Upstream Statin Therapy and Long-Term Recurrence of Atrial Fibrillation after Cardioversion: A Propensity-Matched Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
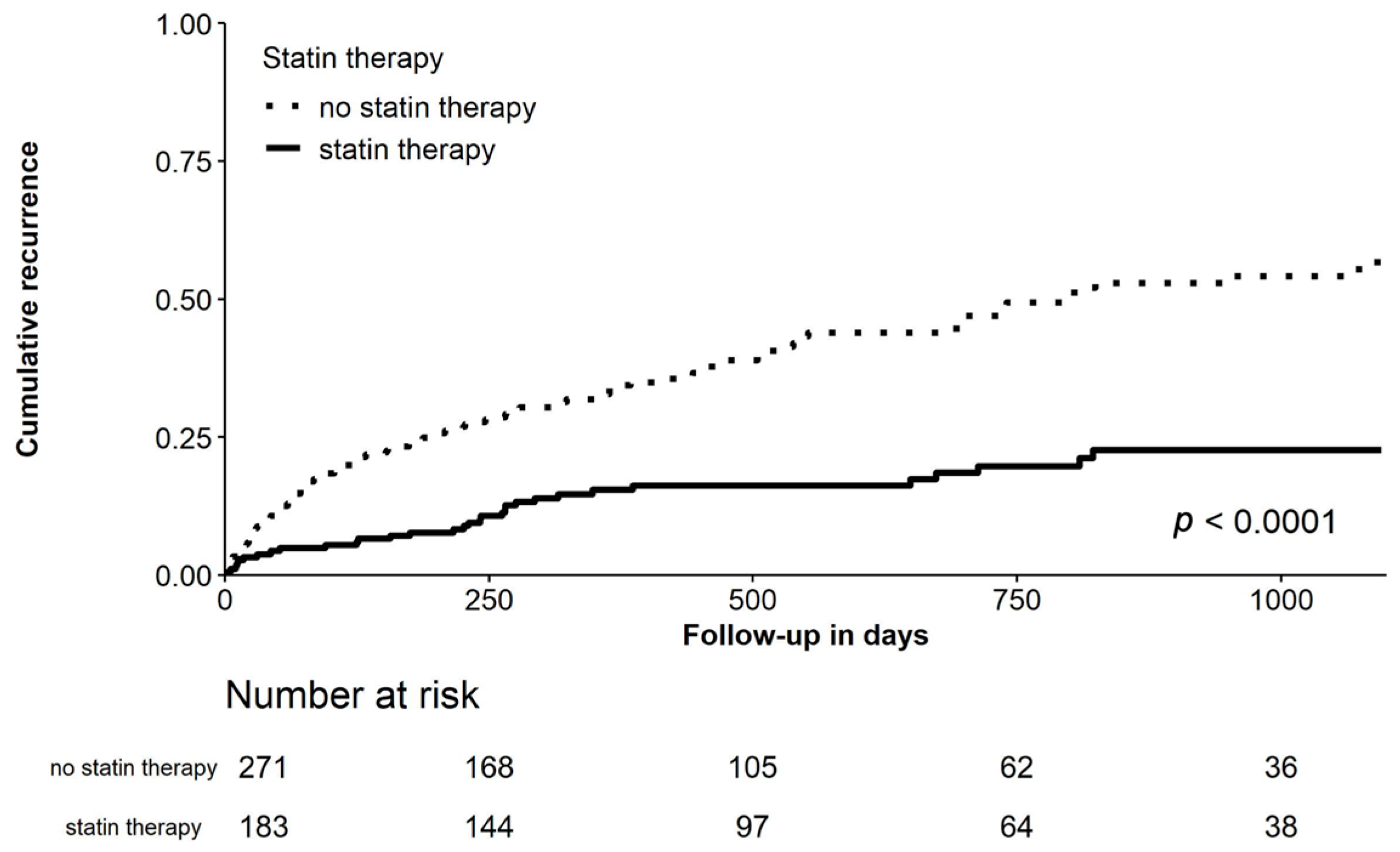
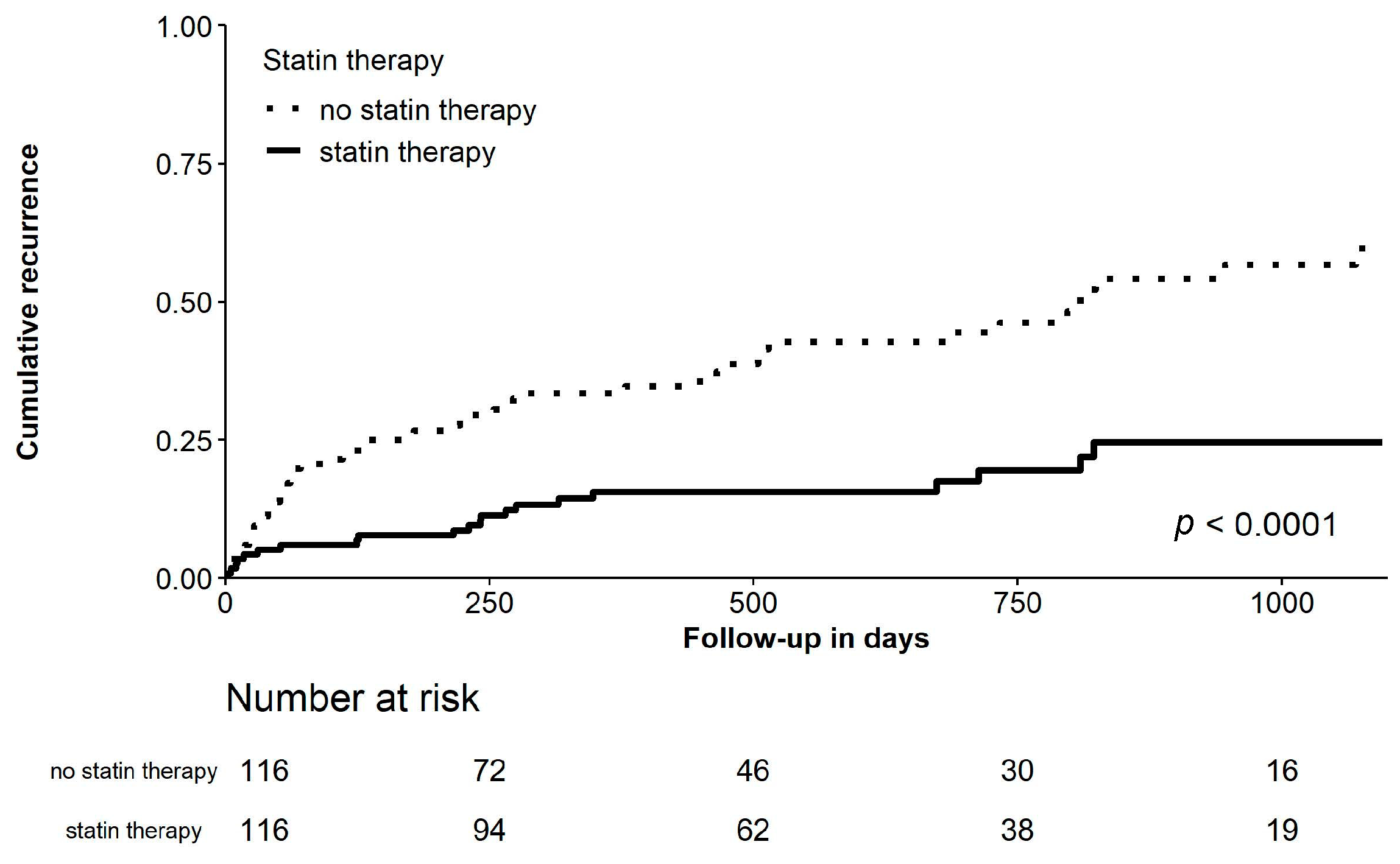
Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heeringa, J.; Van Der Kuip, D.A.M.; Hofman, A.; Kors, J.A.; Van Herpen, G.; Stricker, B.H.C.; Stijnen, T.; Lip, G.Y.H.; Witteman, J.C.M. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Bai, Y.; Shantsila, A.; Fauchier, L.; Potpara, T.S.; Lip, G.Y.H. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: A systematic review. Clin. Res. Cardiol. 2017, 106, 813–823. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Ecker, V.; Knoery, C.; Rushworth, G.; Rudd, I.; Ortner, A.; Begley, D.; Leslie, S.J. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin. Cardiol. 2018, 41, 862–870. [Google Scholar] [CrossRef]
- Rienstra, M.; Hobbelt, A.H.; Alings, M.; Tijssen, J.G.P.; Smit, M.D.; Brügemann, J.; Geelhoed, B.; Tieleman, R.G.; Hillege, H.L.; Tukkie, R.; et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: Results of the RACE 3 trial. Eur. Heart J. 2018, 39, 2987–2996. [Google Scholar] [CrossRef]
- Engelmann, M.D.; Svendsen, J.H. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur. Heart J. 2005, 26, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Preiss, D.; Tobert, J.A.; Hovingh, G.K.; Reith, C. Lipid-Modifying Agents, From Statins to PCSK9 Inhibitors: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Feron, O.; Dessy, C.; Desager, J.P.; Balligand, J.L. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation 2001, 103, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Maas, K.; Youssef, S.; Dürr, C.; Steinman, L.; Negrin, R.S. Regulation of different inflammatory diseases by impacting the mevalonate pathway. Immunology 2009, 127, 18–25. [Google Scholar] [CrossRef]
- Blum, A.; Shamburek, R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 2009, 203, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Millar, P.J.; Floras, J.S. Statins and the autonomic nervous system. Clin. Sci. 2014, 126, 401–415. [Google Scholar] [CrossRef]
