Months | 6 Months | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ER | Ward | ICU | Total | ER | Ward | ICU | Total | ||
| No | 43 (56.6%) | 126 (46.5%) | 137 (15.9%) | 306 (25.4%) | 36 (47.4%) | 125 (46.1%) | 115 (13.4%) | 276 (22.9%) | 0.61 |
| In-patient | 2 (2.6%) | 36 (13.3%) | 303 (35.3%) | 341 (28.3%) | 4 (5.3%) | 21 (7.7%) | 331 (38.5%) | 356 (29.5%) | 0.06 |
| Out-patient | 13 (17.1%) | 38 (14.0%) | 99 (11.5%) | 150 (12.4%) | 15 (19.7%) | 48 (17.7%) | 120 (14.0%) | 183 (15.2%) | 0.98 |
| Missing | 18 (23.7%) | 71 (26.2%) | 320 (37.3%) | 409 (33.9%) | 21 (27.6%) | 77 (28.4%) | 293 (34.1%) | 391 (32.4%) | |
| Total | 76 (6.3%) | 271 (22.5%) | 859 (71.2%) | 1206 (100%) | 76 (6.3%) | 271 (22.5%) | 859 (71.2%) | 1206 (100%) | |
References
- Andelic, N.; Sigurdardottir, S.; Schanke, A.-K.; Sandvik, L.; Sveen, U.; Roe, C. Disability, Physical Health and Mental Health 1 Year after Traumatic Brain Injury. Disabil. Rehabil. 2010, 32, 1122–1131. [Google Scholar] [CrossRef]
- Sigurdardottir, S.; Andelic, N.; Roe, C.; Schanke, A.-K. Cognitive Recovery and Predictors of Functional Outcome 1 Year after Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2009, 15, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Phillips, V.L.; Greenspan, A.I.; Stringer, A.Y.; Stroble, A.K.; Lehtonen, S. Severity of Injury and Service Utilization Following Traumatic Brain Injury: The First 3 Months. J. Head Trauma Rehabil. 2004, 19, 217–225. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Pick, A.; Nair, A.; Disler, P.B.; Wade, D.T. Multi-Disciplinary Rehabilitation for Acquired Brain Injury in Adults of Working Age. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Andelic, N.; Ye, J.; Tornas, S.; Roe, C.; Lu, J.; Bautz-Holter, E.; Moger, T.; Sigurdardottir, S.; Schanke, A.-K.; Aas, E. Cost-Effectiveness Analysis of an Early-Initiated, Continuous Chain of Rehabilitation after Severe Traumatic Brain Injury. J. Neurotrauma 2014, 31, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Andelic, N.; Bautz-Holter, E.; Ronning, P.; Olafsen, K.; Sigurdardottir, S.; Schanke, A.-K.; Sveen, U.; Tornas, S.; Sandhaug, M.; Roe, C. Does an Early Onset and Continuous Chain of Rehabilitation Improve the Long-Term Functional Outcome of Patients with Severe Traumatic Brain Injury? J. Neurotrauma 2012, 29, 66–74. [Google Scholar] [CrossRef]
- Jacob, L.; Cogné, M.; Tenovuo, O.; Røe, C.; Andelic, N.; Majdan, M.; Ranta, J.; Ylen, P.; Dawes, H.; Azouvi, P.; et al. Predictors of Access to Rehabilitation in the Year Following Traumatic Brain Injury: A European Prospective and Multicenter Study. Neurorehabil. Neural Repair 2020, 34, 814–830. [Google Scholar] [CrossRef]
- Ponsford, J.; Carrier, S.; Hicks, A.; McKay, A. Assessment and Management of Patients in the Acute Stages of Recovery after Traumatic Brain Injury in Adults—A World-Wide Survey. J. Neurotrauma 2020. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, C.; Bayen, E.; Darnoux, E.; Ghout, I.; Azerad, S.; Ruet, A.; Vallat-Azouvi, C.; Pradat-Diehl, P.; Aegerter, P.; Weiss, J.-J.; et al. Patterns of Post-Acute Health Care Utilization after a Severe Traumatic Brain Injury: Results from the PariS-TBI Cohort. Brain Inj. 2015, 29, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Andelic, N.; Soberg, H.L.; Berntsen, S.; Sigurdardottir, S.; Roe, C. Self-Perceived Health Care Needs and Delivery of Health Care Services 5 Years after Moderate-to-Severe Traumatic Brain Injury. PM&R 2014, 6, 1013–1021. [Google Scholar] [CrossRef]
