Fertility-Sparing Methods in Adolescents Affected by Endometrial Cancer: A Comprehensive Review
Abstract
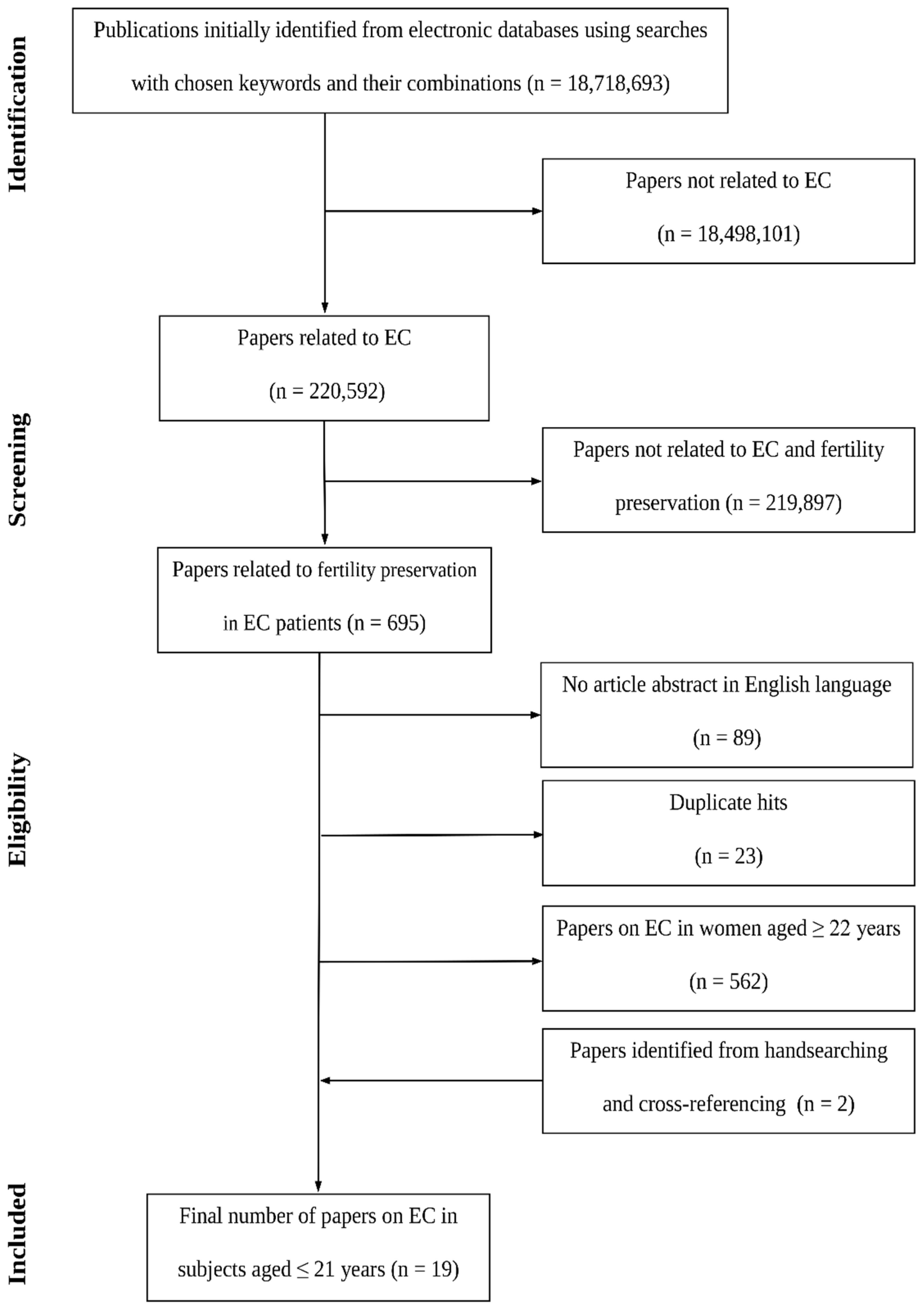
1. Introduction
1.1. Fertility Issues in Adolescents and Young Adults with Cancer
1.2. Selection Criteria
- -
- Well-differentiated (Grade 1) endometrioid endometrial adenocarcinoma on dilatation and curettage confirmed by expert pathology review;
- -
- Disease limited to the endometrium on magnetic resonance imaging (preferred) or transvaginal ultrasound;
- -
- Absence of suspicious or metastatic disease on imaging;
- -
- No contraindications to medical therapy or pregnancy;
- -
- Counseling that fertility-sparing options are not a standard of care in the treatment of this cancer.
