The Impact of Patient Age ≥80 Years on Postoperative Outcomes and Treatment Costs Following Pancreatic Surgery
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Inclusion Criteria
2.2. Preoperative Management
2.3. Surgical Procedure
2.4. Postoperative Management
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and Postoperative Outcomes in the Entire Cohort
3.2. Comparison of Patients Undergoing Pancreaticoduodenectomy
3.3. Comparison of Patients Undergoing Surgery for Pancreatic Ductal Adenocarcinoma
3.4. Factors Associated with Postoperative Mortality
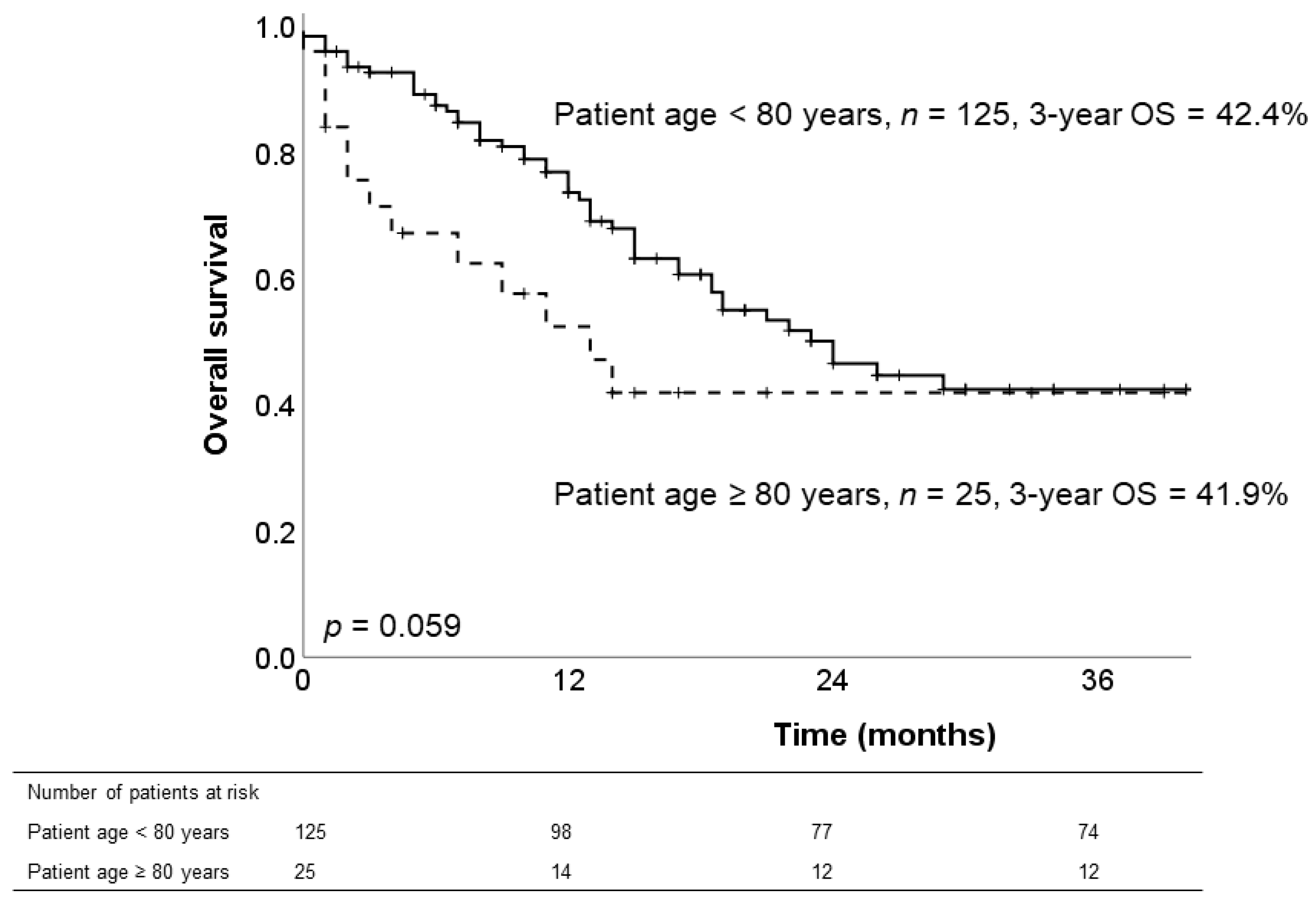
3.5. Long-Term Survival of Patients with PDAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Balcom, J.H.R.D., 4th; Warshaw, A.L.; Chang, Y.; Fernandez-del Castillo, C. Ten-year experience with 733 pancreatic resections: Changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001, 136, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, C.; Takaori, K.; Abu Hilal, M.; Kooby, D.A.; Wakabayashi, G.; Agarwal, A.; Berti, S.; Besselink, M.G.; Chen, K.H.; Gumbs, A.A.; et al. International Summit on Laparoscopic Pancreatic Resection (ISLPR) “Coimbatore Summit Statements”. Surg. Oncol. 2018, 27, A10–A15. [Google Scholar] [CrossRef]
- Cameron, J.L.; He, J. Two Thousand Consecutive Pancreaticoduodenectomies. J. Am. Coll. Surg. 2015, 220, 530–536. [Google Scholar] [CrossRef]
- van Rijssen, L.B.; Zwart, M.J.; van Dieren, S.; de Rooij, T.; Bonsing, B.A.; Bosscha, K.; van Dam, R.M.; van Eijck, C.H.; Gerhards, M.F.; Gerritsen, J.J.; et al. Variation in hospital mortality after pancreatoduodenectomy is related to failure to rescue rather than major complications: A nationwide audit. HPB 2018, 20, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Clinical Management and Surgical Decision-Making of IPMN of the Pancreas. Methods Mol. Biol. 2019, 1882, 9–22. [Google Scholar] [PubMed]
- Schnelldorfer, T.; Lewin, D.N.; Adams, D.B. Operative Management of Chronic Pancreatitis: Longterm Results in 372 Patients. J. Am. Coll. Surg. 2007, 204, 1039–1045. [Google Scholar] [CrossRef]
- Belyaev, O.; Herzog, T.; Kaya, G.; Chromik, A.M.; Meurer, K.; Uhl, W.; Müller, C.A. Pancreatic Surgery in the Very Old: Face to Face With a Challenge of the Near Future. World J. Surg. 2013, 37, 1013–1020. [Google Scholar] [CrossRef]
- Makary, M.A.; Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Chang, D.; Cunningham, S.C.; Riall, T.S.; Yeo, C.J. Pancreaticoduodenectomy in the Very Elderly. J. Gastrointest. Surg. 2006, 10, 347–356. [Google Scholar] [CrossRef]
