Prognostic Significance of Survivin Expression in Patients with Ovarian Carcinoma: A Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
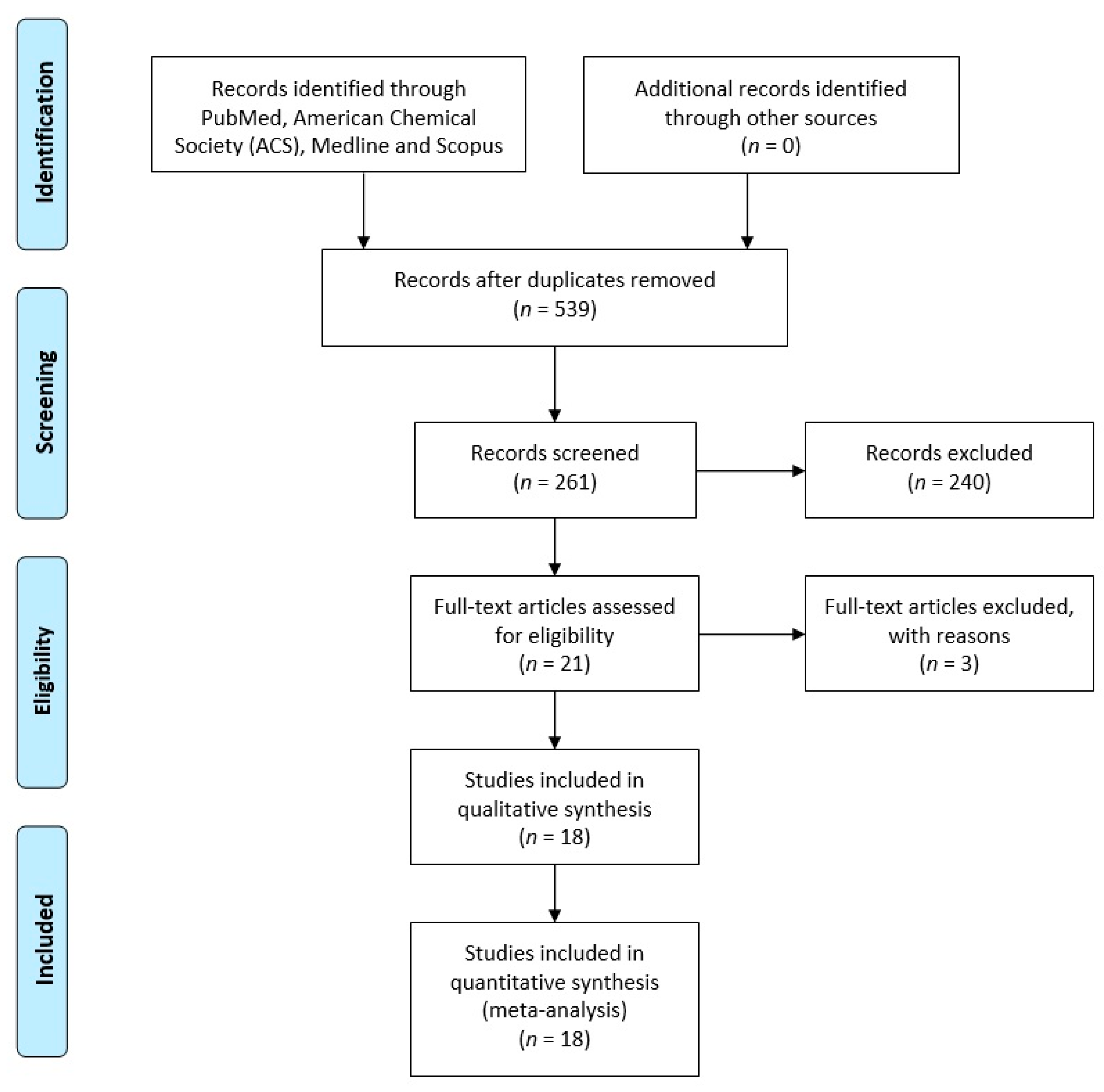
3.1. Search Results
3.2. Basic Information for Inclusion in the Literature
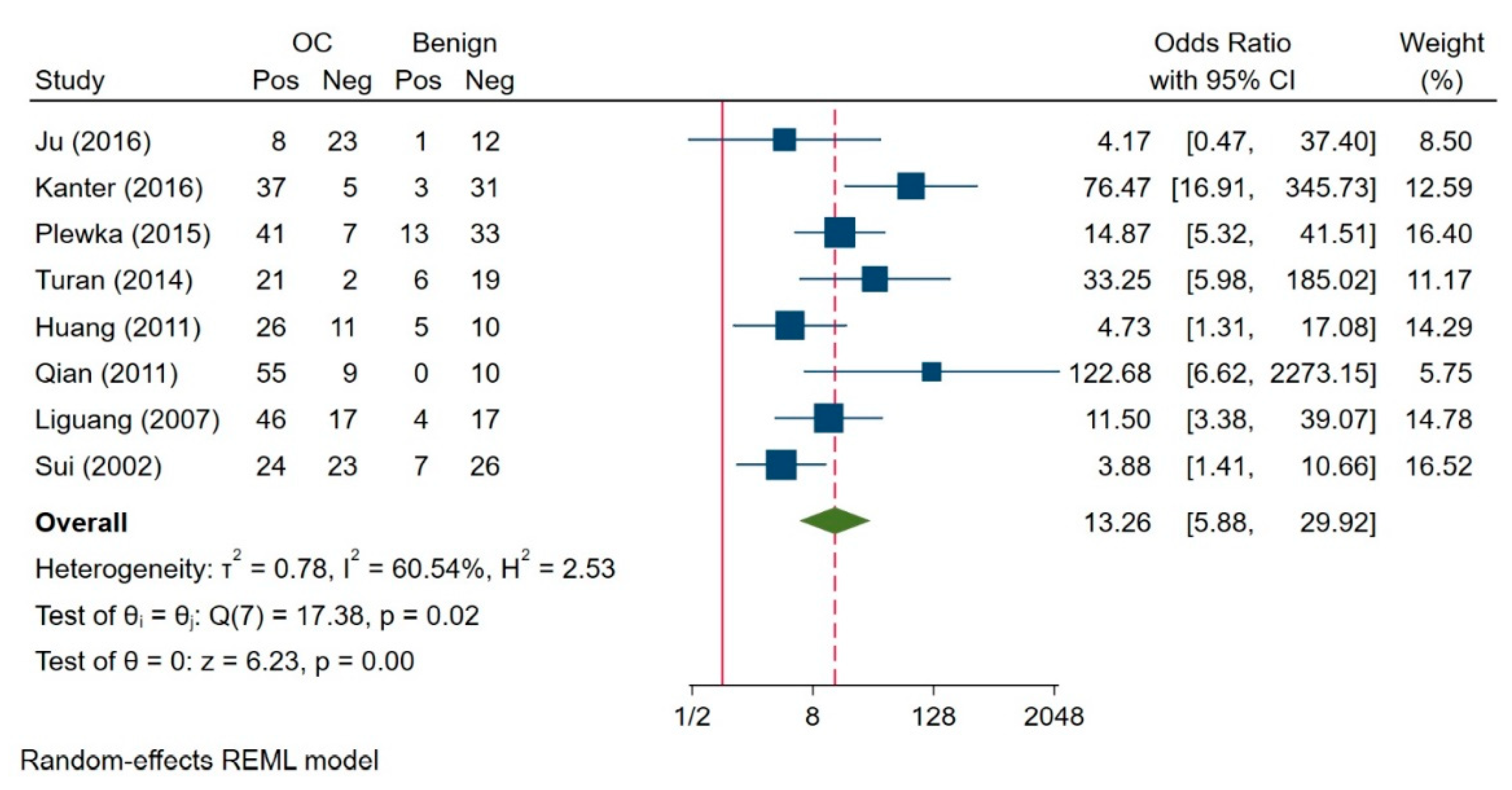
3.3. Survivin Expression Assessment in Ovarian Benign Tumour, Borderline Ovarian Tumour and Ovarian Carcinoma
3.4. Survivin Expression and FIGO Stage
3.5. Association between Survivin Expression and Histological Grade
3.6. Association between Survivin Expression and Histological Subtype
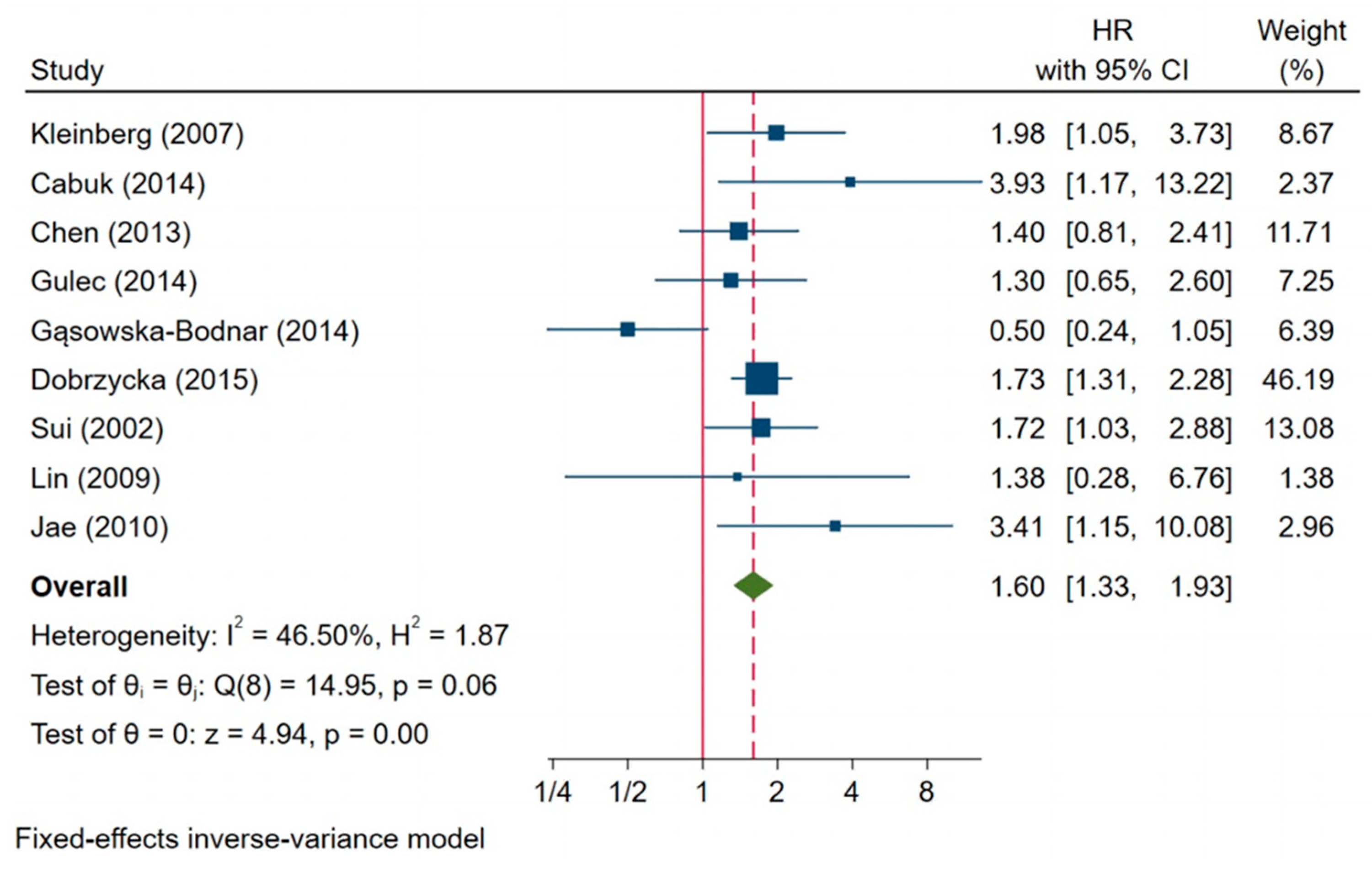
3.7. Impact of Survivin Expression on Prognosis in Ovarian Cancer
3.8. Sensitivity Analysis
3.9. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer Statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Nossov, V.; Amneus, M.; Su, F.; Lang, J.; Janco, J.M.T.; Reddy, S.T.; Farias-Eisner, R. The early detection of ovarian cancer: From traditional methods to proteomics. Can we really do better than serum CA-125? Am. J. Obstet. Gynecol. 2008, 199, 215–223. [Google Scholar] [CrossRef]
- Li, X.-J.; Pang, J.-S.; Li, Y.-M.; Ahmed, F.A.; He, R.-Q.; Ma, J.; Ma, F.-C.; Chen, G. Clinical value of survivin and its underlying mechanism in ovarian cancer: A bioinformatics study based on GEO and TCGA data mining. Pathol. Res. Pract. 2018, 214, 385–401. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN esti-mates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Goff, B.A.; Mandel, L.; Muntz, H.G.; Melancon, C.H. Ovarian carcinoma diagnosis. Cancer 2000, 89, 2068–2075. [Google Scholar] [CrossRef]
- Ju, L.-L.; Zhao, C.Y.; Ye, K.-F.; Yang, H.; Zhang, J. Expression and clinical implication of Beclin1, HMGB1, p62, survivin, BRCA1 and ERCC1 in epithelial ovarian tumor tissues. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1993–2003. [Google Scholar] [PubMed]
- Leahy, Y. Are Serum Protein Biomarkers Effective in Detecting Ovarian Cancer in Its Early Stages? Clin. J. Oncol. Nurs. 2009, 13, 443–445. [Google Scholar] [CrossRef]