1.3. Existing Scarce Evidence
2. Pharmacological Fertility-Sparing Treatment
2.1. Oral Progestins
2.2. Levonorgestrel Intrauterine Device
2.3. Anti-Estrogen Treatment
2.4. Gonadotropin-Releasing Hormone Agonist
3. Surgical Fertility-Sparing Treatment
3.1. Hysteroscopic Resection of Endometrium
3.2. Ovarian Preservation
4. Assisted Reproductive Technology
5. Surveillance
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Accosta-Torres, S.; Murdock, T.; Matsuno, R.; Beavis, A.L.; Stone, R.L.; Wethington, S.L.; Levison, K.; Grumbine, F.; Ferriss, J.S.; Tanner, E.J.; et al. The addition of metformin to progestin therapy in the fertility-sparing treatment of women with atypical hyperplasia/endometrial intraepithelial neoplasia or endometrial cancer: Little impact on response and low live-birth rates. Gynecol. Oncol. 2020, 157, 348–356. [Google Scholar] [CrossRef]
- American Cancer Society®. Available online: https://cancerstatisticscenter.cancer.org (accessed on 2 December 2020).
- Corzo, C.; Santillan, N.B.; Westin, S.N.; Ramirez, P.T. Updates on conservative management of endometrial cancer. J. Minim. Invasive Gynecol. 2018, 25, 308–313. [Google Scholar] [CrossRef]
- Park, J.Y.; Seong, S.J.; Kim, T.J.; Kim, J.W.; Bae, D.S.; Nam, J.H. Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol. Oncol. 2017, 146, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.F.; Weiguo, H.; Fu, S.; Zhao, H.; Sun, C.; Suidan, R.S.; Woodard, T.L.; Rauh-Hain, A.; Westin, S.N.; Giordano, S.H.; et al. National patterns of care and fertility outcomes for reproductive-aged women with endometrial cancer or atypical hyperplasia. Am. J. Obstet. Gynecol. 2019, 221, 474.e1–474.e11. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.B. Carcinoma of the body of the uterus in childhood. Am. J. Obstet. Gynecol. 1932, 24, 402–405. [Google Scholar] [CrossRef]
- Kim, S.M.; Shin, S.J.; Bae, J.G.; Kwon, K.Y.; Rhee, J.H. Endometrial adenocarcinoma in a 13-year-old girl. Obstet. Gynecol. Sci. 2016, 59, 152–156. [Google Scholar] [CrossRef]
- Elizondo-Montemayor, L.; Hernandez-Escobar, C.; Lara-Torre, E.; Nieblas, B.; Gomez-Carmona, M. Gynecologic and obstetric consequences of obesity in adolescent girls. J. Pediatr. Adolesc. Gynecol. 2017, 30, 156–168. [Google Scholar] [CrossRef]
- Yang, B.; Xie, L.; Zhang, H.; Zhu, Q.; Du, Y.; Luo, X.; Chen, X. Insulin resistance and overweight prolonged fertility-sparing treatment duration in endometrial atypical hyperplasia patients. J. Gynecol. Oncol. 2018, 29, e35. [Google Scholar] [CrossRef]
- Gałczyński, K.; Nowakowski, Ł.; Rechberger, T.; Semczuk, A. Should we be more aware of endometrial cancer in adolescents? Dev. Period Med. 2016, 20, 169–173. [Google Scholar]
- Rosen, M.W.; Tasset, J.; Kobernik, E.K.; Smith, Y.R.; Johnston, C.; Quint, E.H. Risk factors for endometrial cancer or hyperplasia in adolescents and women 25 years old or younger. J. Pediatr. Adolesc. Gynecol. 2019, 32, 546–549. [Google Scholar] [CrossRef]
