Negative Impact of Elevated DNA Fragmentation and Human Papillomavirus (HPV) Presence in Sperm on the Outcome of Intra-Uterine Insemination (IUI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patient Selection
2.3. Semen Analysis and Capacitation
2.4. Detection of HPV DNA by Type-Specific Real-Time Quantitative PCR (qPCR) Analysis in Sperm
2.5. Sperm Chromatin Structure Assay (SCSA)
2.6. IUI Protocol
2.7. Statistical Analysis
3. Results
3.1. Semen Parameters, IUI and Pregnancy
3.2. ROC Analysis and Clinical DFI% Cutoff
3.3. DFI vs. HPV Positivity in Sperm
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Bungum, M.; Bungum, L.; Giwercman, A. Sperm chromatin structure assay (SCSA): A tool in diagnosis and treatment of infertility. Asian J. 2011, 13, 69–75. [Google Scholar] [CrossRef]
- Irvine, D.S. Epidemiology and aetiology of male infertility. Hum. Reprod. 1998, 13, 33–44. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Gizzo, S.; Ferrari, B.; Noventa, M.; Ferrari, E.; Patrelli, T.S.; Gangemi, M.; Nardelli, G.B. Male and couple fertility impairment due to HPV-DNA sperm infection: Update on molecular mechanism and clinical impact—Systematic review. BioMed Res. Int. 2014, 2014, 230263. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Chen, Y.X.; Cheng, M.J.; He, W.Q.; Chen, Q. The risk of human papillomavirus infection for male fertility abnormality: A meta-analysis. Asian J. 2018, 20, 493–497. [Google Scholar] [CrossRef]
- Garolla, A.; Engl, B.; Pizzol, D.; Ghezzi, M.; Bertoldo, A.; Bottacin, A.; Noventa, M.; Foresta, C. Spontaneous fertility and in vitro fertilization outcome: New evidence of human papillomavirus sperm infection. Fertil. Steril. 2016, 105, 65–72. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Donders, G.G.G.; Verstraete, L.; Vanden Broeck, D.; Beert, J.F.A.; Salembier, G.; Bosmans, E.; Ombelet, W. Infectious human papillomavirus virions in semen reduce clinical pregnancy rates in women undergoing intrauterine insemination. Fertil. Steril. 2019, 111, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Criel, A.M.; Benoy, I.H.; Arbyn, M.; Vereecken, A.J.; Bogers, J.J. Changes in type-specific human papillomavirus load predict progression to cervical cancer. J. Cell. Mol. Med. 2012, 16, 3096–3104. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, C.E.; Thys, S.; Beert, J.; Jonckheere, J.; Salembier, G.; Bogers, J.J. Linear viral load increase of a single HPV-type in women with multiple HPV infections predicts progression to cervical cancer. Int. J. Cancer 2016, 139, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, C.E.; Jonckheere, J.; Berth, M.; Salembier, G.M.; Vereecken, A.J.; Bogers, J.J. Serial type-specific human papillomavirus (HPV) load measurement allows differentiation between regressing cervical lesions and serial virion productive transient infections. Cancer Med. 2015, 4, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Perez-Andino, J.; Buck, C.B.; Ribbeck, K. Adsorption of human papillomavirus 16 to live human sperm. PLoS ONE 2009, 4, e5847. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Lenzi, A.; Palu, G.; Pizzol, D.; Bertoldo, A.; De, T.L.; Foresta, C. Human papillomavirus sperm infection and assisted reproduction: A dangerous hazard with a possible safe solution. Hum. Reprod. 2012, 27, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Patassini, C.; Bertoldo, A.; Menegazzo, M.; Francavilla, F.; Barzon, L.; Ferlin, A. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS ONE 2011, 6, e15036. [Google Scholar] [CrossRef]
- Rintala, M.A.; Grénman, S.E.; Pöllänen, P.P.; Suominen, J.J.; Syrjänen, S.M. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int. J. STD AIDS 2004, 15, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Garolla, A.; Zuccarello, D.; Pizzol, D.; Moretti, A.; Barzon, L.; Palù, G. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil. Steril. 2010, 93, 802–806. [Google Scholar] [CrossRef]
- Lai, Y.M.; Lee, J.F.; Huang, H.Y.; Soong, Y.K.; Yang, F.P.; Pao, C.C. The effect of human papillomavirus infection on sperm cell motility. Fertil. Steril. 1997, 67, 1152–1155. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Cazzaniga, W.; Chierigo, F.; Pozzi, E.; Clementi, M.; Vigano, P.; Montanari, E.; et al. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum. Reprod. 2019, 34, 209–217. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Beert, J.; Bosmans, E.; Salembier, G. Human Papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. Facts Views Vis. ObGyn 2016, 8, 211–222. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: A guideline. Fertil. Steril. 2013, 99, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Rex, A.S.; Aagaard, J.; Fedder, J. DNA fragmentation in spermatozoa: A historical review. Andrology 2017, 5, 622–630. [Google Scholar] [CrossRef]
- Evenson, D.P.; Darzynkiewicz, Z.; Melamed, M.R. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980, 210, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef]