- Coleman, M.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J.; et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef]
- Ambrosini, G.; Adida, C.; Altieri, D.C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997, 3, 917–921. [Google Scholar] [CrossRef]
- Altieri, D.C.; Marchisio, P.C.; Marchisio, C. Survivin apoptosis: An interloper between cell death and cell proliferation in cancer. Lab. Investig. 1999, 79, 1327–1333. [Google Scholar] [PubMed]
- Dallaglio, K.; Petrachi, T.; Marconi, A.; Truzzi, F.; Lotti, R.; Saltari, A.; Morandi, P.; Puviani, M.; Maiorana, A.; Pincelli, C. Expression of nuclear survivin in normal skin and squamous cell carcinoma: A possible role in tumour invasion. Br. J. Cancer 2014, 110, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Pižem, J.; Cör, A. Survivin—An inhibitor of apoptosis and a new therapeutic target in cancer. Radiol. Oncol. 2003, 37, 195–201. Available online: https://www.radioloncol.com/index.php/ro/article/view/1366 (accessed on 15 May 2020).
- Monzó, M.; Rosell, R.; Felip, E.; Astudillo, J.; Sánchez, J.J.; Maestre, J.; Martín, C.; Font, A.; Barnadas, A.; Abad, A. A Novel Anti-Apoptosis Gene: Re-expression of Survivin Messenger RNA as a Prognosis Marker in Non–Small-Cell Lung Cancers. J. Clin. Oncol. 1999, 17, 2100. [Google Scholar] [CrossRef]
- Sarela, A.I.; Macadam, R.C.A.; Farmery, S.M.; Markham, A.F.; Guillou, P.J. Expression of the antiapoptosis gene, Survivin, predicts death from recurrent colorectal carcinoma. Gut 2000, 46, 645–650. [Google Scholar] [CrossRef]
- Swana, H.S.; Grossman, U.; Anthony, J.N.; Altieri, D.C.; Weiss, R.M. Tumor Content of the Antiapoptosis Molecule Survivin and Recurrence of Bladder Cancer. N. Engl. J. Med. 1999, 341, 452–453. [Google Scholar] [CrossRef]
- Krajewska, M.; Krajewski, S.; Banares, S.; Huang, X.; Turner, B.; Bubendorf, L.; Kallioniemi, O.-P.; Shabaik, A.; Vitiello, A.; Peehl, D.; et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin. Cancer Res. 2003, 9, 4914–4925. [Google Scholar] [PubMed]