- Kocova, M.; Basheska, N.; Papazovska, A.; Jankova, R.; Toncheva, D.; Popovska, S. Girls with Turner’s syndrome with spontaneous menarche have an increased risk of endometrial carcinoma: A case report and review from the literature. Gynecol. Oncol. 2005, 96, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ostor, A.G.; Adam, R.; Gutteridge, B.H.; Fortune, D.W. Endometrial carcinoma in young women. Aust. N. Z. J. Obstet. Gynaecol. 1982, 22, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Trojano, G.; Olivieri, C.; Tinelli, R.; Damiani, G.R.; Pellegrino, A.; Cicinelli, E. Conservative treatment in early stage endometrial cancer: A review. Acta BioMed. 2019, 90, 405–410. [Google Scholar] [PubMed]
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. FIGO Cancer Report 2018. Cancer of the corpus uteri. Int. J. Gynecol. Obstet. 2018, 143 (Suppl. 2), 37–50. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.D.; Kennard, J.A.; Ahmad, S. Fertility preserving options for gynecologic malignancies: A review of current understanding and future directions. Crit. Rev. Oncol. Hematol. 2018, 132, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Paracchini, S.; Chauvet, P.; Gałczyński, K.; Boulay, E.; Jaillet, L.; Canis, M.; Bourdel, N. Surgical technique for the sentinel lymph node (SLN) mapping in 10 steps. Gynecol. Oncol. 2020, 156, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Di Spiezio Sardo, A.; Mollo, A.; Raffone, A.; Travaglino, A.; Boccellino, A.; Zizolfi, B.; Insabato, L.; Zullo, F.; De Placido, G.; et al. Hysteroscopic endometrial focal resection followed by levonorgestrel intrauterine device insertion as a fertility-sparing treatment of atypical endometrial hyperplasia and early endometrial cancer: A retrospective study. J. Minim. Invasive Gynecol. 2019, 26, 648–656. [Google Scholar] [CrossRef]
- Kohler, T.S.; Kondapalli, L.A.; Shah, A.; Chan, S.; Woodruff, T.K.; Branningan, R.E. Results from the survey for preservation of adolescent reproduction (SPARE) study: Gender disparity in delivery of fertility preservation message to adolescents with cancer. J. Assist. Reprod. Genet. 2011, 28, 269–277. [Google Scholar] [CrossRef]
- Carneiro, M.M.; Lamaita, R.M.; Ferreira, M.C.F.; Silva-Filho, A.L. Fertility-preservation in endometrial cancer: Is it safe? Review of the literature. JBRA Assist. Reprod. 2016, 20, 232–239. [Google Scholar] [CrossRef]
- UNICEF. Convention on the Rights of the Child. Available online: https://www.unicef.org/child-rights-convention/convention-text (accessed on 10 December 2020).
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The age of adolescence. Lancet Child Adolesc. Health 2018, 2, 223–228. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Medical Subject Headings. 2021. Available online: https://meshb.nlm.nih.gov/record/ui?ui=D000293 (accessed on 22 December 2020).