- Giwercman, A.; Lindstedt, L.; Larsson, M.; Bungum, M.; Spano, M.; Levine, R.J.; Rylander, L. Sperm chromatin structure assay as an independent predictor of fertility in vivo: A case-control study. Int. J. Androl. 2010, 33, e221–e227. [Google Scholar] [CrossRef]
- Spano, M.; Bonde, J.P.; Hjollund, H.I.; Kolstad, H.A.; Cordelli, E.; Leter, G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil. Steril. 2000, 73, 43–50. [Google Scholar] [CrossRef]
- Evenson, D.P.; Jost, L.K.; Marshall, D.; Zinaman, M.J.; Clegg, E.; Purvis, K.; de Angelis, P.; Claussen, O.P. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum. Reprod. 1999, 14, 1039–1049. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, Y.; Sun, X.P.; Cui, Y.G.; Qian, X.Q.; Mao, Y.D.; Liu, J.Y. Sperm chromatin structure assay predicts the outcome of intrauterine insemination. Zhonghua Nan Ke Xue 2011, 17, 977–983. [Google Scholar] [PubMed]
- Bungum, M.; Humaidan, P.; Axmon, A.; Spano, M.; Bungum, L.; Erenpreiss, J.; Giwercman, A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum. Reprod. 2007, 22, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, A.; Van Avermaete, A.F.; Roelant, E.; Punjabi, U.; De Neubourg, N.D. The role of sperm DNA fragmentation testing in predicting intra-uterine insemination outcome: A systematic review and meta-analysis. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 244, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Noonan, E.; von Eckardstein, E.S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, C.E.; Verstraete, L.; Berth, M.; Beert, J.; Bogers, J.P.; Salembier, G.; Vereecken, A.J.; Bosmans, E. Human Papillomavirus Positivity in Women Undergoing Intrauterine Insemination Has a Negative Effect on Pregnancy Rates. Gynecol. Obs. Invest. 2016, 81, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W.; Vandeput, H.; Van de Putte, G.; Cox, A.; Janssen, M.; Jacobs, P.; Bosmans, E.; Steeno, O.; Kruger, T. Intrauterine insemination after ovarian stimulation with clomiphene citrate: Predictive potential of inseminating motile count and sperm morphology. Hum. Reprod. 1997, 12, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, C.E.; Benoy, I.H.; Beert, J.F.A.; Criel, A.M.; Bogers, J.J.; Arbyn, M. Clinical Validation of a Type-Specific Real-Time Quantitative Human Papillomavirus PCR against the Performance of Hybrid Capture 2 for the Purpose of Cervical Cancer Screening. J. Clin. Microbiol. 2012, 50, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Micalessi, I.M.; Boulet, G.A.; Bogers, J.J.; Benoy, I.H.; Depuydt, C.E. High-throughput detection, genotyping and quantification of the human papillomavirus using real-time PCR. Clin. Chem. Lab. Med. 2011, 50, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, C.E.; Boulet, G.A.; Horvath, C.A.; Benoy, I.H.; Vereecken, A.J.; Bogers, J.J. Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J. Cell. Mol. Med. 2007, 11, 881–891. [Google Scholar] [CrossRef]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm chromatin structure assay: Its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef]
- Thijssen, A.; Creemers, A.; Van der Elst, W.; Creemers, E.; Vandormael, E.; Dhont, N.; Ombelet, W. Predictive value of different covariates influencing pregnancy rate following intrauterine insemination with homologous semen: A prospective cohort study. Reprod. Biomed. Online 2017, 34, 463–472. [Google Scholar] [CrossRef]
- Thijssen, A.; Creemers, A.; Van der Elst, W.; Creemers, E.; Vandormael, E.; Dhont, N.; Ombelet, W. Predictive factors influencing pregnancy rates after intrauterine insemination with frozen donor semen: A prospective cohort study. Reprod. Biomed. Online 2017, 34, 590–597. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, M.J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, F.; Zalata, A.; Depuydt, C.E.; Comhaire, F.H. MedCalc: A new computer program for medical statistics. Comput. Methods Programs Biomed. 1995, 48, 257–262. [Google Scholar] [CrossRef]
- De Kretser, D.M. Male infertility. Lancet 1997, 349, 787–790. [Google Scholar] [CrossRef]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European Association of Urology guidelines on Male Infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef]
- Hamada, A.; Esteves, S.C.; Nizza, M.; Agarwal, A. Unexplained male infertility: Diagnosis and management. Int. Braz. J. Urol. 2012, 38, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Duran, E.H.; Morshedi, M.; Taylor, S.; Oehninger, S. Sperm DNA quality predicts intrauterine insemination outcome: A prospective cohort study. Hum. Reprod. 2002, 17, 3122–3128. [Google Scholar] [CrossRef]
- Laprise, C.; Trottier, H.; Monnier, P.; Coutlee, F.; Mayrand, M.H. Prevalence of human papillomaviruses in semen: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Kaspersen, M.D.; Larsen, P.B.; Ingerslev, H.J.; Fedder, J.; Petersen, G.B.; Bonde, J.; Höllsberg, P. Identification of multiple HPV types on spermatozoa from human sperm donors. PLoS ONE 2011, 6, e18095. [Google Scholar] [CrossRef]