- Weinman, E.C.; Roche, P.C.; Kasperbauer, J.L.; Cha, S.S.; Sargent, D.J.; Cheville, J.; Murphy, L.M.; Chen, L.; Wettstein, P.J.; Gostout, B.; et al. Characterization of antigen processing machinery and Survivin expression in tonsillar squamous cell carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 2203–2211. [Google Scholar] [CrossRef]
- Saxena, A.; Yashar, C.; Taylor, U.D.; Gercel-Taylor, C. Cellular response to chemotherapy and radiation in cervical cancer. Am. J. Obstet. Gynecol. 2005, 192, 1399–1403. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Cohen, C.; Lohmann, C.M.; Cotsonis, G.; Lawson, D.; Santoianni, R. Survivin Expression in Ovarian Carcinoma: Correlation with Apoptotic Markers and Prognosis. Mod. Pathol. 2003, 16, 574–583. [Google Scholar] [CrossRef]
- Ferrandina, G.; Legge, F.; Martinelli, E.; Ranelletti, F.O.; Zannoni, G.F.; Lauriola, L.; Gessi, M.; Gallotta, V.; Scambia, G. Survivin expression in ovarian cancer and its correlation with clinico-pathological, surgical and apoptosis-related parameters. Br. J. Cancer 2005, 92, 271–277. [Google Scholar] [CrossRef]
- Kleinberg, L.; Flørenes, V.A.; Silins, I.; Haug, K.; Trope, C.G.; Nesland, J.M.; Davidson, B. Nuclear expression of survivin is associated with improved survival in metastatic ovarian carcinoma. Cancer 2007, 109, 228–238. [Google Scholar] [CrossRef]
- Athanassiadou, P.; Grapsa, D.; Athanassiades, P.; Gonidi, M.; Athanassiadou, A.-M.; Tsipis, A.; Patsouris, E. The prognostic significance of COX-2 and survivin expression in ovarian cancer. Pathol. Res. Pract. 2008, 204, 241–249. [Google Scholar] [CrossRef]
- Felisiak-Golabek, A.; Rembiszewska, A.; Rzepecka, I.K.; Szafron, L.; Madry, R.; Murawska, M.; Napiorkowski, T.; Sobiczewski, P.; Osuch, B.; Kupryjańczyk, J. Nuclear survivin expression is a positive prognostic factor in taxane-platinum-treated ovarian cancer patients. J. Ovarian Res. 2011, 4, 20. [Google Scholar] [CrossRef]
- Çabuk, F.K.; Yiğit, S.; Demir, L.; Çakalağaoğlu, F.; Tarhan, O. Correlation of survivin and MMP9 expressions with prognosis and clinicopathological parameters in surface epithelial ovarian carcinomas. Turk. J. Pathol. 2014, 30, 30–37. [Google Scholar] [CrossRef]