- McVeigh, T.P.; Sundar, R.; Diamantis, N.; Kaye, S.B.; Banerji, U.; Lopez, J.S.; de Bono, J.; van der Graaf, W.T.A.; George, A.J. The role of genomic profiling in adolescents and young adults (AYAs) with advanced cancer participating in phase I clinical trials. Eur. J. Cancer 2018, 95, 20–29. [Google Scholar] [CrossRef]
- Benedict, C.; Shuk, E.; Ford, S.J. Fertility issues in adolescent and young adult cancer survivors. J. Adolesc. Young Adult Oncol. 2016, 1, 48–57. [Google Scholar] [CrossRef]
- Feichtinger, M.; Rodriguez-Wallberg, K.A. Fertility preservation in women with cervical, endometrial or ovarian cancers. Gynecol. Oncol. Res. Pract. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Su, H.I.; Lee, Y.T.; Barr, R. Oncofertility: Meeting the fertility goals of adolescents and young adults with cancer. Cancer J. 2018, 24, 328–335. [Google Scholar]
- Goodman, A. Oncofertility for adolescents: When parents and physicians disagree about egg cryopreservation for a mature minor. AMA J. Ethics 2015, 17, 826–833. [Google Scholar]
- Krawczuk-Rybak, M.; Latoch, E. Risk factors for premature aging in childhood cancer survivors. Dev. Period Med. 2019, 23, 97–103. [Google Scholar]
- Bovicelli, A.; D’Andrilli, G.; Giordano, A.; De Iaco, P. Conservative treatment of early endometrial cancer. J. Cell. Physiol. 2013, 228, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Jóźwik, M.; Jóźwik, M.; Zaręba, K.; Semczuk, A.; Modzelewska, B.; Jóźwik, M. Congenital vesicouterine fistulas—A PRISMA-compliant systematic review. Neurourol. Urodyn. 2018, 37, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Richards, D. Handsearching still a valuable element of the systematic review. Evid. Based Dent. 2008, 9, 85. [Google Scholar] [CrossRef]
- Hirst, B.C. Malignant growths of the uterus in young girls. Am. J. Obstet. Gynecol. 1929, 18, 104–105. [Google Scholar] [CrossRef]
- Smith, A.A. Malignant tumors of the uterus in the young. Neb. State Med. J. 1933, 18, 19–20. [Google Scholar]
- Mazzola, V.P. Endometrial hyperplasia (puberty), adeno-carcinoma, fifteen years’ follow-up. Am. J. Obstet. Gynecol. 1938, 36, 698–701. [Google Scholar] [CrossRef]
- Healy, W.P.; Brown, R.L. Experience with surgical and radiation therapy in carcinoma of the corpus uteri. Am. J. Obstet. Gynecol. 1939, 38, 1–13. [Google Scholar] [CrossRef]
- Sommers, S.C.; Hertig, A.T.; Bengloff, H. Genesis of endometrial carcinoma. II. Cases 19 to 35 years old. Cancer 1949, 2, 957–963. [Google Scholar] [CrossRef]
- Silverberg, S.G.; Makowski, E.L. Endometrial carcinoma in young women taking oral contraceptive agents. Obstet. Gynecol. 1975, 46, 503–506. [Google Scholar]
- Westin, S.N.; Fellman, B.; Sun, C.C.; Broaddus, R.R.; Woodall, M.L.; Pal, N.; Urbauer, D.L.; Ramondetta, L.M.; Schmeler, K.M.; Soliman, P.T.; et al. Prospective phase II trial of levonorgestrel intrauterine device: Nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am. J. Obstet. Gynecol. 2021, 224, e1–e191. [Google Scholar] [CrossRef]
- Baker, W.D.; Soisson, A.P.; Dodson, M.K. Endometrial cancer in 14-year-old girl with Cowden syndrome. A case report. J. Obstet. Gynaecol. Res. 2013, 3, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Westin, S.; Broaddus, R.; Schmeler, K. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obstet. Gynecol. 2012, 119, 423–425. [Google Scholar] [CrossRef]
- Cohn, D.E.; Resnick, K.E.; Ramirez, N.C.; Morrison, C.D. Advanced endometrial cancer with serous metastasis in a 17-year-old. Gynecol. Oncol. 2006, 101, 356–359. [Google Scholar] [CrossRef]
- ElNaggar, A.C.; Spunt, S.L.; Smith, W.; Depas, M.; Santoso, J.T. Endometrial cancer in 15-year-old girl: A complication of Cowden syndrome. Gynecol. Oncol. Case Rep. 2013, 3, 18–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farhi, D.C.; Nosanchuk, J.; Silverberg, S.G. Endometrial adenocarcinoma in women under 25 years of age. Obstet. Gynecol. 1986, 68, 741–745. [Google Scholar] [CrossRef]
