Efficacy and Safety of Tranexamic Acid in Emergency Trauma: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Assessment of Risk of Bias
2.5. Outcomes and Subgroups
2.6. Statistical Analysis
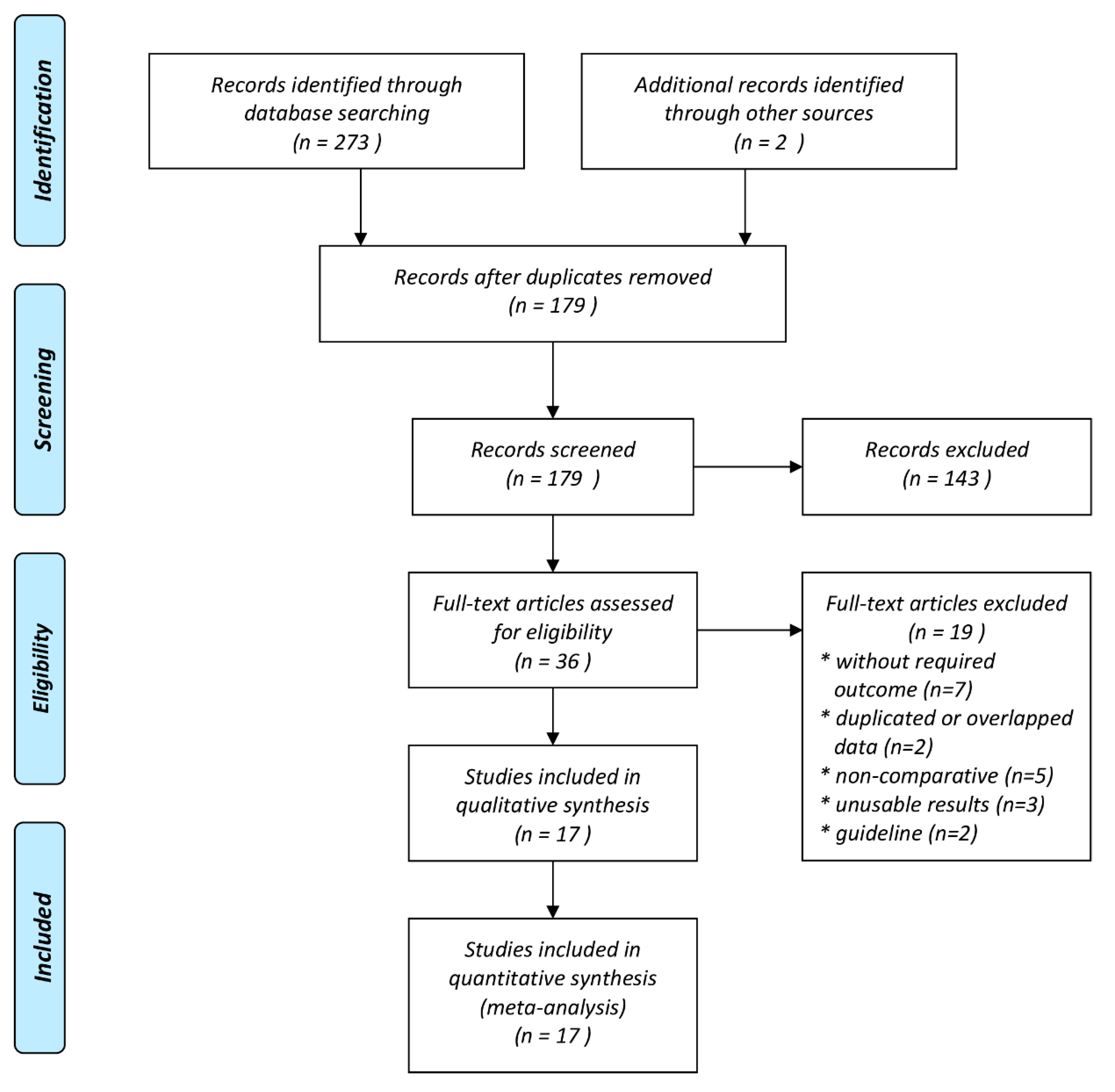
3. Results
3.1. Characteristics of Studies Included in the Meta-Analysis
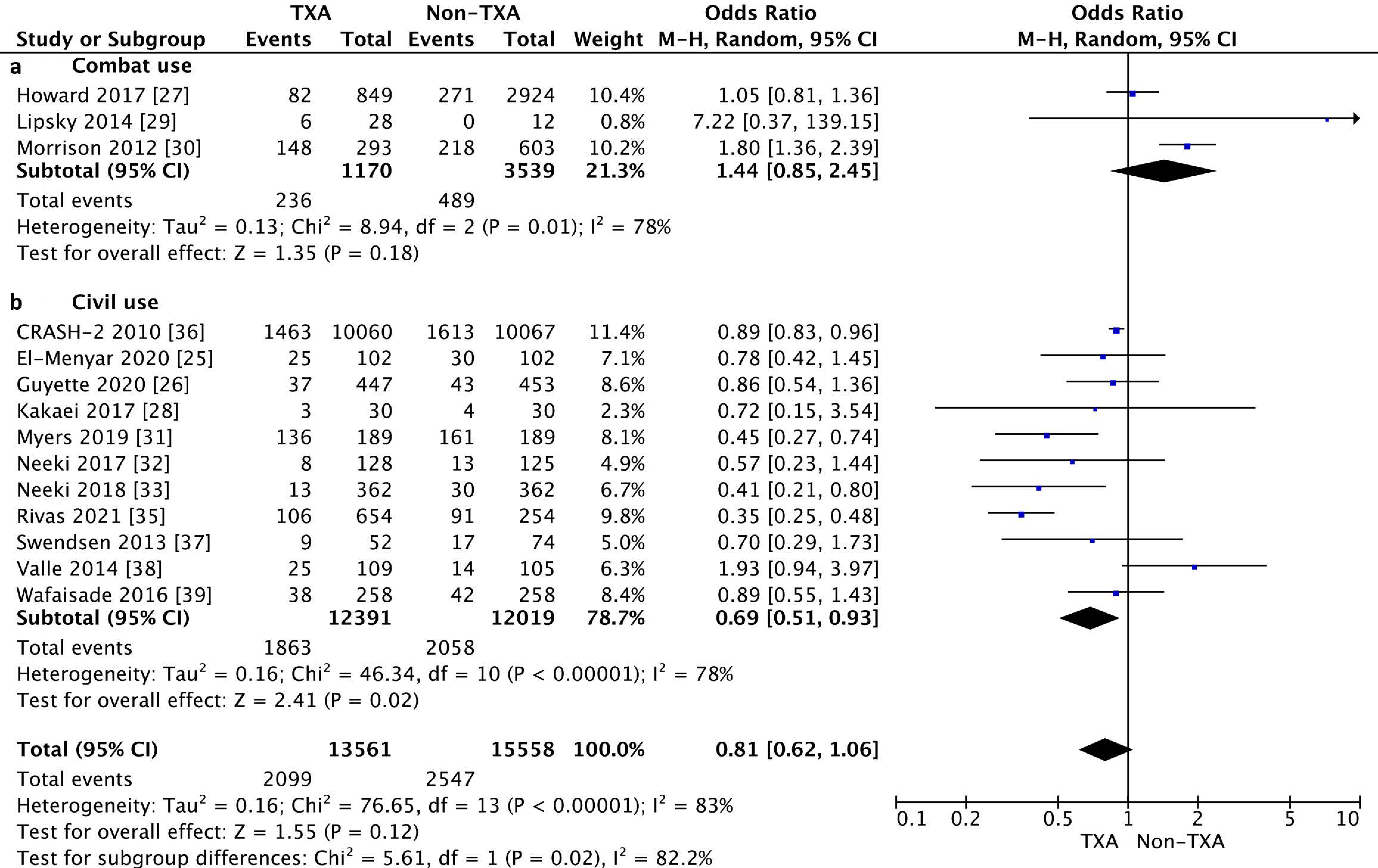
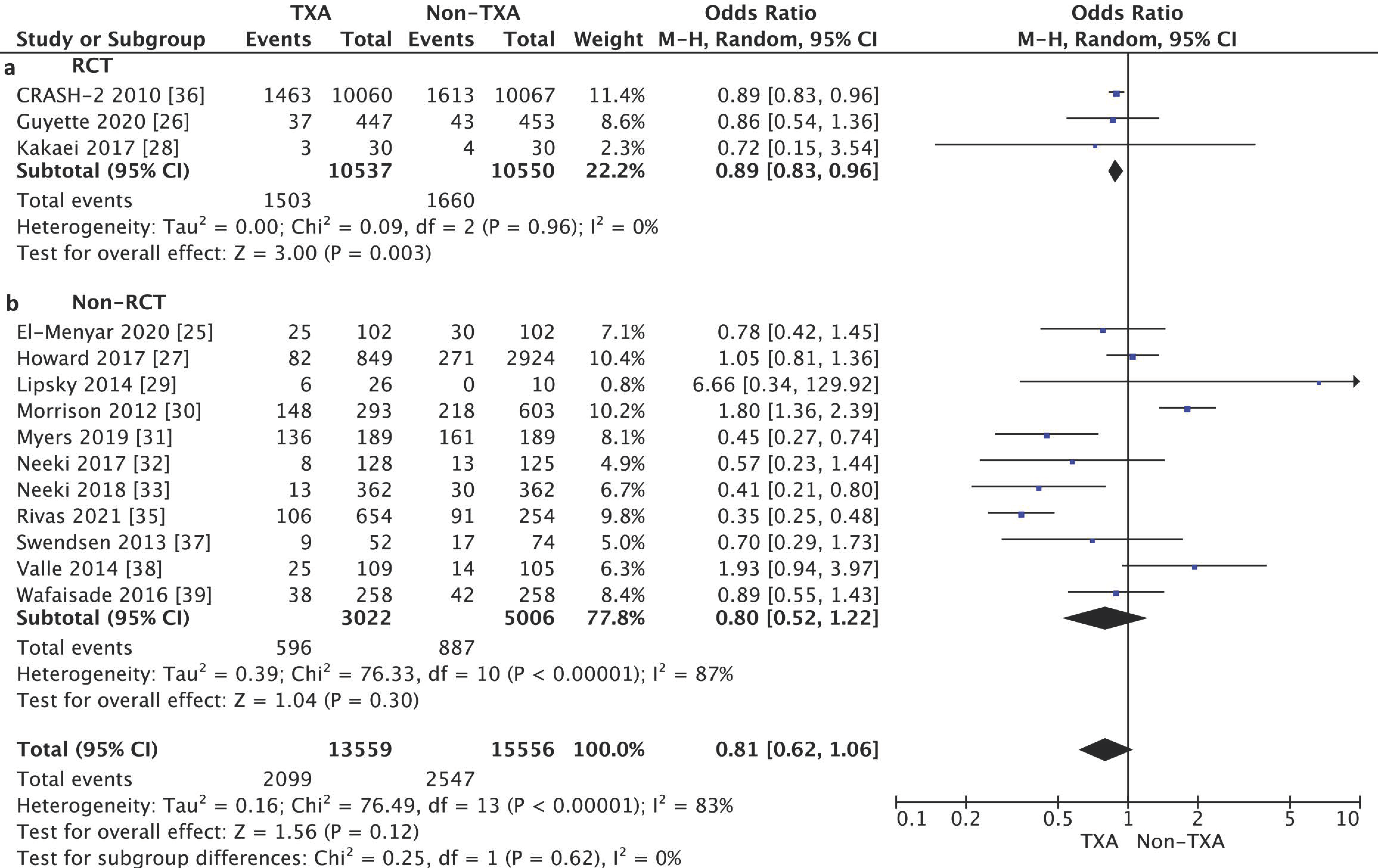
3.2. Primary Outcome
3.3. Secondary Outcomes
3.4. Risk of Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kool, B.; Lilley, R.; Davie, G.; Reid, P.; Civil, I.; Branas, C.; de Graaf, B.; Dicker, B.; Ameratunga, S.N. Evaluating the impact of prehospital care on mortality following major trauma in New Zealand: A retrospective cohort study. Inj. Prev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Prieto-Merino, D.; Shakur, H.; Clayton, T.; Lecky, F.; Bouamra, O.; Russell, R.; Faulkner, M.; Steyerberg, E.W.; Roberts, I. Predicting early death in patients with traumatic bleeding: Development and validation of prognostic model. BMJ 2012, 345, e5166. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, P.; Smith, J.; Robinson, M.; South, A.; Higginson, I.; Reuben, A.; Shaffee, J.; Black, S.; Logan, S. Tranexamic acid in major trauma. Eur. J. Emerg. Med. 2017, 24, 44–48. [Google Scholar] [CrossRef]
- Al-Jeabory, M.; Gasecka, A.; Wieczorek, W.; Mayer-Szary, J.; Jaguszewski, M.J.; Szarpak, L. Efficacy and safety of tranexamic acid in pediatric trauma patients: Evidence from meta-analysis. Am. J. Emerg. Med. 2021, 1–3. [Google Scholar] [CrossRef]