- Li, J.; Solus, J.; Chen, Q.; Rho, Y.H.; Milne, G.; Stein, C.M.; Darbar, D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010, 7, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, L.; Sun, A.; Jiang, H.; Qian, J.; Ge, J. Efficacy of statin therapy in chronic systolic cardiac insufficiency: A meta-analysis. Eur. J. Intern. Med. 2011, 22, 478–484. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, X.; Dang, Y.; Li, Y.; Song, X.; Hao, X. Effects of atorvastatin on atrial remodeling in a rabbit model of atrial fibrillation produced by rapid atrial pacing. BMC Cardiovasc. Disord. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Feng, W.; Qu, X. Effects of rosuvastatin on serum asymmetric dimethylarginine levels and atrial structural remodeling in atrial fibrillation dogs. PACE Pacing Clin. Electrophysiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Shiroshita-Takeshita, A.; Schram, G.; Lavoie, J.; Nattel, S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 2004, 110, 2313–2319. [Google Scholar] [CrossRef]
- Loffredo, L.; Angelico, F.; Perri, L.; Violi, F. Upstream therapy with statin and recurrence of atrial fibrillation after electrical cardioversion. Review of the literature and meta-analysis. BMC Cardiovasc. Disord. 2012, 12, 1. [Google Scholar] [CrossRef]
- Dentali, F.; Gianni, M.; Squizzato, A.; Ageno, W.; Castiglioni, L.; Maroni, L.; Hylek, E.M.; Grandi, A.M.; Cazzani, E.; Venco, A.; et al. Use of statins and recurrence of atrial fibrillation after catheter ablation or electrical cardioversion; A systematic review and meta-analysis. Thromb. Haemost. 2011, 106, 363–370. [Google Scholar] [CrossRef]
- Rahimi, K.; Emberson, J.; McGale, P.; Majoni, W.; Merhi, A.; Asselbergs, F.W.; Krane, V.; Macfarlane, P.W. Effect of statins on atrial fibrillation: Collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. BMJ 2011, 342, 693. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Kirchhof, P.; Lip, G.Y.H.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; Hindricks, G.; Prendergast, B.; et al. Guidelines for the management of atrial fibrillation. Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dunkler, D.; Plischke, M.; Leffondré, K.; Heinze, G. Augmented backward elimination: A pragmatic and purposeful way to develop statistical models. PLoS ONE 2014, 9, e113677. [Google Scholar] [CrossRef]