- Koh, K.S.; Blajer, S.; Vadas, G. Adenocarcinoma of the endometrium in a teenager. Can. Med. Assoc. J. 1975, 112, 980–981. [Google Scholar] [PubMed]
- Liu, G.; Wang, Y.; Zhang, X.; Yuan, B.; Han, C.; Xue, F. Endometrial carcinoma in 15-year-old obese patient with persistent uterine bleeding. Gynecol. Endocrinol. 2014, 30, 277–279. [Google Scholar] [CrossRef]
- Mitamura, T.; Watari, H.; Todo, Y.; Koshida, T.; Sakuragi, N. A 14-year-old female patient with FIGO stage IB endometrial carcinoma. A case report. Int. J. Gynecol. Cancer 2009, 19, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.P. Endometrial carcinoma in young women. A clinical profile. Obstet. Gynecol. 1968, 31, 702–707. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Daniels, M.S.; Brandt, A.C.; Lu, K.H. Endometrial cancer in an adolescent: A possible manifestation of Cowden syndrome. Obstet. Gynecol. 2009, 114, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Uda, H.; Kitai, M.; Kogiku, A.; Kobayashi, A.; Sakuma, T.; Nagao, S.; Yamaguchi, S. Endometrial carcinoma in a 14-year-old: A case report. Gynecol. Oncol. Rep. 2019, 29, 7–9. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef]
- Voss, M.A.; Ganesan, R.; Ludeman, L.; McCarthy, K.; Gornall, R.; Schaller, G.; Wei, W.; Sundar, S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer—A clinical and pathological evaluation. Gynecol. Oncol. 2012, 124, 15–20. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Lu, K.H.; Schorge, J.O.; Rodabaugh, K.J.; Daniels, M.S.; Sun, C.C.; Soliman, P.T.; White, K.G.; Luthra, R.; Gershenson, D.M.; Broaddus, R.R. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J. Clin. Oncol. 2007, 25, 5158–5164. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Nam, J.H. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist 2015, 20, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.J.; Thiel, K.; Yang, S.; Leslie, K.K. Catch it before it kills: Progesterone, obesity, and the prevention of endometrial cancer. Discov. Med. 2012, 14, 215–222. [Google Scholar] [PubMed]
- Kim, J.J.; Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Van der Zanden, C.; Ikiz, H.; Kuzelijevic, B.; Havelock, J.; Kwon, J.S. Fertility-sparing management using progestin for young women with endometrial cancer from a population-based study. J. Obstet. Gynecol. Can. 2018, 40, 328–333. [Google Scholar] [CrossRef]
- Terzic, M.; Norton, M.; Terzic, S.; Bapayeva, G.; Aimagambetova, G. Fertility preservation in endometrial cancer patients: Options, challenges and perspectives. Ecancermedicalscience 2020, 14, 1030. [Google Scholar] [CrossRef]
- Kalogera, E.; Dowdy, S.C.; Bakkum-Gamez, J.N. Preserving fertility in young patients with endometrial cancer: Current perspectives. Int. J. Womens Health 2014, 6, 691–701. [Google Scholar] [PubMed]
- Ohyagi-Hara, C.; Sawada, K.; Aki, I.; Mabuchi, S.; Kobayashi, E.; Ueda, Y.; Yoshino, K.; Fujita, M.; Tsutsui, T.; Kimura, T. Efficacies and pregnant outcomes of fertility-sparing treatment with medroxyprogesterone acetate for endometrioid adenocarcinoma and complex atypical hyperplasia: Our experience and a review of the literature. Arch. Gynecol. Obstet. 2015, 291, 151–157. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, K.R.; Kim, Y.T.; Seong, S.J.; Kim, T.J.; Kim, J.W.; Kim, S.M.; et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer. Eur. J. Cancer 2013, 49, 868–874. [Google Scholar] [CrossRef]
- Vitale, S.G.; Rossetti, D.; Tropea, A.; Biondi, A.; Lagana, A.S. Fertility sparing surgery for stage IA type I and G2 endometrial cancer in reproductive-aged patients: Evidence-based approach and future perspectives. Updates Surg. 2017, 69, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, J.X.; Wu, M.; Lang, J.H.; Huo, Z.; Shen, K. Fertility-preserving treatment in young women with well-differentiated endometrial carcinoma and severe atypical hyperplasia of endometrium. Fertil. Steril. 2009, 92, 2122–2124. [Google Scholar] [CrossRef]