- Soberg, H.; Finset, A.; Roise, O.; Bautz-Holter, E. Identification and Comparison of Rehabilitation Goals after Multiple Injuries: An ICF Analysis of the Patients’, Physiotherapists’ and other Allied Professionals’ Reported Goals. J. Rehabil. Med. 2008, 40, 340–346. [Google Scholar] [CrossRef]
- Schumacher, R.; Walder, B.; Delhumeau, C.; Müri, R.M. Predictors of Inpatient (Neuro)Rehabilitation after Acute Care of Severe Traumatic Brain Injury: An Epidemiological Study. Brain Inj. 2016, 30, 1186–1193. [Google Scholar] [CrossRef]
- Cnossen, M.C.; Lingsma, H.F.; Tenovuo, O.; Maas, A.I.R.; Menon, D.; Steyerberg, E.W.; Ribbers, G.M.; Polinder, S. Rehabilitation after Traumatic Brain Injury: A Survey in 70 European Neurotrauma Centres Participating in the CENTER-TBI Study. J. Rehabil. Med. 2017, 49, 395–401. [Google Scholar] [CrossRef]
- Corrigan, J.D.; Whiteneck, G.; Mellick, D. Perceived Needs Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2004, 19, 205–216. [Google Scholar] [CrossRef]
- Ta’eed, G.; Skilbeck, C.; Slatyer, M. Service Utilisation in a Public Post-Acute Rehabilitation Unit Following Traumatic Brain Injury. Neuropsychol. Rehabil. 2015, 25, 841–863. [Google Scholar] [CrossRef]
- Andelic, N.; Forslund, M.V.; Perrin, P.B.; Sigurdardottir, S.; Lu, J.; Howe, E.; Sveen, U.; Rasmussen, M.S.; Søberg, H.L.; Røe, C. Long-Term Follow-Up of Use of Therapy Services for Patients with Moderate-to-Severe Traumatic Brain Injury. J. Rehabil. Med. 2020. [Google Scholar] [CrossRef]
- Prang, K.-H.; Ruseckaite, R.; Collie, A. Healthcare and Disability Service Utilization in the 5-Year Period Following Transport-Related Traumatic Brain Injury. Brain Inj. 2012, 26, 1611–1620. [Google Scholar] [CrossRef]
- Johnstone, B.; Nossaman, L.D.; Schopp, L.H.; Holmquist, L.; Rupright, S.J. Distribution of Services and Supports for People with Traumatic Brain Injury in Ruraland Urban Missouri. J. Rural Health 2002, 18, 109–117. [Google Scholar] [CrossRef]
- Jennekens, N.; de Casterlé, B.D.; Dobbels, F. A Systematic Review of Care Needs of People with Traumatic Brain Injury (TBI) on a Cognitive, Emotional and Behavioural Level. J. Clin. Nurs. 2010, 19, 1198–1206. [Google Scholar] [CrossRef]
- Rathmann, K.; Nellen, C.; Wetzel, L.D. Behinderungsspezifischer Gradient in der Psychischen Gesundheit und dem Gesundheitsbewusstsein: Ergebnisse der Repräsentativen GEDA-Studie für Deutschland. Rehabilitation 2020, 59, 223–230. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Steyerberg, E.W.; Citerio, G.; Lecky, F.; Manley, G.T.; Hill, S.; Legrand, V.; Sorgner, A. Collaborative European Neurotrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): A Prospective Longitudinal Observational Study. Neurosurgery 2015, 76, 67–80. [Google Scholar] [CrossRef]
- Wilson, J.T.L.; Pettigrew, L.E.L.; Teasdale, G.M. Structured Interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: Guidelines for Their Use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Wiegers, E.; Sewalt, C.; Buki, A.; Citerio, G.; De Keyser, V.; Ercole, A.; Kunzmann, K.; Lanyon, L.; Lecky, F.; et al. Case-Mix, Care Pathways, and Outcomes in Patients with Traumatic Brain Injury in CENTER-TBI: A European Prospective, Multicentre, Longitudinal, Cohort Study. Lancet Neurol. 2019, 18, 923–934. [Google Scholar] [CrossRef]
- Wilson, J.T.L.; Edwards, P.; Fiddes, H.; Stewart, E.; Teasdale, G.M. Reliability of Postal Questionnaires for the Glasgow Outcome Scale. J. Neurotrauma 2002, 19, 999–1005. [Google Scholar] [CrossRef]
- Publications Office of the European Union EuroVoc Thesaurus. Available online: http://publications.europa.eu/resource/dataset/eurovoc (accessed on 9 January 2020).