- Gąsowska-Bodnar, A.; Bodnar, L.; Dąbek, A.; Cichowicz, M.; Jerzak, M.; Cierniak, S.; Kozłowski, W.; Baranowski, W. Survivin Expression as a Prognostic Factor in Patients With Epithelial Ovarian Cancer or Primary Peritoneal Cancer Treated With Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer 2014, 24, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Gulec, U.K.; Gumurdulu, D.; Guzel, A.B.; Paydas, S.; Seydaoglu, G.; Açıkalın, A.; Khatib, G.; Zeren, H.; Vardar, M.A.; Altintas, A.; et al. Prognostic importance of survivin, Ki-67, and topoisomerase IIα in ovarian carcinoma. Arch. Gynecol. Obstet. 2013, 289, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycka, B.; Mackowiak-Matejczyk, B.; Terlikowska, K.M.; Kulesza-Bronczyk, B.; Kinalski, M.; Terlikowski, S.J. Prognostic significance of pretreatment VEGF, survivin, and Smac/DIABLO serum levels in patients with serous ovarian carcinoma. Tumor Biol. 2015, 36, 4157–4165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Turan, G.; Usta, C.S.; Usta, A.; Kanter, M.; Tavli, L.; Karacan, M.; Celik, C.; Eser, M. The expression of HER-2/neu (c-erbB2), survivin and cycline D1 in serous ovarian neoplasms: Their correlation with clinicopathological variables. J. Mol. Histol. 2014, 45, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Turan, G.; Usta, C.; Usta, A.; Esen, H.H.; Tavlı, L.; Celik, C.; Demirkol, Y.; Kanter, B. Survivin and cycline D1 expressions are associated with malignant potential in mucinous ovarian neoplasms. J. Mol. Histol. 2016, 47, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, H.; Lin, M.E. Evaluation the expression of three genes to epithelial ovarian cancer risk in chinese population. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 81–87. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Liang, L.; Yan, X.; Liu, N.; Gong, L.; Pan, S.; Lin, F.; Zhang, Q.; Zhao, H.; Zheng, F. Survivin Status Affects Prognosis and Chemosensitivity in Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2013, 23, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Markwitz, E.; Puła, B.; Szajnik, M.; Dziegiel, P.; Piotrowska, A.; Zabel, M.; Spaczyński, M. Expression of survivin, SDF-1 and CXCR4 on tumor cells in ovarian cancer. Ginekol. Polska 2010, 81, 674–677. [Google Scholar]