- Frith, D.; Goslings, J.C.; Gaarder, C.; Maegele, M.; Cohen, M.J.; Allard, S.; Johansson, P.I.; Stanworth, S.; Thiemermann, C.; Brohi, K. Definition and drivers of acute traumatic coagulopathy: Clinical and experimental investigations. J. Thromb. Haemost. 2010, 8, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Cardenas, J.C.; Wade, C.E.; Holcomb, J.B. Advances in the understanding of trauma-induced coagulopathy. Blood 2016, 128, 1043–1049. [Google Scholar] [CrossRef]
- Singh, R.A.; Asprou, F.; Patel, A.; Trickett, R.W. Haemorrhage control in extremity stab injury. J. Surg. Case Rep. 2013, 2013, rjt093. [Google Scholar] [CrossRef][Green Version]
- Skitch, S.; Engels, P.T. Acute Management of the Traumatically Injured Pelvis. Emerg. Med. Clin. N. Am. 2018, 36, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Vermylen, J.; Verhaegen-Declercq, M.L.; Fierens, F.; Verstraete, M. A double blind study of the effect of tranexamic acid in essential menorrhagia. Bull. Soc. R. Belg. Gynecol. d’obstetrique 1968, 38, 385–390. [Google Scholar] [CrossRef]
- Myles, P.S.; Smith, J.A.; Forbes, A.; Silbert, B.; Jayarajah, M.; Painter, T.; Cooper, D.J.; Marasco, S.; McNeil, J.; Bussières, J.S.; et al. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N. Engl. J. Med. 2017, 376, 136–148. [Google Scholar] [CrossRef]
- Tiede, A.; Rand, J.H.; Budde, U.; Ganser, A.; Federici, A.B. How I treat the acquired von Willebrand syndrome. Blood 2011, 117, 6777–6785. [Google Scholar] [CrossRef] [PubMed]