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A Review of ASA Physical Status—Historical Perspectives and Modern Developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of Coma and Impaired Consciousness: A Practical Scale. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Gennarelli, T.A.; Wodzin, E. AIS 2005: A Contemporary Injury Scale. Injury 2006, 37, 1083–1091. [Google Scholar] [CrossRef]
- Gennarelli, T.A.; Wodzin, E.; Association for the Advancement of Automotive Medicine. Abbreviated Injury Scale 2005: Update 2008; Association for the Advancement of Automative Medicine: Barrington, IL, USA, 2008; ISBN 978-0-00-000202-0. [Google Scholar]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Long, W.B. The Injury Severity Score: A Method for Describing Patients with Multiple Injuries and Evaluating Emergency Care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Von Steinbuechel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Höfer, S.; Schmidt, S.; Bullinger, M.; Maas, A.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Validity and Correlates of Quality of Life. J. Neurotrauma 2010, 27, 1157–1165. [Google Scholar] [CrossRef]
- Wilson, J.T.L.; Marsden-Loftus, I.; Koskinen, S.; Bakx, W.; Bullinger, M.; Formisano, R.; Maas, A.; Neugebauer, E.; Powell, J.; Sarajuuri, J.; et al. Interpreting Quality of Life after Brain Injury Scores: Cross-Walk with the Short Form-36. J. Neurotrauma 2017, 34, 59–65. [Google Scholar] [CrossRef] [PubMed]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A Measure of Symptoms Commonly Experienced after Head Injury and Its Reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Voormolen, D.C.; Cnossen, M.C.; Polinder, S.; von Steinbuechel, N.; Vos, P.E.; Haagsma, J.A. Divergent Classification Methods of Post-Concussion Syndrome after Mild Traumatic Brain Injury: Prevalence Rates, Risk Factors, and Functional Outcome. J. Neurotrauma 2018, 35, 1233–1241. [Google Scholar] [CrossRef]
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma. Stress 2015, 28, 489–498. [Google Scholar] [CrossRef]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Stein, M.B.; Jain, S.; Giacino, J.T.; Levin, H.; Dikmen, S.; Nelson, L.D.; Vassar, M.J.; Okonkwo, D.O.; Diaz-Arrastia, R.; Robertson, C.S.; et al. Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients after Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA Psychiatry 2019, 76, 249–258. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Rubin, D.B. (Ed.) Multiple Imputation for Nonresponse in Surveys; Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987; ISBN 978-0-470-31669-6. [Google Scholar]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple Imputation by Chained Equations: What Is It and How Does It Work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef]
- Heinze, G.; Dunkler, D. Five Myths about Variable Selection. Transpl. Int. 2017, 30, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, N.J.D. A Note on a General Definition of the Coefficient of Determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Bamber, D. The Area above the Ordinal Dominance Graph and the Area below the Receiver Operating Characteristic Graph. J. Math. Psychol. 1975, 12, 387–415. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Heymans, M. Psfmi: Prediction Model Selection and Performance Evaluation in Multiple Imputed Datasets; R Package Version 0.5.0; Available online: https://CRAN.R-project.org/package=psfmi (accessed on 12 January 2020).