- Plewka, D.; Jakubiec-Bartnik, B.; Morek, M.; Bogunia, E.; Bienioszek, M.; Wolski, H.; Kotrych, D.; Dziekan, K.; Seremak-Mrozikiewicz, A.; Plewka, A. Survivin in ovary tumors. Ginekol. Polska 2015, 86, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Liguang, Z.; Peishu, L.; Hongluan, M.; Hong, J.; Rong, W.; Wachtel, M.S.; Frezza, E.E. Survivin expression in ovarian cancer. Exp. Oncol. 2007, 29, 121–125. [Google Scholar]
- Sui, L.; Dong, Y.; Ohno, M.; Watanabe, Y.; Sugimoto, K.; Tokuda, M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int. J. Oncol. 2002, 21, 315–320. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society|Cancer Facts & Statistics. American Cancer Society|Cancer Facts & Statistics. Available online: http://cancerstatisticscenter.cancer.org/ (accessed on 27 July 2020).
- Braný, D.; Dvorská, D.; Slávik, P.; Školka, R.; Adamkov, M. Survivin and gynaecological tumours. Pathol. Res. Pract. 2017, 213, 295–300. [Google Scholar] [CrossRef]
- Fukuda, S.; Pelus, L.M. Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34+ cells by hematopoietic growth factors: Implication of survivin expression in normal hematopoiesis. Blood 2001, 98, 2091–2100. [Google Scholar] [CrossRef]
- González-Cámpora, R.; Ruiz, M.R.G.; Ramírez, F.V.; Martín, J.J.R.; Santos, J.M.F.; Martos, M.D.M.R.; Pascual, A.G. Apoptosis in Breast Carcinoma. Pathol. Res. Pract. 2000, 196, 167–174. [Google Scholar] [CrossRef]
- Lu, C.D.; Altieri, D.C.; Tanigawa, N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998, 58, 1808–1812. [Google Scholar] [PubMed]
- Krieg, A.; Mahotka, C.; Krieg, T.; Grabsch, H.; Müller, W.; Takeno, S.; Suschek, C.V.; Heydthausen, M.; Gabbert, H.E.; Gerharz, C.D. Expression of different survivin variants in gastric carcinomas: First clues to a role of survivin-2B in tumour progression. Br. J. Cancer 2002, 86, 737–743. [Google Scholar] [CrossRef]
- Chiou, S.-K.; Moon, W.S.; Jones, M.K.; Tarnawski, A.S. Survivin expression in the stomach: Implications for mucosal integrity and protection. Biochem. Biophys. Res. Commun. 2003, 305, 374–379. [Google Scholar] [CrossRef]