- Wu, W.; He, X.; Yang, L.; Wang, Q.; Bian, X.; Ye, J.; Li, Y.; Li, L. Rising trends in pancreatic cancer incidence and mortality in 2000–2014. Clin. Epidemiol. 2018, 10, 789–797. [Google Scholar] [CrossRef]
- Riall, T.S.; Reddy, D.M.; Nealon, W.H.; Goodwin, J.S. The effect of age on short-term outcomes after pancreatic resection: A population-based study. Ann. Surg. 2008, 248, 459–467. [Google Scholar] [CrossRef]
- Janssen, Q.P.; Buettner, S.; Suker, M.; Beumer, B.R.; Addeo, P.; Bachellier, P.; Bahary, N.; Bekaii-Saab, T.; Bali, M.A.; Besselink, M.G.; et al. Neoadjuvant FOLFIRINOX in Patients with Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 782–794. [Google Scholar] [CrossRef]
- Hatzaras, I.; Schmidt, C.; Klemanski, D.; Muscarella, P.; Melvin, W.S.; Ellison, E.C.; Bloomston, M. Pancreatic Resection in the Octogenarian: A Safe Option for Pancreatic Malignancy. J. Am. Coll. Surg. 2011, 212, 373–377. [Google Scholar] [CrossRef]
- Finlayson, E.; Fan, Z.; Birkmeyer, J.D. Outcomes in Octogenarians Undergoing High-Risk Cancer Operation: A National Study. J. Am. Coll. Surg. 2007, 205, 729–734. [Google Scholar] [CrossRef]
- Groen, J.V.; Douwes, T.A.; Van Eycken, E.; Van Der Geest, L.G.M.; Johannesen, T.B.; Besselink, M.G.; Koerkamp, B.G.; Wilmink, J.W.; Bonsing, B.A.; on behalf of the Dutch Pancreatic Cancer Group; et al. Treatment and Survival of Elderly Patients with Stage I–II Pancreatic Cancer: A Report of the EURECCA Pancreas Consortium. Ann. Surg. Oncol. 2020, 27, 5337–5346. [Google Scholar] [CrossRef]
- Kachare, S.D.; Liner, K.R.; Vohra, N.A.; Zervos, E.E.; Hickey, T.; Fitzgerald, T.L. Assessment of health care cost for complex surgical patients: Review of cost, re-imbursement and revenue involved in pancreatic surgery at a high-volume academic medical centre. HPB 2015, 17, 311–317. [Google Scholar] [CrossRef]
- Wang, J.; Ma, R.; Churilov, L.; Eleftheriou, P.; Nikfarjam, M.; Christophi, C.; Weinberg, L. The cost of perioperative complications following pancreaticoduodenectomy: A systematic review. Pancreatology 2018, 18, 208–220. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Gøtzsche, P.C.; Vandenbroucke, J.