- Pradat-Diehl, P.; Joseph, P.-A.; Beuret-Blanquart, F.; Luauté, J.; Tasseau, F.; Remy-Neris, O.; Azouvi, P.; Sengler, J.; Bayen, É.; Yelnik, A.; et al. Physical and Rehabilitation Medicine (PRM) Care Pathways: Adults with Severe Traumatic Brain Injury. Ann. Phys. Rehabil. Med. 2012, 55, 546–556. [Google Scholar] [CrossRef]
- Majdan, M.; Plancikova, D.; Brazinova, A.; Rusnak, M.; Nieboer, D.; Feigin, V.; Maas, A. Epidemiology of Traumatic Brain Injuries in Europe: A Cross-Sectional Analysis. Lancet Public Health 2016, 1, e76–e83. [Google Scholar] [CrossRef]
- Jennett, B.; Snoek, J.; Bond, M.R.; Brooks, N. Disability after Severe Head Injury: Observations on the Use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry 1981, 44, 285–293. [Google Scholar] [CrossRef]
- Wilson, J.T.L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The Chronic and Evolving Neurological Consequences of Traumatic Brain Injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef]
- Sveen, U.; Røe, C.; Sigurdardottir, S.; Skandsen, T.; Andelic, N.; Manskow, U.; Berntsen, S.A.; Soberg, H.L.; Anke, A. Rehabilitation Pathways and Functional Independence One Year after Severe Traumatic Brain Injury. Eur. J. Phys. Rehabil. Med. 2016, 52, 650–661. [Google Scholar] [PubMed]
- Althubaiti, A. Information Bias in Health Research: Definition, Pitfalls, and Adjustment Methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef]
- Arnould, A.; Dromer, E.; Rochat, L.; Van der Linden, M.; Azouvi, P. Neurobehavioral and Self-Awareness Changes after Traumatic Brain Injury: Towards New Multidimensional Approaches. Ann. Phys. Rehabil. Med. 2016, 59, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of Important Variables and Determination of Functional Form for Continuous Predictors in Multivariable Model Building: Selection of Variables and Functional Forms. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
| Variable | Group/Values | N (%) or M (SD) or Md [Min, Max] |
|---|---|---|
| Sex | Female | 390 (32.3%) |
| Male | 816 (67.7%) | |
| Age in years | M (SD) | 49.3 (18.9) |
| Md [Min, Max] | 50.0 [16.00, 93.00] | |
| Missing | 0 (0%) | |
| Education in years | M (SD) | 13.1 (3.84) |
| Md [Min, Max] | 13.0 [1.00, 30.00] | |
| Missing | 321 (26.6%) | |
| Employment status | Employed | 633 (52.5%) |
| Unemployed | 104 (8.6%) | |
| Other | 341 (28.3%) | |
| Missing | 128 (10.6%) | |
| Living status | Alone | 948 (78.6%) |
| Not alone | 255 (21.1%) | |
| Missing | 3 (0.2%) | |
| Geographical regions | Western Europe | 593 (49.2%) |
| Northern Europe | 306 (25.4%) | |
| Southern/Eastern Europe | 307 (25.5%) | |
| Premorbid somatic health status | Healthy | 632 (52.4%) |
| Mild disease | 417 (34.6%) | |
| Severe disease | 130 (10.8%) | |
| Missing | 27 (2.2%) | |
| Premorbid psychological problems | No | 968 (80.3%) |
| Yes | 198 (16.4%) | |
| Missing | 40 (3.3%) | |
| Injury cause | Road traffic accident | 550 (45.6%) |
| Fall | 455 (37.7%) | |
| Violent/other | 196 (16.3%) | |
| Missing | 5 (0.4%) | |
| Clinical care pathways | ER | 76 (6.3%) |
| ward | 271 (22.5%) | |
| ICU | 859 (71.2%) | |
| GOSE (6 months) | M (SD) | 4.66 (1.17) |
| Md [Min, Max] | 5.00 [3.00, 6.00] | |
| Missing | 0 (0%) | |
| TBI severity | Mild | 592 (49.1%) |
| Moderate | 141 (11.7%) | |
| Severe | 425 (35.2%) | |