- Nattel, S.; Guasch, E.; Savelieva, I.; Cosio, F.G.; Valverde, I.; Halperin, J.L.; Conroy, J.M.; Al-Khatib, S.M.; Hess, P.L.; Kirchhof, P.; et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J. 2014, 35, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Cho, K.I.; Koo, S.H.; Cha, T.J.; Heo, J.H.; Kim, H.S.; Jo, G.B.; Lee, J.W. Simvastatin attenuates the oxidative stress, endothelial thrombogenicity and the inducibility of atrial fibrillation in a rat model of ischemic heart failure. Int. J. Mol. Sci. 2014, 15, 14803–14818. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Deng, W.; Bukiya, A.N.; Rodríguez-Menchaca, A.A.; Zhang, Z.; Baumgarten, C.M.; Logothetis, D.E.; Levitan, I.; Rosenhouse-Dantsker, A. Hypercholesterolemia induces up-regulation of KACh cardiac currents via a mechanism independent of phosphatidylinositol 4,5-bisphosphate and Gβγ. J. Biol. Chem. 2012, 287, 4925–4935. [Google Scholar] [CrossRef]
- Ridker, P.M. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: Rationale and design of the JUPITER trial. Circulation 2003, 108, 2292–2297. [Google Scholar] [CrossRef]
- Tsai, C.-T.; Lai, L.-P.; Kuo, K.-T.; Hwang, J.-J.; Hsieh, C.-S.; Hsu, K.-L.; Tseng, C.-D.; Tseng, Y.-Z.; Chiang, F.-T.; Lin, J.-L. Angiotensin II Activates Signal Transducer and Activators of Transcription 3 via Rac1 in Atrial Myocytes and Fibroblasts. Circulation 2008, 117, 344–355. [Google Scholar] [CrossRef]
- Reilly, S.N.; Jayaram, R.; Nahar, K.; Antoniades, C.; Verheule, S.; Channon, K.M.; Alp, N.J.; Schotten, U.; Casadei, B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: Implications for the antiarrhythmic effect of statins. Circulation 2011, 124, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Yin, Z.; Li, J.; Song, Y.; Qu, X. Effects of rosuvastatin on asymmetric dimethylarginine levels and early atrial fibrillation recurrence after electrical cardioversion. PACE Pacing Clin. Electrophysiol. 2009, 32, 1562–1566. [Google Scholar] [CrossRef]
- Almroth, H.; Höglund, N.; Boman, K.; Englund, A.; Jensen, S.; Kjellman, B.; Tornvall, P.; Rosenqvist, M. Atorvastatin and persistent atrial fibrillation following cardioversion: A randomized placebo-controlled multicentre study. Eur. Heart J. 2009, 30, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin, M.; Varol, E.; Aslan, S.M.; Kucuktepe, Z.; Dogan, A.; Ozturk, M.; Altinbas, A. Effect of Atorvastatin on the Recurrence Rates of Atrial Fibrillation After Electrical Cardioversion. Am. J. Cardiol. 2006. [Google Scholar] [CrossRef]