- Nadler, R.; Gendler, S.; Benov, A.; Strugo, R.; Abramovich, A.; Glassberg, E. Tranexamic acid at the point of injury. J. Trauma Acute Care Surg. 2014, 77, S146–S150. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 10 January 2021).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Safiejko, K.; Smereka, J.; Filipiak, K.J.; Szarpak, A.; Dabrowski, M.; Ladny, J.R.; Jaguszewski, M.J.; Szarpak, L. Effectiveness and safety of hypotension fluid resuscitation in traumatic hemorrhagic shock: A systematic review and meta-analysis of randomized controlled trials. Cardiol. J. 2020. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Adair, K.E.; Patrick, J.D.; Kliber, E.J.; Peterson, M.N.; Holland, S.R. TXA (Tranexamic Acid) Risk Evaluation in Combat Casualties (TRECC). Trauma Surg. Acute Care Open 2020, 5, e000353. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.; Davenport, R.; Willett, K.; Brohi, K. Tranexamic Acid Use in Severely Injured Civilian Patients and the Effects on Outcomes. Ann. Surg. 2015, 261, 390–394. [Google Scholar] [CrossRef] [PubMed]
- El-Menyar, A.; Sathian, B.; Wahlen, B.M.; Abdelrahman, H.; Peralta, R.; Al-Thani, H.; Rizoli, S. Prehospital administration of tranexamic acid in trauma patients: A 1:1 matched comparative study from a level 1 trauma center. Am. J. Emerg. Med. 2020, 38, 266–271. [Google Scholar] [CrossRef]
- Guyette, F.X.; Brown, J.B.; Zenati, M.S.; Early-Young, B.J.; Adams, P.W.; Eastridge, B.J.; Nirula, R.; Vercruysse, G.A.; O’Keeffe, T.; Joseph, B.; et al. Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury. JAMA Surg. 2020, 156, 11–20. [Google Scholar] [CrossRef]
- Howard, J.T.; Stockinger, Z.T.; Cap, A.P.; Bailey, J.A.; Gross, K.R. Military use of tranexamic acid in combat trauma. J. Trauma Acute Care Surg. 2017, 83, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kakaei, F.; Virani, P.; Hashemzadeh, S.; Zarrintan, S.; Beheshtirouy, S.; Asvadi, T. Effects of Tranexamic Acid on Mortality and Blood Transfusion in Trauma Patients with Significant Hemorrhage: A Clinical Trial. Adv. Biosci. Clin. Med. 2017, 5, 24. [Google Scholar] [CrossRef][Green Version]