| Missing | 48 (4.0%) | |
| Abnormalities on the first CT scan | Absent | 223 (18.5%) |
| Present | 750 (62.2%) | |
| Missing | 233 (19.3%) | |
| Brain Injury AIS | M (SD) | 3.82 (1.25) |
| Md [Min, Max] | 4.00 [1.00, 6.00] | |
| Missing | 19 (1.6%) | |
| Total ISS | M (SD) | 27.7 (16.2) |
| Md [Min, Max] | 25.0 [1.00, 75.0] | |
| Missing | 19 (1.6%) | |
| Total | 1206 (100%) |
| Outcome Type | Impairment | Three Months | Six Months | p |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Problems with activities of daily life | Not impaired | 283 (23.5%) | 361 (29.9%) | 0.01 |
| Impaired | 411 (34.1%) | 400 (33.2%) | ||
| Missing | 512 (42.5%) | 445 (36.9%) | ||
| Physical problems | Not impaired | 289 (24.0%) | 333 (27.6%) | 0.06 |
| Impaired | 506 (42.0%) | 480 (39.8%) | ||
| Missing | 411 (34.1%) | 393 (32.6%) | ||
| Cognition problems | Not impaired | 209 (17.3%) | 215 (17.8%) | 0.41 |
| Impaired | 498 (41.3%) | 567 (47.0%) | ||
| Missing | 499 (41.4%) | 424 (35.2%) | ||
| Speech and language problems | Not impaired | 579 (48.0%) | 607 (50.3%) | 0.49 |
| Impaired | 215 (17.8%) | 207 (17.2%) | ||
| Missing | 412 (34.2%) | 392 (32.5%) | ||
| Psychological problems | Not impaired | 411 (34.1%) | 454 (37.6%) | 0.70 |
| Impaired | 277 (23.0%) | 292 (24.2%) | ||
| Missing | 518 (43.0%) | 460 (38.1%) |
| Outcome Type | Type of Professional Help | Three Months | Six Months | p | ||||
|---|---|---|---|---|---|---|---|---|
| Impaired | Help Provided | % | Impaired | Help Provided | % | |||
| Problems with activities of daily life | Occupational therapy | 411 | 153 | 37% | 400 | 170 | 43% | 0.34 |
| Physical problems | Physiotherapy | 506 | 334 | 66% | 480 | 340 | 71% | 0.82 |
| Cognition problems | Cognitive rehabilitation | 498 | 166 | 33% | 567 | 212 | 37% | 0.02 |
| Speech and language problems | Speech therapy | 215 | 84 | 39% | 207 | 89 | 43% | 0.70 |
| Psychological problems | Psychological services | 277 | 73 | 26% | 292 | 89 | 30% | 0.21 |
| Recovery Status | Rehabilitation | Three Months | Six Months | p |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Total | No | 306 (25.4%) | 276 (22.9%) | 0.07 ‡ |
| In-patient | 341 (28.3%) | 356 (29.5%) | ||
| Out-patient | 150 (12.4%) | 183 (15.2%) | ||
| Missing | 409 (33.9%) | 391 (32.4%) | ||
| Total | 1206 (100.0%) | 1206 (100.0%) | ||
| GOSE 2/3 (vegetative state/lower severe disability) | No | 30 (9.7%) | 18 (5.8%) | 0.05 † |
| In-patient | 103 (33.3%) | 93 (30.1%) | ||
| Out-patient | 9 (2.9%) | 18 (5.8%) | ||
| Missing | 167 (54.0%) | 180 (58.3%) | ||
| Total | 309 (100.0%) | 309 (100.0%) | ||
| GOSE 4 (upper severe disability) | No | 35 (20.1%) | 33 (19.0%) | 0.34 † |
| In-patient | 51 (29.3%) | 68 (39.1%) | ||
| Out-patient | 14 (8.0%) | 23 (13.2%) | ||
| Missing | 74 (42.5%) | 50 (28.7%) | ||
| Total | 174 (100.0%) | 174 (100.0%) | ||
| GOSE 5 (lower moderate disability) | No | 95 (28.0%) | 75 (22.1%) | 0.12 † |
| In-patient | 100 (29.5%) | 98 (28.9%) | ||
| Out-patient | 52 (15.3%) | 67 (19.8%) | ||
| Missing | 92 (27.1%) | 99 (29.2%) | ||
| Total | 339 (100.0%) | 339 (100.0%) | ||
| GOSE 6 (upper moderate disability) | No | 146 (38.0%) | 150 (39.1%) | 0.89 † |
| In-patient | 87 (22.7%) | 97 (25.3%) | ||
| Out-patient | 75 (19.5%) | 75 (19.5%) | ||
| Missing | 76 (19.8%) | 62 (16.1%) | ||