- Negi, S.; Shukrullah, I.; Veledar, E.; Bloom, H.L.; Jones, D.P.; Dudley, S.C. Statin Therapy for the Prevention of Atrial Fibrillation Trial (SToP AF trial). J. Cardiovasc. Electrophysiol. 2011, 22, 414–419. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, J.A.; Blazing, M.A.; Wiviott, S.D.; Lewis, E.F.; Fox, K.A.A.; White, H.D.; Rouleau, J.-L.; Pedersen, T.R.; Gardner, L.H.; Mukherjee, R.; et al. Early Intensive vs. a Delayed Conservative Simvastatin Strategy in Patients With Acute Coronary Syndromes. JAMA 2004, 292, 1307. [Google Scholar] [CrossRef]
- Veronese, G.; Montomoli, J.; Schmidt, M.; Horváth-Puhó, E.; Sørensen, H.T. Statin Use and Risk of Atrial Fibrillation or Flutter: A Population-based Case-Control Study. Am. J. Ther. 2015, 22, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Kim, B.J.; Cha, T.J.; Heo, J.H.; Kim, H.S.; Lee, J.W. Impact of duration and dosage of statin treatment and epicardial fat thickness on the recurrence of atrial fibrillation after electrical cardioversion. Heart Vessel. 2015, 30, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Balse, E.; El-Haou, S.; Dillanian, G.; Dauphin, A.; Eldstrom, J.; Fedida, D.; Coulombe, A.; Hatem, S.N. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 14681–14686. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.M.; Hernán, M.A.; Siebert, U. Estimations of the Effects of Multiple Interventions. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 2191–2230. [Google Scholar]
- Kuehne, F.; Jahn, B.; Conrads-Frank, A.; Bundo, M.; Arvandi, M.; Endel, F.; Popper, N.; Endel, G.; Urach, C.; Gyimesi, M.; et al. Guidance for a causal comparative effectiveness analysis emulating a target trial based on big real world evidence: When to start statin treatment. J. Comp. Eff. Res. 2019, 8, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
| Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|
| All Patients | No Statin | Statin | p-Value | No Statin | Statin | p-Value | |
| n = 454 | n = 271 | n = 183 | n = 116 | n = 116 | |||