- Lipsky, A.M.; Abramovich, A.; Nadler, R.; Feinstein, U.; Shaked, G.; Kreiss, Y.; Glassberg, E. Tranexamic acid in the prehospital setting: Israel Defense Forces’ initial experience. Injury 2014, 45, 66–70. [Google Scholar] [CrossRef]
- Morrison, J.J.; DuBose, J.J.; Rasmussen, T.E.; Midwinter, M.J. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch. Surg. 2012, 147, 113–119. [Google Scholar] [CrossRef]
- Myers, S.P.; Kutcher, M.E.; Rosengart, M.R.; Sperry, J.L.; Peitzman, A.B.; Brown, J.B.; Neal, M.D. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J. Trauma Acute Care Surg. 2019, 86, 20–27. [Google Scholar] [CrossRef]
- Neeki, M.M.; Dong, F.; Toy, J.; Vaezazizi, R.; Jabourian, N.; Jabourian, A.; Wong, D.; Vara, R.; Seiler, K.; Pennington, T.W.; et al. Efficacy and Safety of Tranexamic Acid in Prehospital Traumatic Hemorrhagic Shock: Outcomes of the Cal-PAT Study. West. J. Emerg. Med. 2017, 18, 673–683. [Google Scholar] [CrossRef]
- Neeki, M.M.; Dong, F.; Toy, J.; Vaezazizi, R.; Powell, J.; Wong, D.; Mousselli, M.; Rabiei, M.; Jabourian, A.; Niknafs, N.; et al. Tranexamic Acid in Civilian Trauma Care in the California Prehospital Antifibrinolytic Therapy Study. West. J. Emerg. Med. 2018, 19, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Perrott, J.; Burgess, S. Evaluation of tranexamic acid in trauma patients: A retrospective quantitative analysis. Am. J. Emerg. Med. 2019, 37, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Estroff, J.; Sparks, A.; Nahmias, J.; Allen, R.; Smith, S.R.; Kutcher, M.; Carter, K.; Grigorian, A.; Albertson, S.; et al. The incidence of venous thromboembolic events in trauma patients after tranexamic acid administration: An EAST multicenter study. Blood Coagul. Fibrinolysis 2021, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Shakur, H.; Roberts, I.; Bautista, R.; Caballero, J.; Coats, T.; Dewan, Y.; El-Sayed, H.; Gogichaishvili, T.; Gupta, S.; Herrera, J.; et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Galante, H.S.J.M.; Swendsen, J.M.G.H. Tranexamic Acid use in Trauma: Effective but not Without Consequences. J. Trauma Treat. 2013, 2. [Google Scholar] [CrossRef]
- Valle, E.J.; Allen, C.J.; Van Haren, R.M.; Jouria, J.M.; Li, H.; Livingstone, A.S.; Namias, N.; Schulman, C.I.; Proctor, K.G. Do all trauma patients benefit from tranexamic acid? J. Trauma Acute Care Surg. 2014, 76, 1373–1378. [Google Scholar] [CrossRef]
- Wafaisade, A.; Dgu, T.; Lefering, R.; Bouillon, B.; Böhmer, A.B.; Gäßler, M.; Ruppert, M. Prehospital administration of tranexamic acid in trauma patients. Crit. Care 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Ng, W.C.K.; Jerath, A.; Wasowicz, M. Tranexamic acid: A clinical review. Anestezjol. Intensywna Ther. 2015, 47, 339–350. [Google Scholar] [CrossRef] [PubMed]
- US Army Institute of Surgical Research Damage Control Resuscitation (CPG ID: 18). Available online: https://jts.amedd.army.mil/assets/docs/cpgs/JTS_Clinical_Practice_Guidelines(CPGs)/Damage_Control_Resuscitation_12_Jul_2019_ID18.pdf (accessed on 17 January 2021).