- Fedder, J.; Ørnskov, D.; Engvad, B.; Kristensen, T.K.; Lomholt, M.; Marcussen, N.; Waldström, M. Seminal human papillomavirus originates from the body surface and is not a frequent aetiological factor in azoospermia. Andrologia 2019, 51, e13202. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Donders, G.; Verstraete, L.; Vanden Broeck, D.; Beert, J.; Salembier, G.; Bosmans, E.; Dhont, N.; Van Der Auwera, I.; Vandenborne, K.; et al. Time has come to include Human Papillomavirus (HPV) testing in sperm donor banks. Facts Views Vis. ObGyn 2018, 10, 201–205. [Google Scholar]
- Pellavio, G.; Todaro, F.; Alberizzi, P.; Scotti, C.; Gastaldi, G.; Lolicato, M.; Omes, C.; Caliogna, L.; Nappi, R.E.; Laforenza, U. HPV Infection Affects Human Sperm Functionality by Inhibition of Aquaporin-8. Cells 2020, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Huang, C.T.; King, A.; Chan, P.J. Differential effects of human papillomavirus DNA types on p53 tumor-suppressor gene apoptosis in sperm. Gynecol. Oncol. 2002, 85, 511–516. [Google Scholar] [CrossRef]
- Connelly, D.A.; Chan, P.J.; Patton, W.C.; King, A. Human sperm deoxyribonucleic acid fragmentation by specific types of papillomavirus. Am. J. Obs. Gynecol. 2001, 184, 1068–1070. [Google Scholar] [CrossRef]
- Kaspersen, M.D.; Bungum, M.; Fedder, J.; Bonde, J.; Larsen, P.B.J.; Ingerslev, H.; Höllsberg, P. No increased sperm DNA fragmentation index in semen containing human papillomavirus or herpesvirus. Andrology 2013, 1, 361–364. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; Fernández, J.L.; de la Pérez, L.O.; Garza-Flores, M.E.; Eguren-Garza, R.; Gosálvez, J. The presence of human papillomavirus in semen does not affect the integrity of sperm DNA. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Chan, P.J.; Seraj, I.M.; Kalugdan, T.H.; King, A. Evidence for ease of transmission of human papillomavirus DNA from sperm to cells of the uterus and embryo. J. Assist. Reprod. Genet. 1996, 13, 516–519. [Google Scholar] [CrossRef]
- Henneberg, A.A.; Patton, W.C.; Jacobson, J.D.; Chan, P.J. Human papilloma virus DNA exposure and embryo survival is stage-specific. J. Assist. Reprod. Genet. 2006, 23, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lee, J.H.; Fulp, W.; Villa, L.L.; Lazcano, E.; Papenfuss, M.R.; Abrahamsen, M.; Salmeron, J.; Anic, G.M.; Rollison, D.E.; et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): A cohort study. Lancet 2011, 377, 932–940. [Google Scholar] [CrossRef]
- Hamilton, T.R.D.S.; Assumpcao, M.E.O.D. Sperm DNA fragmentation: Causes and identification. Zygote 2020, 28, 1–8. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Mitra, A.; Arbyn, M.; Stasinou, S.M.; Martin-Hirsch, P.; Bennett, P.; Paraskevaidis, E. Fertility and early pregnancy outcomes after treatment for cervical intraepithelial neoplasia: Systematic review and meta-analysis. BMJ 2014, 349, g6192. [Google Scholar] [CrossRef]