- Xu, X.-L.; Tao, K.-Y.; Li, X.-X.; Xu, W.-Z.; Wang, Y.; Zhu, S.-M.; Xie, H.-X.; Luo, W.-H.; Xu, Y.-J. Prognostic role of apoptosis-related gene functional variants in advanced non-small-cell lung cancer patients treated with first-line platinum-based chemotherapy. OncoTargets Ther. 2015, 8, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Altieri, D.C.; Lu, C.D.; Toyoda, M.; Tenjo, T.; Tanigawa, N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998, 58, 5071–5074. [Google Scholar]
- Xie, S.; Xu, H.; Shan, X.; Liu, B.; Wang, K.; Cai, Z. Clinicopathological and Prognostic Significance of Survivin Expression in Patients with Oral Squamous Cell Carcinoma: Evidence from a Meta-Analysis. PLoS ONE 2015, 10, e0116517. [Google Scholar] [CrossRef]
- Zaffaroni, N.; Pennati, M.; Colella, G.; Perego, P.; Supino, R.; Gatti, L.; Pilotti, S.; Zunino, F.; Daidone, M.G. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell. Mol. Life Sci. 2002, 59, 1406–1412. [Google Scholar] [CrossRef]
- Liu, T.; Brouha, B.; Grossman, D. Rapid induction of mitochondrial events and caspase-independent apoptosis in Survivin-targeted melanoma cells. Oncogene 2004, 23, 39–48. [Google Scholar] [CrossRef]
- Watson, J.L.; Greenshields, A.; Hill, R.; Hilchie, A.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol. Carcinog. 2009, 49, 13–24. [Google Scholar] [CrossRef]
- Glienke, W.; Maute, L.; Wicht, J.; Bergmann, L. Curcumin Inhibits Constitutive STAT3 Phosphorylation in Human Pancreatic Cancer Cell lines and Downregulation of Survivin/BIRC5 Gene Expression. Cancer Investig. 2009, 28, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karathanasi, A.; Cooke, D.; Neille, C.; Sadauskaite, A.; Moschetta, M.; Zakynthinakis-Kyriakou, N.; Pavlidis, N. PARP Inhibitors in Ovarian Cancer: The Route to “Ithaca”. Diagnostics 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Suzuki, S.; Togashi, K.; Sanomachi, T.; Seino, S.; Kitanaka, C.; Okada, M. AS602801, an Anticancer Stem Cell Candidate Drug, Reduces Survivin Expression and Sensitizes A2780 Ovarian Cancer Stem Cells to Carboplatin and Paclitaxel. Anticancer Res. 2018, 38, 6699–6706. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, K.; Wang, H.; Chen, X.; Wu, H.; Yao, L.; Ma, S. Expression and clinical significance of survivin in ovarian cancer: A meta-analysis. PLoS ONE 2018, 13, e0194463. [Google Scholar] [CrossRef] [PubMed]




| Autor | Reference | Country | Year | No. of P. | Mean Age (Range) | Method | Tumour Grade G1/G2/G3 | FIGO Stage (I–II/III–IV) |
|---|---|---|---|---|---|---|---|---|
| Huang Ju | [33] | China | 2016 | 60 | - | IHC | - | - |
| Kanter M. | [32] | Turkey | 2016 | 98 | 52 (18–87) | IHC | 17/25 * | 15/27 |
| Ju L.-L. | [7] | China | 2016 | 60 | 48 (25–72) | IHC | - | 19/20 |
| Plewka D. | [36] | Poland | 2015 | 137 | 45 (34–58) | IHC | - | - |
| Dobrzycka B. | [29] | Poland | 2015 | 92 | 56 (32–76) | IHC | 33 **/59 | 21/71 |
| Gąsowska-Bodnar A. | [27] | Poland | 2014 | 66 | 60 (39–80) | IHC | - | -/60 |
| Turan G. | [31] | Turkey | 2014 | 62 | 48 (16–88) benign 39 (17–77) borderline 52 (36–82) malignant | IHC | - | 6/17 |
| Gulec U.K. | [28] | Turkey | 2014 | 73 | 52.6 (17–78) | IHC | 11 **/47 | 41/15 |
| Chen L. | [34] | China | 2014 | 90 | 50 (22–75) | IHC, | 22/21/47 | 42/48 |
| Çabuk F.K. | [26] | Turkey | 2014 | 60 | 54.5 (36–80) | IHC | 8/21/31 | 25/35 |
| Felisiak-Gołabek A. | [25] | Poland | 2011 | 435 | 54.3 (20–78) | IHC | 54/263/118 | 27/408 |
| Nowak-Markwitz E. | [35] | Poland | 2010 | 82 | 49 (49–75) | IHC | 17/33/24 | 20/62 |
| Athanassiadou P. | [24] | Greece | 2008 | 100 | 62 (38–82) | IHC | 34/39/27 | 44/56 |
| Liguang Z. | [37] | China, USA | 2007 | 114 | 56 (19–72) | RT-PCR | 30 **/33 | 28/35 |
| Kleinberg L. | [23] | Norway | 2006 | 220 | 63 (38–87) + 59 (25–81) ++ | IHC | 14/31/106 | 3/172 |
| Ferrandina G. | [22] | Italy | 2005 | 110 | 58.5 (25–84) | IHC | 26 **/74 | 19/91 |
| Cohen C. | [21] | USA | 2003 | 49 | 57 (29–76) • 63 (45–79) •• | IHC | 7/15/27 | 5/41 |
| Sui L. | [38] | Japan | 2002 | 103 | 49 (16–77) | IHC | 21/13/13 | 19/28 |
| Studies | References | HR | 95% CI | Heterogeneity Test | |
|---|---|---|---|---|---|