- Morrison, J.J.; Ross, J.D.; DuBose, J.J.; Jansen, J.O.; Midwinter, M.J.; Rasmussen, T.E. Association of Cryoprecipitate and Tranexamic Acid With Improved Survival Following Wartime Injury. JAMA Surg. 2013, 148, 218–225. [Google Scholar] [CrossRef]
- Yao, Y.T.; Yuan, X.; Shao, K. Acute Myocardial Infarction After Tranexamic Acid: Review of Published Case Reports. Chin. Med Sci. J. 2020, 35, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Chen, M.; Zhou, Y.; Yu, X.; Zhou, H.; Chen, G. The safety and efficiency of intravenous administration of tranexamic acid in coronary artery bypass grafting (CABG): A meta-analysis of 28 randomized controlled trials. BMC Anesthesiol. 2019, 19, 104. [Google Scholar] [CrossRef]
- Nejad, M.H.G.; Baharestani, B.; Esfandiari, R.; Hashemi, J.; Panahipoor, A. Evaluation and Comparison of Using Low-Dose Aprotinin and Tranexamic Acid in CABG: A Double Blind Randomized Clinical Trial. J. Tehran Univ. Hear. Cent. 2012, 7, 15–18. [Google Scholar]
- Yoshizaki, S.; Kijima, K.; Hara, M.; Saito, T.; Tamaru, T.; Tanaka, M.; Konno, D.-J.; Nakashima, Y.; Okada, S. Tranexamic acid reduces heme cytotoxicity via the TLR4/TNF axis and ameliorates functional recovery after spinal cord injury. J. Neuroinflammation 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Xue, P.; Yang, J.; Xu, X.; Liu, T.; Huang, Y.; Qiao, F.; Huang, X. The efficacy and safety of tranexamic acid in reducing perioperative blood loss in patients with multilevel thoracic spinal stenosis. Medicine 2018, 97, e13643. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.; Duncan, C.; Whiting, D.; Brown, M.; Warner, M.; Smith, H.; Kremers, H.; Stewart, T. Tranexamic acid is associated with decreased transfusion, hospital length of stay, and hospital cost in simultaneous bilateral total knee arthroplasty. Bosn. J. Basic Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ismail, K.; Moll, N.; Koch, L.; Swanson, K.; Walsh, G. Intra-operative tranexamic acid reduces blood loss and length of hospital stay after unilateral knee replacement surgery. Eur. J. Anaesthesiol. 2012, 29, 95–96. [Google Scholar] [CrossRef]
| Study | Country | Study Design | TXA Place of Infusion | TXA Group | Non-TXA Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age | Sex, Male | ISS | No. | Age | Sex, Male | ISS | ||||
| Adair et al., 2020 [23] | USA | Retrospective, observational analysis | Combat | 318 | 24.5 ± 4.6 | 318 (100%) | NS | 38 | 25.6 ± 5.2 | 38 (100%) | NS |
| Cole et al., 2020 [24] | UK | Prospective cohort study | Civil | 160 | 42 ± 17.2 | 125 (78.1%) | 33 ± 13 | 225 | 40 ± 18.6 | 185 (82.2%) | 29 ± 10 |
| Shakur et al., 2010 “CRASH-2” [36] | Multi-country | A randomized, placebo-controlled trial | Civil | 10,093 | 34.6 ± 14.1 | 8439 (83.6%) | NS | 10,114 | 34.5 ± 14.4 | 8496 (84.0%) | NS |
| El-Menyar et al., 2020 [25] | Qatar | Retrospective study | Civil | 102 | 31.5 ± 0.8 | 98 (96.1%) | 24.0 ± 0.8 | 102 | 31.5 ± 0.8 | 91 (89.2%) | 24.99 ± 0.9 |
| Guyette et al., 2020 “STAAMP” [26] | USA | Pragmatic, phase 3, multicenter double-blind placebo-controlled, superiority randomized clinical trial | Civil | 447 | 41 ± 17 | 327 (73.2%) | 13 (5–22) | 456 | 42 ± 18 | 341 (74.8%) | 11 (4–22) |