- Garolla, A.; De Toni, L.; Bottacin, A.; Valente, U.; De Rocco Ponce, M.; Di Nisio, A.; Foresta, C. Human Papillomavirus Prophylactic Vaccination improves reproductive outcome in infertile patients with HPV semen infection: A retrospective study. Sci. Rep. 2018, 8, 912. [Google Scholar] [CrossRef]
- Rex, A.S.; Wu, C.; Aagaard, J.; Fedder, J. Implementation of an in-house flow cytometric analysis of DNA fragmentation in spermatozoa. Asian J. 2020, 22, 246–251. [Google Scholar] [CrossRef]
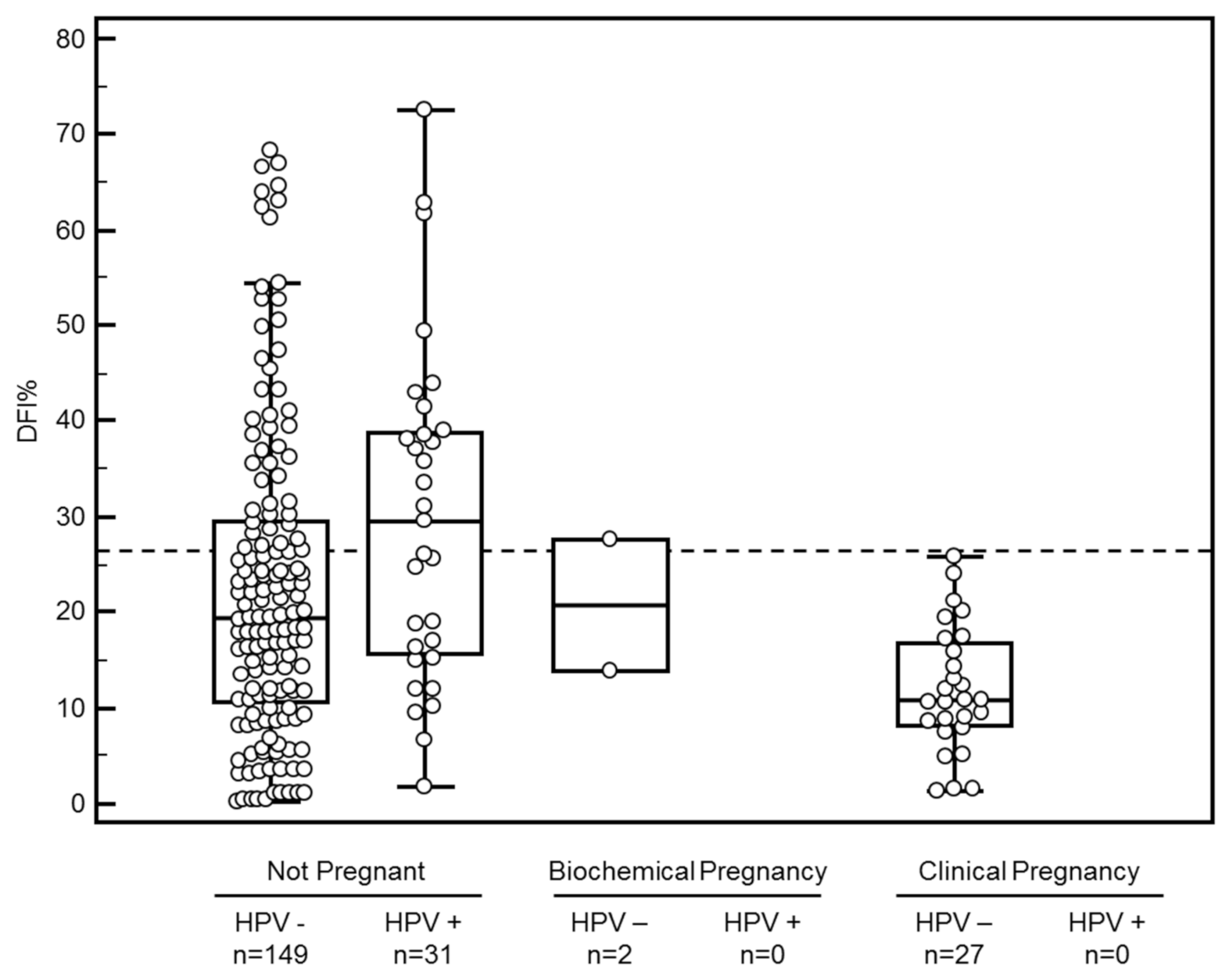
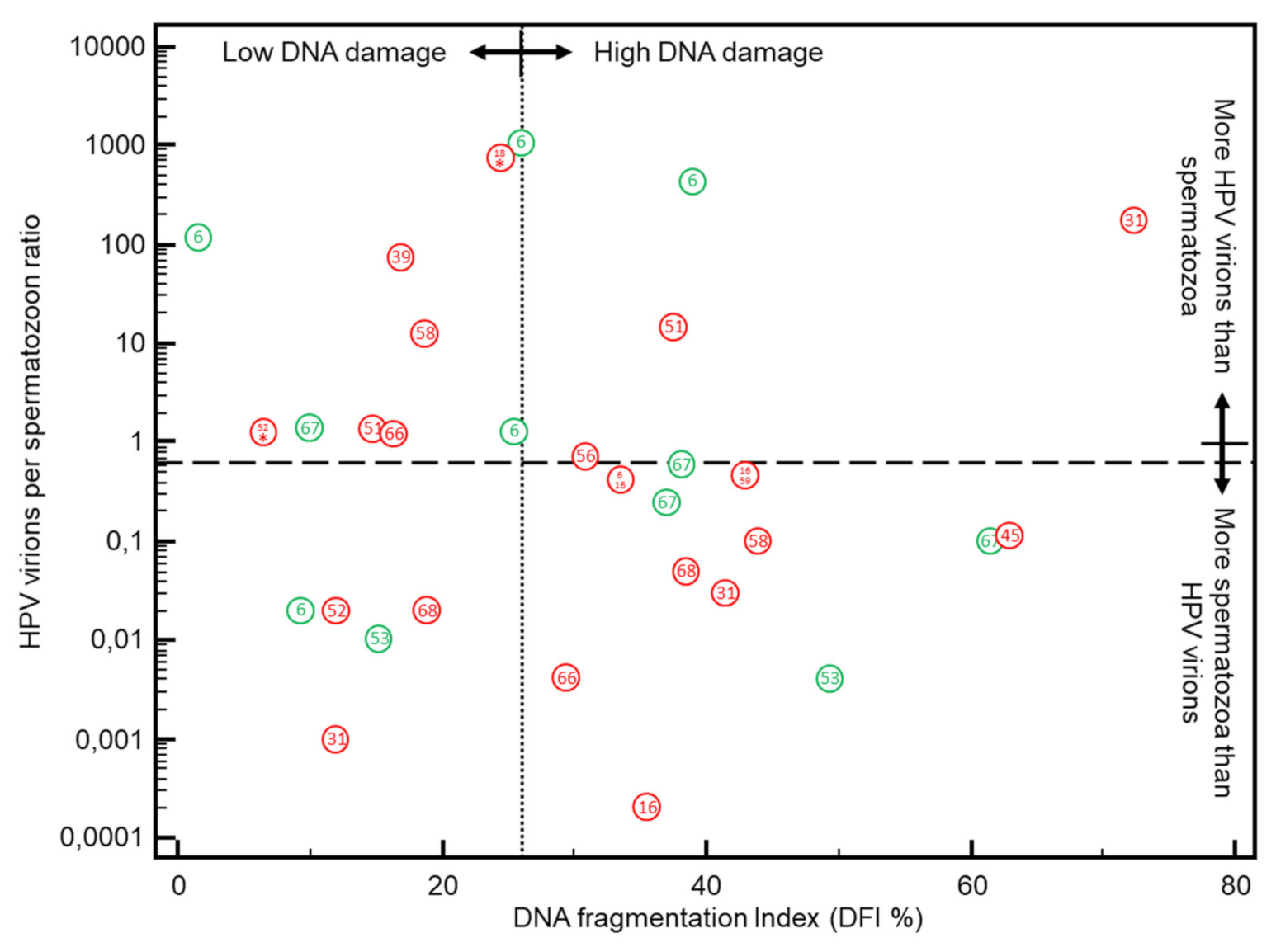
| All | DFI ≤ 26% | DFI > 26% | p-Value | HPV-Negative | HPV-Positive | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IUI Cycles Included (n; %) | 209 | 100 | 144 | 68.9 | 65 | 31.1 | 178 | 85.2 | 31 | 14.8 | ||
| Female Age (mean; 95% CI)(Years) | 32.3 | 31.5 to 33.1 | 32.3 | 31.3 to 33.2 | 32.3 | 30.9 to 33.7 | NS | 32.2 | 31.3 to 33.1 | 32.7 | 30.7 to 34.7 | NS |
| Male Age (mean; 95% CI)(Years) | 34.9 | 34.0 to 35.9 | 34.1 | 33.1 to 35.2 | 36.5 | 34.5 to 38.4 | 0.0259 | 34.8 | 33.8 to 35.8 | 35.6 | 32.7 to 38.6 | NS |
| Sperm Concentration (Mean; 95% CI) (106/mL) | 47.3 | 40.3 to 54.4 | 49.4 | 40.6 to 58.3 | 43.1 | 31.3 to 54.9 | NS | 49.4 | 41.9 to 56.8 | 35.9 | 14.9 to 56.9 | NS |
| Progressive Sperm Concentration (Mean; 95% CI) (106/mL) | 58.3 | 48.1 to 68.4 | 61.8 | 49.2 to 74.4 | 51.0 | 33.4 to 68.5 | NS | 61.1 | 49.9 to 72.3 | 42.2 | 18.8 to 66.7 | NS |
| Percentage Progressive Sperm (Mean; 95% CI) (%) | 40.5 | 38.0 to 43.0 | 43.5 | 40.6 to 46.4 | 34.2 | 29.7 to 38.7 | 0.0007 | 42.2 | 39.6 to 44.7 | 30.9 | 23.4 to 38.3 | 0.0058 |
| Sperm Morphology (Mean; 95% CI) (%) | 3.8 | 3.5 to 4.2 | 4.1 | 3.7 to 4.6 | 3.2 | 2.6 to 3.7 | 0.0069 | 3.9 | 3.5 to 4.3 | 3.3 | 2.3 to 4.3 | NS |
| IMC (mean; 95% CI)(106/mL) | 9.5 | 7.7 to 11.4 | 10.4 | 8.0 to 12.9 | 8.1 | 5.6 to 10.6 | NS | 10.6 | 8.6 to 12.7 | 2.8 | 1.1 to 4.3 | 0.0086 |
| DFI (mean; 95% CI) (%) | 22.2 | 20.0 to 24.4 | 13.3 | 12.1 to 14.6 | 41.9 | 38.7 to 45.1 | <0.0001 | 20.9 | 18.6 to 23.2 | 29.8 | 23.4 to 36.2 | 0.0111 |
| HDS (mean; 95% CI) (%) | 3.6 | 3.2 to 4.0 | 3.4 | 2.9 to 3.9 | 3.8 | 3.2 to 4.4 | NS | 3.3 | 2.9 to 3.7 | 5.0 | 3.5 to 6.5 | 0.0025 |
| Normal Semen Parameters (WHO 2010) (n) (%/IUI cycle) | 78 | 37.3 | 64 | 82.1 | 17 | 17.9 | <0.0001 | 72 | 92.3 | 6 | 7.7 | <0.0001 |