| Recurrence of AF, No. (%) | 150 (33.0%) | 118 (43.5%) | 32 (17.5%) | <0.001 | 53 (45.7%) | 21 (18.1%) | <0.001 |
| Age, years | 65 (57–72) | 64 (54–71) | 67 (61–72) | 0.001 | 67 (61–72) | 66 (60–71) | 0.427 |
| Female patients, No. (%) | 146 (32.2%) | 87 (32.1%) | 59 (32.2%) | 1.000 | 43 (37.1%) | 38 (32.8%) | 0.582 |
| Clinical presentation, No. (%) | |||||||
| persistent AF | 256 (56.4%) | 155 (57.2%) | 101 (55.2%) | 0.744 | 66 (56.9%) | 61 (52.6%) | 0.598 |
| paroxysmal AF | 198 (43.6%) | 116 (42.8%) | 82 (44.8%) | 50 (43.1%) | 55 (47.4%) | ||
| Type of cardioversion, No. (%) | |||||||
| Electrical | 438 (96.5%) | 257 (94.8%) | 181 (98.9%) | 0.040 | 109 (94.0%) | 115 (99.1%) | 0.073 |
| Pharmacological | 16 (3.5%) | 14 (5.2%) | 2 (1.1%) | 7 (6.0%) | 1 (0.9%) | ||
| CHA2DS2-VASc | 2 (1–2) | 2 (1–2) | 2 (2–3) | <0.001 | 2 (2–3) | 2 (1–2) | 0.477 |
| Prior ablation, No. (%) | 78 (17.2%) | 47 (17.3%) | 31 (16.9%) | 1.000 | 19 (16.4%) | 19 (16.4%) | 1.000 |
| Arterial hypertension, No. (%) | 313 (68.9%) | 165 (60.9%) | 148 (80.9%) | <0.001 | 92 (79.3%) | 88 (75.9%) | 0.637 |
| Diabetes mellitus, No. (%): | 66 (14.5%) | 25 (9.23%) | 41 (22.4%) | <0.001 | 20 (17.2%) | 24 (20.7%) | 0.503 |
| Coronary artery disease, No. (%) | 75 (16.5%) | 26 (9.6%) | 49 (26.8%) | <0.001 | 22 (19.0%) | 22 (19.0%) | 1.000 |
| Heart failure, No. (%) | 83 (18.3%) | 41 (15.1%) | 42 (23.0%) | 0.046 | 27 (23.3%) | 22 (19.0%) | 0.520 |
| Hyperlipidemia, No. (%) | 111 (24.4%) | 23 (8.49%) | 88 (48.1%) | <0.001 | 23 (19.8%) | 26 (22.4%) | 0.748 |
| Beta blocker, No. (%) | 304 (67.0%) | 178 (65.7%) | 126 (68.9%) | 0.547 | 78 (67.2%) | 77 (66.4%) | 1.000 |
| ACE inhibitor, No. (%) | 175 (38.5%) | 96 (35.4%) | 79 (43.2%) | 0.118 | 63 (54.3%) | 51 (44.0%) | 0.149 |
| ARB, No.(%) | 101 (22.2%) | 53 (19.6%) | 48 (26.2%) | 0.118 | 25 (21.6%) | 26 (22.4%) | 1.000 |
| MRA, No.(%) | 38 (8.41%) | 18 (6.69%) | 20 (10.9%) | 0.155 | 11 (9.48%) | 9 (7.76%) | 0.815 |
| Anti-arrhythmic therapy, No. (%) | 303 (66.7%) | 176 (64.9%) | 127 (69.4%) | 0.375 | 81 (69.8%) | 80 (69.0%) | 1.000 |
| Amiodarone | 220 (48.5%) | 123 (45.4%) | 97 (53.0%) | 0.111 | 54 (46.6%) | 59 (50.9%) | 0.599 |
| Sotalol | 27 (5.9%) | 18 (6.6%) | 9 (4.9%) | 0.446 | 8 (6.9%) | 7 (6.0%) | 1.000 |
| Dronedarone | 23 (5.1%) | 14 (5.2%) | 9 (4.9%) | 0.906 | 5 (4.3%) | 6 (5.2%) | 1.000 |