| I2 (%) | P | ||||
| Overall Survival | |||||
| All studies (n = 9) | 1.60 | 1.33–1.99 | 46.50 | 0.06 | |
| Omitting Kleinberg (2007) | [23] | 1.57 | 1.29–1.91 | 51.66 | 0.04 |
| Omitting Cabuk (2014) | [26] | 1.57 | 1.30–1.89 | 45.30 | 0.08 |
| Omitting Chen (2013) | [34] | 1.63 | 1.34–1.99 | 52.35 | 0.04 |
| Omitting Gulec (2014) | [28] | 1.63 | 1.34–1.98 | 51.99 | 0.04 |
| Omitting Gąsowska-Bodnar (2014) | [27] | 1.73 | 1.43–2.10 | 0.00 | 0.69 |
| Omitting Dobrzycka (2015) | [29] | 1.50 | 1.16–1.93 | 51.34 | 0.04 |
| Omitting Sui (2002) | [38] | 1.58 | 1.30–1.93 | 52.92 | 0.04 |
| Omitting Lin (2009) | [3] | 1.60 | 1.33–1.94 | 53.08 | 0.04 |
| Omitting Jae (2010) | [3] | 1.56 | 1.29–1.89 | 46.27 | 0.07 |
| Disease-Free Survival/ Recurrent-Free Survival | |||||
| All studies (n = 3) | 1.06 | 0.55–2.05 | 84.59 | 0.00 | |
| Omitting Felisiak-Gołąbek (2011) | [25] | 1.39 | 0.66–2.94 | 75.17 | 0.04 |
| Omitting Gąsowska-Bodnar (2014) | [27] | 1.13 | 0.38–3.36 | 93.12 | 0.00 |
| Omitting Dobrzycka (2015) | [29] | 0.72 | 0.54–0.96 | 2.81 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąsowska-Bajger, B.; Gąsowska-Bodnar, A.; Knapp, P.; Bodnar, L. Prognostic Significance of Survivin Expression in Patients with Ovarian Carcinoma: A Meta-Analysis. J. Clin. Med. 2021, 10, 879. https://doi.org/10.3390/jcm10040879
Gąsowska-Bajger B, Gąsowska-Bodnar A, Knapp P, Bodnar L. Prognostic Significance of Survivin Expression in Patients with Ovarian Carcinoma: A Meta-Analysis. Journal of Clinical Medicine. 2021; 10(4):879. https://doi.org/10.3390/jcm10040879
Chicago/Turabian StyleGąsowska-Bajger, Beata, Agnieszka Gąsowska-Bodnar, Paweł Knapp, and Lubomir Bodnar. 2021. "Prognostic Significance of Survivin Expression in Patients with Ovarian Carcinoma: A Meta-Analysis" Journal of Clinical Medicine 10, no. 4: 879. https://doi.org/10.3390/jcm10040879
APA StyleGąsowska-Bajger, B., Gąsowska-Bodnar, A., Knapp, P., & Bodnar, L. (2021). Prognostic Significance of Survivin Expression in Patients with Ovarian Carcinoma: A Meta-Analysis. Journal of Clinical Medicine, 10(4), 879. https://doi.org/10.3390/jcm10040879