| Biochemical Pregnancies (n) (%/IUI cycle) | 2 | 1.0 | 1 | 0.7 | 1 | 1.5 | NS | 2 | 1.1 | 0 | 0 | NS |
| Clinical Pregnancies (n) (%/IUI cycle) | 27 | 12.9 | 27 | 18.8 | 0 | 0 | 0.0004 | 27 | 15.2 | 0 | 0 | 0.0417 |
| HPV Type | Single | Multiple | Total | DFI ≤ 26 | DFI > 26 | |||
|---|---|---|---|---|---|---|---|---|
| n | n | n | % | n | % | n | % | |
| 6 | 5 | 2 | 7 | 22.6 | 4 | 80 | 1 | 20 |
| 11 | - | - | - | - | - | - | - | |
| 16 | 1 | 2 | 3 | 9.7 | 0 | 0 | 3 | 100 |
| 18 | - | 1 | 1 | 3.2 | 1 | 100 | 0 | 0 |
| 31 | 3 | - | 3 | 9.7 | 1 | 33 | 2 | 67 |
| 33 | - | - | - | - | - | - | - | - |
| 35 | - | - | - | - | - | - | - | - |
| 39 | 1 | - | 1 | 3.2 | 1 | 100 | 0 | 0 |
| 45 | 1 | - | 1 | 3.2 | 0 | 0 | 1 | 100 |
| 51 | 2 | - | 2 | 6.5 | 1 | 50 | 1 | 50 |
| 52 | 1 | 1 | 2 | 6.5 | 2 | 100 | 0 | 0 |
| 53 | 2 | 1 | 3 | 9.7 | 1 | 50 | 1 | 50 |
| 56 | 1 | 1 | 2 | 6.5 | 0 | 0 | 1 | 100 |
| 58 | 2 | - | 2 | 6.5 | 1 | 50 | 1 | 50 |
| 59 | - | 2 | 2 | 6.5 | - | - | - | - |
| 66 | 2 | 1 | 3 | 9.7 | 1 | 50 | 1 | 50 |
| 67 | 4 | - | 4 | 12.9 | 1 | 25 | 3 | 75 |
| 68 | 2 | - | 2 | 6.5 | 1 | 50 | 1 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depuydt, C.; Donders, G.; Verstraete, L.; Beert, J.; Salembier, G.; Bosmans, E.; Dhont, N.; Kerkhofs, C.; Ombelet, W. Negative Impact of Elevated DNA Fragmentation and Human Papillomavirus (HPV) Presence in Sperm on the Outcome of Intra-Uterine Insemination (IUI). J. Clin. Med. 2021, 10, 717. https://doi.org/10.3390/jcm10040717
Depuydt C, Donders G, Verstraete L, Beert J, Salembier G, Bosmans E, Dhont N, Kerkhofs C, Ombelet W. Negative Impact of Elevated DNA Fragmentation and Human Papillomavirus (HPV) Presence in Sperm on the Outcome of Intra-Uterine Insemination (IUI). Journal of Clinical Medicine. 2021; 10(4):717. https://doi.org/10.3390/jcm10040717
Chicago/Turabian StyleDepuydt, Christophe, Gilbert Donders, Ludo Verstraete, Johan Beert, Geert Salembier, Eugene Bosmans, Nathalie Dhont, Carmen Kerkhofs, and Willem Ombelet. 2021. "Negative Impact of Elevated DNA Fragmentation and Human Papillomavirus (HPV) Presence in Sperm on the Outcome of Intra-Uterine Insemination (IUI)" Journal of Clinical Medicine 10, no. 4: 717. https://doi.org/10.3390/jcm10040717
APA StyleDepuydt, C., Donders, G., Verstraete, L., Beert, J., Salembier, G., Bosmans, E., Dhont, N., Kerkhofs, C., & Ombelet, W. (2021). Negative Impact of Elevated DNA Fragmentation and Human Papillomavirus (HPV) Presence in Sperm on the Outcome of Intra-Uterine Insemination (IUI). Journal of Clinical Medicine, 10(4), 717. https://doi.org/10.3390/jcm10040717