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.; Moir, J.; Damm, M.; Maggino, L.; Kordes, M.; Rosendahl, J.; Ceyhan, G.O.; Schorn, S.; Schmid, D.; D’Haese, J.G.; et al. Respect—A multicenter retrospective study on preoperative chemotherapy in locally advanced and borderline resectable pancreatic cancer. Pancreatology 2020, 20, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Trestini, I.; Paiella, S.; Sandini, M.; Sperduti, I.; Elio, G.; Pollini, T.; Melisi, D.; Auriemma, A.; Soldà, C.; Bonaiuto, C.; et al. Prognostic Impact of Preoperative Nutritional Risk in Patients Who Undergo Surgery for Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 5325–5334. [Google Scholar] [CrossRef] [PubMed]
- Keck, T.; Wellner, U.F.; Bahra, M.; Klein, F.; Sick, O.; Niedergethmann, M.; Wilhelm, T.J.; Farkas, S.A.; Börner, T.; Bruns, C.J.; et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann. Surg. 2016, 263, 440–449. [Google Scholar] [CrossRef]
- Yang, P.-C.; Huang, K.-W.; Pua, U.; Kim, M.-D.; Li, S.-P.; Li, X.-Y.; Liang, P.-C. Prognostic factor analysis of irreversible electroporation for locally advanced pancreatic cancer—A multi-institutional clinical study in Asia. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 811–817. [Google Scholar] [CrossRef]
- Holzgang, M.; Eigl, B.; Erdem, S.; Gloor, B.; Worni, M. Irreversible Electroporation in Pancreatic Cancer. Adv. Pancreat. Cancer 2018. [Google Scholar] [CrossRef][Green Version]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.J.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH)–An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2012, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Bettelli, G. Preoperative evaluation of the elderly surgical patient and anesthesia challenges in the XXI century. Aging Clin. Exp. Res. 2018, 30, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Shahrokni, A.; Vishnevsky, B.M.; Jang, B.; Sarraf, S.; Alexander, K.; Kim, S.J.; Downey, R.; Afonso, A.; Korc-Grodzicki, B. Geriatric Assessment, Not ASA Physical Status, Is Associated With 6-Month Postoperative Survival in Patients with Cancer Aged >/=75 Years. J. Natl. Compr. Cancer Netw. 2019, 17, 687–694. [Google Scholar] [CrossRef]
- Augustin, T.; Burstein, M.D.; Schneider, E.B.; Morris-Stiff, G.; Wey, J.; Chalikonda, S.; Walsh, R.M. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery 2016, 160, 987–996. [Google Scholar] [CrossRef]
- Merath, K.; Mehta, R.; Tsilimigras, D.I.; Farooq, A.; Sahara, K.; Paredes, A.Z.; Wu, L.; Ejaz, A.; Pawlik, T.M. In-hospital Mortality Following Pancreatoduodenectomy: A Comprehensive Analysis. J. Gastrointest. Surg. 2019, 24, 1119–1126. [Google Scholar] [CrossRef]