- Fastrez, M.; Houba, C.; Vandromme, J.; Rozenberg, S. Fertility-sparing management of gynecological cancers. Maturitas 2015, 82, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Gallos, I.D.; Yap, J.; Rajkhowa, M.; Luesley, D.M.; Coomarasamy, A.; Gupta, J.K. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2012, 207, e1–e266. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Fader, A.N.; Carson, K.A.; Bristow, R.E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol. Oncol. 2012, 125, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, M.; Yang, J.; Cao, D.; Yuan, Z.; Zhou, H.; Zhang, Y.; Li, L.; Shen, K.; Wu, H. Prolonged conservative treatment in patients with recurrent endometrial cancer after primary fertility-sparing therapy: 15-year experience. Int. J. Clin. Oncol. 2019, 24, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhan, W.; Feng, I.; Gao, W. Comparision of fertility-sparing treatments in patients with early endometrial cancer and atypical complex hyperplasia: A meta-analysis and systematic review. Medicine 2017, 96, e8034. [Google Scholar] [CrossRef]
- Obermair, A.; Baxter, E.; Brennan, D.J.; Mcalpine, J.N.; Mueller, J.J.; Amant, F.; Van Gent, M.D.J.M.; Coleman, R.L.; Westin, S.N.; Yates, M.N.; et al. Fertility-sparing treatment in early endometrial cancer: Current state and future strategies. Obstet. Gynecol. Sci. 2020, 63, 417–431. [Google Scholar] [CrossRef]
- Jones, R.L.; Critchley, H.O.D. Morphological and functional changes in human endometrium following intrauterine levonorgestrel delivery. Hum. Reprod. 2000, 15 (Suppl. 3), 162–172. [Google Scholar] [CrossRef]
- Pakarinen, P.I.; Lähteenmäki, P.; Lehtonen, E.; Reima, I. The ultrastructure of human endometrium is altered by administration of intrauterine levonorgestrel. Hum. Reprod. 1998, 13, 1846–1853. [Google Scholar] [CrossRef]
- Boruban, M.C.; Altundag, K.; Kilic, G.S.; Blankstein, J. From endometrial hyperplasia to endometrial cancer: Insight into the biology and possible medical preventive measures. Eur. J. Cancer Prev. 2008, 17, 133–138. [Google Scholar] [CrossRef]
- Melvin, L.; Scott, J.; Craik, J. Jaydess® levonorgestrel intrauterine system. J. Fam. Plann. Reprod. Health Care 2014, 40, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, W.J.; Massuger, L.F.A.G.; Enitec; Pijnenborg, J.M.A.; Romano, A. Anti-estrogen treatment in endometrial cancer: A systematic review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, H.; Feng, F.; Wang, J.; Cheng, N. A pilot study of gonadotropin-releasing hormone agonist combined with aromatase inhibitor as fertility-sparing treatment in obese patients with endometrial cancer. J. Gynecol. Oncol. 2019, 30, e61. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, P.; Donnez, J. Conservative treatment may be beneficial for young women with atypical endometrial hyperplasia or endometrial adenocarcinoma. Fertil. Steril. 2003, 80, 1315–1324. [Google Scholar] [CrossRef]
- Tock, S.; Jadoul, P.; Squifflet, J.-L.; Marbaix, E.; Baurain, J.-F.; Luyckx, M. Fertility sparing treatment in patients with early stage endometrial cancer, using a combination of surgery and GnRH agonist: A monocentric retrospective study and review of the literature. Front. Med. 2018, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, I.; Corrado, G.; Morricone, D.; Scambia, G. Reproductive preservation for treatment of stage IA endometrial cancer in a young woman: Hysteroscopic resection. Int. J. Gynecol. Cancer 2005, 15, 974–978. [Google Scholar] [CrossRef]
- Laurelli, G.; Di Vagno, G.; Scaffa, C.; Losito, S.; Del Giudice, M.; Greggi., S. Conservative treatment of early endometrial cancer: Preliminary results of a pilot study. Gynecol. Oncol. 2011, 120, 43–46. [Google Scholar] [CrossRef]