| Howard et al., 2017 [27] | USA | Retrospective study | Combat | 849 | 24.8 ± 8.3 | 835 (98.4%) | 21.5 ± 12.5 | 2924 | 25.1 ± 10.7 | 2794 (95.6%) | 18.2 ± 11.2 |
| Kakaei et al., 2017 [28] | Iran | Randomized-controlled trial | Civil | 30 | 37.8 ± 10.1 | 23 (76.7%) | NS | 30 | 35.8 ± 10.5 | 22 (73.3%) | NS |
| Lipsky et al., 2014 [29] | Israel | Retrospective study | Combat | 28 | 28.25 ± 3.8 | 25 (89.3%) | 17.8 ± 3.7 | 12 | 28.75 ± 5.5 | 10 (83.3%) | 7 ± 2.3 |
| Morrison et al., 2012 [30] | Afghanistan | Retrospective observational study | Combat | 293 | 24.9 ± 9.6 | 285 (97.3%) | 25.2 ± 16.6 | 603 | 23.1 ± 10.1 | 568 (94.2%) | 22.5 ± 18.5 |
| Myers et al., 2019 [31] | USA | Retrospective study | Civil | 189 | 37.25 ± 4.8 | 142 (75.1%) | NS | 189 | 35.25 ± 5.5 | 132 (69.8%) | NS |
| Neeki et al., 2017 [32] | USA | Multi-centered, prospective, observational cohort study with a retrospective chart-review comparison | Civil | 128 | 38.23 ± 15.48 | 103 (80.5%) | 12.96 ± 9.03 | 125 | 39.06 ± 16.66 | 104 (83.2%) | 17 ± 10.74 |
| Neeki et al., 2018 [33] | USA | Multi-centered, prospective, observational cohort study with a retrospective comparison | Civil | 362 | 37.96 ± 16.11 | 293 (80.9%) | 16.08 ± 10.69 | 362 | 37.64 ± 16.33 | 293 (80.9%) | 17.15 ± 11.71 |
| Ng et al., 2019 [34] | Canada | Retrospective study | Civil | 67 | 42.3 ± 17.9 | 54 (80.6%) | 27 ± 16 | 50 | 43.6 ± 20.4 | 42 (84.0%) | 25 ± 16 |
| Rivas et al., 2021 [35] | USA | A multicenter retrospective study | Civil | 887 | 41 ± 18.1 | 480 (54.1%) | 25 ± 3 | 446 | 40.3 ± 18.2 | 361 (80.9%) | 27.3 ± 3.5 |
| Swendsen et al., 2012 [37] | USA | Retrospective study | Civil | 52 | 44.6 ± 20.3 | 37 (71.2%) | 27.1 ± 15.0 | 74 | 47.6 ± 18.9 | 49 (66.2%) | 20.5 ± 16.8 |
| Valle et al., 2014 [38] | USA | Prospective study | Civil | 150 | 43 ± 20 | 128 (85.3%) | 28 ± 16 | 150 | 43 ± 20 | 129 (86.0%) | 28 ± 17 |
| Wafaisade et al., 2016 [39] | Germany | Retrospective study | Civil | 258 | 43 ± 19 | 187 (72.5%) | 24 ± 14 | 258 | 41 ± 18 | 187 (72.5%) | 24 ± 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jeabory, M.; Szarpak, L.; Attila, K.; Simpson, M.; Smereka, A.; Gasecka, A.; Wieczorek, W.; Pruc, M.; Koselak, M.; Gawel, W.; et al. Efficacy and Safety of Tranexamic Acid in Emergency Trauma: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1030. https://doi.org/10.3390/jcm10051030
Al-Jeabory M, Szarpak L, Attila K, Simpson M, Smereka A, Gasecka A, Wieczorek W, Pruc M, Koselak M, Gawel W, et al. Efficacy and Safety of Tranexamic Acid in Emergency Trauma: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(5):1030. https://doi.org/10.3390/jcm10051030
Chicago/Turabian StyleAl-Jeabory, Mahdi, Lukasz Szarpak, Kecskes Attila, Michael Simpson, Adam Smereka, Aleksandra Gasecka, Wojciech Wieczorek, Michal Pruc, Maciej Koselak, Wladyslaw Gawel, and et al. 2021. "Efficacy and Safety of Tranexamic Acid in Emergency Trauma: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 5: 1030. https://doi.org/10.3390/jcm10051030
APA StyleAl-Jeabory, M., Szarpak, L., Attila, K., Simpson, M., Smereka, A., Gasecka, A., Wieczorek, W., Pruc, M., Koselak, M., Gawel, W., Checinski, I., Jaguszewski, M. J., & Filipiak, K. J. (2021). Efficacy and Safety of Tranexamic Acid in Emergency Trauma: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(5), 1030. https://doi.org/10.3390/jcm10051030