- De Hert, S.; Staender, S.; Fritsch, G.; Hinkelbein, J.; Afshari, A.; Bettelli, G.; Bock, M.; Chew, M.S.; Coburn, M.; de Robertis, E.; et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: Updated guideline from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2018, 35, 407–465. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, P.; Fumarola, E.M.; Ierardi, A.M.; Carrafiello, G. Bleeding complications after pancreatic surgery: Interventional radiology management. Gland. Surg. 2019, 8, 150–163. [Google Scholar] [CrossRef]
- Akgul, O.; Merath, K.; Mehta, R.; Hyer, J.M.; Chakedis, J.; Wiemann, B.; Johnson, M.; Paredes, A.; Dillhoff, M.; Cloyd, J.M.; et al. Postoperative Pancreatic Fistula Following Pancreaticoduodenectomy—Stratification of Patient Risk. J. Gastrointest. Surg. 2019, 23, 1817–1824. [Google Scholar] [CrossRef]
- Roberts, K.J.; Hodson, J.; Mehrzad, H.; Marudanayagam, R.; Sutcliffe, R.P.; Muiesan, P.; Isaac, J.; Bramhall, S.R.; Mirza, D.F. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB 2014, 16, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Wellner, U.F.; Kayser, G.; Lapshyn, H.; Sick, O.; Makowiec, F.; Höppner, J.; Hopt, U.T.; Keck, T. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB 2010, 12, 696–702. [Google Scholar] [CrossRef]
- Bicakli, D.H.; Uslu, R.; Güney, S.C.; Coker, A. The Relationship between Nutritional Status, Performance Status, and Survival among Pancreatic Cancer Patients. Nutr. Cancer 2020, 72, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Rangelova, E.; Segersvard, R.; Arnelo, U. Are there still indications for total pancreatectomy? Updates Surg. 2016, 68, 257–263. [Google Scholar] [CrossRef]
- Mackay, T.M.; Smits, F.J.; Roos, D.; Bonsing, B.A.; Bosscha, K.; Busch, R.; Creemers, G.; van Dam, M.; van Eijck, H.J.; Gerhards, F.; et al. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: A nationwide analysis. HPB 2019, 22, 233–240. [Google Scholar] [CrossRef]
- Oliveira-Cunha, M.; Malde, D.J.; Aldouri, A.; Morris-Stiff, G.; Menon, K.V.; Smith, A.M. Results of pancreatic surgery in the elderly: Is age a barrier? HPB 2013, 15, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
| Variable | <80 Years (n = 307) | ≥80 Years (n = 39) | All Patients (n = 346) | p Value |
|---|---|---|---|---|
| Gender, n (%) | 0.106 | |||
| Female | 139 (45) | 23 (59) | 162 (47) | |
| Male | 168 (55) | 16 (41) | 184 (53) | |
| cost recovery, %, median (range) | 102 (38–233) | 94 (46–172) | 101 (38–233) | 0.046 |