| Propafenone | 16 (3.5%) | 10 (3.7%) | 6 (3.3%) | 0.816 | 7 (6.0%) | 6 (5.2%) | 1.000 |
| Flecainide | 17 (3.7%) | 11 (4.1%) | 6 (3.3%) | 0.667 | 7 (6.0%) | 2 (1.7%) | 0.171 |
| Digitalis, No. (%) | 38 (8.4%) | 15 (5.5%) | 23 (12.6%) | 0.013 | 13 (11.2%) | 15 (12.9%) | 0.840 |
| Patients with Statins | |
|---|---|
| n = 183 | |
| Fluvastatin, No. (%) | 17 (9.3) |
| Pravastatin, No. (%) | 18 (9.8) |
| Simvastatin, No. (%) | 112 (61.2) |
| Atorvastatin, No. (%) | 31 (16.9) |
| Rosuvastatin, No. (%) | 5 (2.7) |
| Final Model (Continuous) | ||||
|---|---|---|---|---|
| HR | 95% CI | p-Value | ||
| Statin therapy | 0.313 | 0.188 | 0.521 | <0.001 |
| Hyperlipidemia | 3.018 | 1.860 | 4.895 | <0.001 |
| Anti-arrhythmic therapy | 1.443 | 0.858 | 2.427 | 0.166 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, L.; Hallsson, L.; Tscharre, M.; Oebel, S.; Pfeffer, M.; Schönbauer, R.; Tokarska, L.; Stix, L.; Haiden, A.; Kraus, J.; et al. Upstream Statin Therapy and Long-Term Recurrence of Atrial Fibrillation after Cardioversion: A Propensity-Matched Analysis. J. Clin. Med. 2021, 10, 807. https://doi.org/10.3390/jcm10040807
Fiedler L, Hallsson L, Tscharre M, Oebel S, Pfeffer M, Schönbauer R, Tokarska L, Stix L, Haiden A, Kraus J, et al. Upstream Statin Therapy and Long-Term Recurrence of Atrial Fibrillation after Cardioversion: A Propensity-Matched Analysis. Journal of Clinical Medicine. 2021; 10(4):807. https://doi.org/10.3390/jcm10040807
Chicago/Turabian StyleFiedler, Lukas, Lára Hallsson, Maximilian Tscharre, Sabrina Oebel, Michael Pfeffer, Robert Schönbauer, Lyudmyla Tokarska, Laura Stix, Anton Haiden, Johannes Kraus, and et al. 2021. "Upstream Statin Therapy and Long-Term Recurrence of Atrial Fibrillation after Cardioversion: A Propensity-Matched Analysis" Journal of Clinical Medicine 10, no. 4: 807. https://doi.org/10.3390/jcm10040807
APA StyleFiedler, L., Hallsson, L., Tscharre, M., Oebel, S., Pfeffer, M., Schönbauer, R., Tokarska, L., Stix, L., Haiden, A., Kraus, J., Blessberger, H., Siebert, U., & Roithinger, F. X. (2021). Upstream Statin Therapy and Long-Term Recurrence of Atrial Fibrillation after Cardioversion: A Propensity-Matched Analysis. Journal of Clinical Medicine, 10(4), 807. https://doi.org/10.3390/jcm10040807