- Yang, H.C.; Liu, J.C.; Liu, F.S. Fertility-preserving treatment of stage IA, well-differentiated endometrial carcinoma in young women with hysteroscopic resection and high-dose progesterone therapy. Taiwan J. Obstet. Gynecol. 2019, 58, 90–93. [Google Scholar] [CrossRef]
- Shan, B.; Ren, Y.; Sun, J.; Tu, X.; Jiang, Z.; Ju, X.; Zang, R.; Wang, H. A prospective study of fertility-sparing treatment with megestrol acetate following hysteroscopic curettage for well-differentiated endometrioid carcinoma and atypical hyperplasia in young women. Arch. Gynecol. Obstet. 2013, 288, 1115–1123. [Google Scholar] [CrossRef]
- Navarria, I.; Usel, M.; Rapiti, E.; Neyroud-Caspar, I.; Pelte, M.F.; Bouchardy, C.; Petignat, P. Young patients with endometrial cancer: How many could be eligible for fertility-sparing treatment? Gynecol. Oncol. 2009, 114, 448–451. [Google Scholar] [CrossRef]
- Gonthier, C.; Trefoux-Bourdet, A.; Koskas, M. Impact of conservative managements in young women with grade 2 or 3 endometrial adenocarcinoma confined to the endometrium. Int. J. Gynecol. Cancer 2017, 27, 493–499. [Google Scholar] [CrossRef]
- Levine, J.M. Preserving fertility in children and adolescents with cancer. Children 2014, 1, 166–185. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Oktay, K. Fertility preservation during cancer treatment: Clinical guidelines. Cancer Manag. Res. 2014, 6, 105–117. [Google Scholar]
- Kim, M.J.; Choe, S.A.; Kim, M.K.; Yun, B.S.; Seong, S.J.; Kim, S.K. Outcomes of in vitro fertilization cycles following fertility-sparing treatment in stage IA endometrial cancer. Arch. Gynecol. Obstet. 2019, 300, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, P.; Dolmans, M.-M.; Donnez, J. Fertility preservation in girls during childhood: Is it feasible, efficient and safe and to whom should it be proposed? Hum. Reprod. Update 2010, 16, 617–630. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Fertility preservation in women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Lotz, L.; Reis Barbosa, P.; Knorr, C.; Hofbeck, L.; Hoffman, I.; Beckmann, M.W.; Antoniadis, S.; Dittrich, R. The safety and satisfaction of ovarian tissue cryopreservation in prepubertal and adolescent girls. Reprod. Biomed. Online 2020, 40, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Falcone, T.; Patrizio, P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil. Steril. 2020, 114, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.J.; Picton, H.; Ernst, E.; Andersen, C.Y. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. 2018, 70, 432–435. [Google Scholar]
- Mitsushita, J.; Toki, T.; Kato, K.; Fujii, S.; Konishi, I. Endometrial carcinoma remaining after term pregnancy following conservative treatment with medroxyprogesterone acetate. Gynecol. Oncol. 2000, 79, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Matsuzaki, S.; Kobayashi, E.; Hara, T.; Nakagawa, S.; Takiuchi, T.; Mimura, K.; Ueda, Y.; Tomimatsu, T.; Kimura, T. Endometrial carcinoma in a gravid uterus: A case report and literature review. BMC Pregnancy Childbirth 2019, 19, 425. [Google Scholar] [CrossRef] [PubMed]
| Authors | Patient Age (Years), Comorbidities if Any | Symptom(s) | Anatomopathological Diagnosis | Treatment and Response |
|---|---|---|---|---|
| Baker et al. [41] | 14, Cowden syndrome | Menorrhagia | Grade 1 EC | Surgical treatment upon patient’s request |
| Brown et al. [42] | 18 | Polyp protruding through the external cervical os | Grade 2 EC | Fertility-sparing approach, LNG-IUD |
| Cohn et al. [43] | 17 | Menorrhagia, secondary anemia | Grade 2 EC with serous metastasis | Surgical treatment |
| ElNaggar et al. [44] | 15, Cowden syndrome | Excessive uterine bleeding | Grade 1 EC | Surgical treatment upon patient’s request |
| Farhi et al. [45] | 21, obesity, PCOS | Irregular menses | Grade 1 EC, AEH present | Fertility-sparing approach with unspecified progestin |