| Total | 384 (100.0%) | 384 (100.0%) |
| Imapired Outcome | Rehabilitation ‡ | Three Months | Six Months | p |
|---|---|---|---|---|
| Problems with activities of daily life | No | 153 (37.2%) | 116 (29.0%) | 0.03 |
| In-patient | 164 (39.9%) | 178 (44.5%) | ||
| Outpatient | 82 (20.0%) | 98 (24.5%) | ||
| Missing | 12 (2.9%) | 8 (2.0%) | ||
| Physical problems | No | 175 (34.6%) | 145 (30.2%) | 0.06 |
| In-patient | 240 (47.4%) | 223 (46.5%) | ||
| Outpatient | 87 (17.2%) | 110 (22.9%) | ||
| Missing | 4 (0.8%) | 2 (0.4%) | ||
| Cognition problems | No | 182 (36.5%) | 170 (30.0%) | 0.05 |
| In-patient | 200 (40.2%) | 236 (41.6%) | ||
| Outpatient | 105 (21.1%) | 146 (25.7%) | ||
| Missing | 11 (2.2%) | 15 (2.6%) | ||
| Speech and language problems | No | 62 (28.8%) | 51 (24.6%) | 0.06 |
| In-patient | 118 (54.9%) | 104 (50.2%) | ||
| Outpatient | 34 (15.8%) | 52 (25.1%) | ||
| Missing | 1 (0.5%) | 0 (0.0%) | ||
| Psychological problems | No | 123 (44.4%) | 108 (37.0%) | 0.19 |
| In-patient | 96 (34.7%) | 109 (37.3%) | ||
| Outpatient | 53 (19.1%) | 68 (23.3%) | ||
| Missing | 5 (1.8%) | 7 (2.4%) |
| Variable/Category | Reference Group | Estimate | S.E. | p | OR | CI 2.5% | CI 97.5% |
|---|---|---|---|---|---|---|---|
| Intercept | - | 1.38 | 0.80 | 0.086 | 3.99 | 0.82 | 19.29 |
| GCS | - | −0.24 | 0.04 | <0.001 | 0.79 ‡ | 0.72 | 0.86 |
| Brain Injury AIS | - | 0.56 | 0.16 | 0.001 | 1.75 ☥ | 1.27 | 2.42 |
| ISS | - | −0.02 | 0.01 | 0.063 | 0.98 | 0.96 | 1.00 |
| Physical problems | - | 0.65 | 0.24 | 0.006 | 1.92 ☥ | 1.21 | 3.05 |
| Cognition | - | 1.39 | 0.27 | <0.001 | 4.00 ☥ | 2.34 | 6.83 |
| Psychological problems | - | −0.56 | 0.25 | 0.026 | 0.57 ‡ | 0.35 | 0.93 |
| Ward | ER | −0.86 | 0.44 | 0.052 | 0.42 | 0.18 | 1.00 |
| ICU | ER | −0.38 | 0.55 | 0.499 | 0.69 | 0.23 | 2.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andelic, N.; Røe, C.; Tenovuo, O.; Azouvi, P.; Dawes, H.; Majdan, M.; Ranta, J.; Howe, E.I.; Wiegers, E.J.A.; Tverdal, C.; et al. Unmet Rehabilitation Needs after Traumatic Brain Injury across Europe: Results from the CENTER-TBI Study. J. Clin. Med. 2021, 10, 1035. https://doi.org/10.3390/jcm10051035
Andelic N, Røe C, Tenovuo O, Azouvi P, Dawes H, Majdan M, Ranta J, Howe EI, Wiegers EJA, Tverdal C, et al. Unmet Rehabilitation Needs after Traumatic Brain Injury across Europe: Results from the CENTER-TBI Study. Journal of Clinical Medicine. 2021; 10(5):1035. https://doi.org/10.3390/jcm10051035
Chicago/Turabian StyleAndelic, Nada, Cecilie Røe, Olli Tenovuo, Philippe Azouvi, Helen Dawes, Marek Majdan, Jukka Ranta, Emilie I. Howe, Eveline J.A. Wiegers, Cathrine Tverdal, and et al. 2021. "Unmet Rehabilitation Needs after Traumatic Brain Injury across Europe: Results from the CENTER-TBI Study" Journal of Clinical Medicine 10, no. 5: 1035. https://doi.org/10.3390/jcm10051035
APA StyleAndelic, N., Røe, C., Tenovuo, O., Azouvi, P., Dawes, H., Majdan, M., Ranta, J., Howe, E. I., Wiegers, E. J. A., Tverdal, C., Borgen, I., Forslund, M. V., Kleffelgaard, I., Dahl, H. M., Jacob, L., Cogné, M., Lu, J., von Steinbuechel, N., & Zeldovich, M. (2021). Unmet Rehabilitation Needs after Traumatic Brain Injury across Europe: Results from the CENTER-TBI Study. Journal of Clinical Medicine, 10(5), 1035. https://doi.org/10.3390/jcm10051035