| cardiovascular disease, n (%) | 157 (51) | 33 (85) | 190 (55) | <0.0001 |
| Kidney disease, n (%) | 41 (13) | 13 (33) | 53 (15) | 0.001 |
| Pulmonary disease, n (%) | 71 (23) | 8 (21) | 79 (23) | 0.714 |
| Infectious disease, n (%) | 11 (4) | 1 (3) | 12 (4) | 0.743 |
| Liver disease, n (%) | 43 (14) | 7 (18) | 50 (15) | 0.510 |
| Neurologic disease, n (%) | 49 (16) | 6 (15) | 55 (16) | 0.926 |
| Diabetes, n (%) | 73 (24) | 11 (28) | 84 (24) | 0.544 |
| Genetical alteration, n (%) | 9 (3) | 0 (0) | 9 (3) | 0.279 |
| BMI, kg/m2, median (range) | 24 (16–46) | 24 (16–31) | 24 (16–46) | 0.167 |
| BMI > 30, kg/m2, n (%) | 48 (16) | 4 (19) | 52 (15) | 0.376 |
| Smoking, n (%) | 131 (43) | 3 (8) | 134 (39) | <0.0001 |
| Alcohol consumption, n (%) | 106 (35) | 6 (15) | 112 (32) | 0.016 |
| ASA status, n (%) | 0.009 | |||
| 1 | 7 (2) | 0 (0) | 7 (2) | |
| 2 | 98 (32) | 5 (13) | 103 (30) | |
| 3 | 190 (62) | 29 (74) | 219 (63) | |
| 4 | 12 (4) | 5 (13) | 17 (5) | |
| Resection type, n (%) | 0.122 | |||
| Pancreatoduodenectomy | 159 (52) | 26 (67) | 185 (54) | |
| Distal pancreatectomy | 63 (21) | 6 (15) | 69 (20) | |
| Total pancreatectomy | 38 (12) | 7 (18) | 45 (13) | |
| Enucleation | 3 (1) | 0 (0) | 3 (1) | |
| Duodenal-preserving resection | 24 (8) | 0 (0) | 24 (7) | |
| Segmental pancreatic resection | 20 (6) | 0 (0) | 20 (5) | |
| Operating time, h, median (range) | 5.0 (1–10) | 5.75 (3–9) | 5.2 (1–10) | 0.014 |
| Vascular reconstruction, n (%) | 0.638 | |||
| none | 240 (78) | 28 (72) | 268 (78) | |
| venous | 56 (19) | 10 (26) | 66 (19) | |
| arterial | 7 (2) | 1 (2) | 8 (2) | |
| combined | 4 (1) | 0 (0) | 4 (1) | |
| Histologic type, n (%) | 0.006 | |||
| Adenocarcinoma | 125 (41) | 25 (64) | 150 (43) | |
| other | 182 (59) | 14 (36) | 196 (57) | |
| Pancreatic pathologies, n (%) | 0.127 | |||
| PDAC | 125 (41) | 25 (64) | 150 (43) | |
| periampullary malignancies | 46 (15) | 6 (16) | 52 (15) | |
| cystic lesions | 64 (21) | 5 (13) | 69 (20) | |
| neuroendocrine tumors | 28 (9) | 2(5) | 30 (9) | |
| chronic pancreatitis | 44 (14) | 1 (2) | 45 (13) | |
| Length of ICU stay, days, median (range) | 1 (0–37) | 1 (1–19) | 1 (0–37) | 0.065 |
| Length of hospital stay, days, median (range) | 13 (2–87) | 13 (3–66) | 13 (2–87) | 0.372 |
| Readmission within 90 days, n (%) | 53 (17) | 6 (17) | 59 (17) | 0.908 |
| Postoperative morbidity, n (%) | 185 (60) | 25 (64) | 210 (61) | 0.664 |