| 15 | Irregular menses | Grade 1 EC, AEH present | Fertility-sparing approach with unspecified progestin | |
| 19, PCOS | Irregular menses, hirsutism | Grade 1 EC with areas of squamous differentiation, | Fertility-sparing approach with unspecified progestin, foci of cancer persisted after hormonal therapy, lost to follow-up | |
| 15, PCOS | Irregular menses | Grade 1 EC, | Fertility-sparing approach: 400 mg MPA p.d. for 7 days, followed by weekly 400 mg intramuscular injections for 12 weeks, then 1 mg norethindrone acetate p.d. for 21 days of each month for 6 years, 2 successful term pregnancies, later vaginal hysterectomy | |
| 21 | On oral contraceptives | Grade 1 EC with areas of squamous differentiation | Radiation, lost to follow-up | |
| Kim et al. [7] | 13, PCOS | Irregular heavy menstrual bleedings, severe anemia | Grade 2 EC, AEH present; clinical stage IA | Fertility-sparing approach: 160 mg MA p.d. for 3 months, then 10 mg MPA for 5 months |
| Kocova et al. [12] | 21, Turner syndrome | Prolonged heavy uterine bleeding | EC arising in a hyperplastic endometrial polyp | Fertility-sparing approach: MPA depot for 6 months, then radical treatment upon patient’s request |
| Koh et al. [46] | 17, morbid obesity, arterial hypertension | A 2-day painless, heavy intermenstrual bleeding | Grade 1 EC with areas of squamous differentiation | Surgical treatment |
| Liu et al. [47] | 15, obesity, PCOS | Persistent uterine bleeding | EC with myometrial invasion on MRI | Surgical treatment |
| Mitamura et al. [48] | 14, PCOS | Menorrhagia | Grade 2 EC | Fertility-sparing approach: 400 mg MPA p.d. for a month, then surgical treatment |
| Ostor et al. [13] | 19, Turner syndrome with mosaicism | Heavy menstruations at 2-month intervals, anemia, normal secondary sex characteristics | Grade 1 EC | 600 mg MPA p.d. for 2 weeks only, then surgical treatment |
| Peterson [49] | 16, morbid obesity since early childhood, PCOS | Prolonged periods of amenorrhea, extremely profuse menstrual bleedings | Grade 1 EC | Surgical treatment; follow-up at 21 years of age: free of recurrent or metastatic tumor |
| Rosen et al. [11] | 17, morbid obesity as indicated by BMI = 63 | Menorrhagia | Grade 1 EC | LNG-IUD; continues to be without evidence of disease |
| Schmeler et al. [50] | Adolescent (age not given), genetically confirmed familial PTEN mutation suggestive of Cowden syndrome in her case | Abnormal vaginal bleeding, pelvic pain | Grade 1 EC initially, then changed to Grade 2 | Fertility-sparing approach: high-dose MA for 8 months, then robotic hysterectomy |
| Uda et al. [51] | 14, overweight | Excessive uterine bleeding, severe anemia | Grade 1 EC | Fertility-sparing approach: 600 mg MPA p.d. for 26 weeks, complete response observed after 15 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałczyński, K.; Olcha, P.; Romanek-Piva, K.; Jóźwik, M.; Semczuk, A. Fertility-Sparing Methods in Adolescents Affected by Endometrial Cancer: A Comprehensive Review. J. Clin. Med. 2021, 10, 1020. https://doi.org/10.3390/jcm10051020
Gałczyński K, Olcha P, Romanek-Piva K, Jóźwik M, Semczuk A. Fertility-Sparing Methods in Adolescents Affected by Endometrial Cancer: A Comprehensive Review. Journal of Clinical Medicine. 2021; 10(5):1020. https://doi.org/10.3390/jcm10051020
Chicago/Turabian StyleGałczyński, Krzysztof, Piotr Olcha, Katarzyna Romanek-Piva, Maciej Jóźwik, and Andrzej Semczuk. 2021. "Fertility-Sparing Methods in Adolescents Affected by Endometrial Cancer: A Comprehensive Review" Journal of Clinical Medicine 10, no. 5: 1020. https://doi.org/10.3390/jcm10051020
APA StyleGałczyński, K., Olcha, P., Romanek-Piva, K., Jóźwik, M., & Semczuk, A. (2021). Fertility-Sparing Methods in Adolescents Affected by Endometrial Cancer: A Comprehensive Review. Journal of Clinical Medicine, 10(5), 1020. https://doi.org/10.3390/jcm10051020