| Major postoperative morbidity, n (%) | 73 (24) | 12 (31) | 85 (25) | 0.339 |
| Postoperative mortality, n (%) | 6 (2.0) | 6 (15.4) | 12 (3.5) | <0.0001 |
| POPF, n (%) | 0.035 | |||
| None | 221 (72) | 31 (79) | 252 (73) | |
| Type A | 43 (14) | 3 (8) | 46 (13) | |
| Type B | 9 (28) | 0 (0) | 28 (8) | |
| Type C | 15 (5) | 5 (13) | 20 (6) | |
| Re-operation, n (%) | 42 (14) | 9 (23) | 51 (15) | 0.119 |
| Postoperative hemorrhage, n (%) | 15 (5) | 7 (18) | 22 (6) | 0.002 |
| Wound infection, n (%) | 60 (20) | 8 (21) | 68 (20) | 0.886 |
| Pulmonary complication, n (%) | 34 (11) | 10 (26) | 44 (13) | 0.010 |
| Cardiovascular complication, n (%) | 15 (5) | 8 (21) | 23 (7) | <0.0001 |
| Renal complication, n (%) | 15 (5) | 3 (8) | 18 (5) | 0.457 |
| Variable | <80 Years (n = 159) | ≥80 Years (n = 26) | All Patients (n = 185) | p Value |
|---|---|---|---|---|
| Gender, n (%) | 0.032 | |||
| Female | 74 (47) | 18 (69) | 92 (50) | |
| Male | 85 (53) | 8 (31) | 93 (50) | |
| Cost recovery, %, median (range) | 105 (42–233) | 91 (46–172) | 102 (42–233) | 0.045 |
| Heart disease, n (%) | 82 (52) | 23 (89) | 106 (57) | <0.0001 |
| Kidney disease, n (%) | 21 (13) | 8 (31) | 29 (16) | 0.022 |
| Smoking, n (%) | 58 (37) | 1 (4) | 59 (32) | 0.001 |
| Alcohol consumption, n (%) | 54 (34) | 2 (8) | 56 (30) | 0.007 |
| ASA status, n (%) | 0.123 | |||
| 1 | 1 (1) | 0 (0) | 1 (1) | |
| 2 | 49 (31) | 3 (12) | 52 (28) | |
| 3 | 102 (64) | 20 (77) | 122 (66) | |
| 4 | 7 (4) | 3 (11) | 10 (5) | |
| Diameter pancreatic duct, mm, median (range) | 4 (1–15) | 4 (3–8) | 4 (1–15) | 0.080 |
| Pancreatic stent intraoperative, n (%) | 37 (24) | 1 (4) | 28 (21) | 0.021 |
| Histologic type, n (%) | 0.603 | |||
| Adenocarcinoma | 83 (52) | 15 (58) | 98 (53) | |
| other | 76 (48) | 11 (42) | 87 (47) | |
| Length of ICU stay, days, median (range) | 1 (0–26) | 1 (1–11) | 1 (0–26) | 0.190 |
| Length of hospital stay, days, median (range) | 13 (7–67) | 13 (3–48) | 13 (3–67) | 0.973 |
| Readmission within 90 days, n (%) | 26 (17) | 5 (20) | 31 (17) | 0.661 |
| Postoperative morbidity, n (%) | 86 (54) | 19 (73) | 105 (57) | 0.07 |
| Major postoperative morbidity, n (%) | 36 (23) | 9 (35) | 45 (24) | 0.187 |
| Postoperative mortality, n (%) | 2 (1.3) | 3 (11.5) | 5 (2.7) | 0.003 |
| POPF, n (%) | 0.452 | |||
| None | 123 (77) | 20 (77) | 143 (77) | |
| Type A | 12 (8) | 2 (8) | 14 (8) | |
| Type B | 10 (6) | 0 (0) | 10 (5) | |
| Type C | 14 (9) | 4 (15) | 18 (10) | |
| Re-operation, n (%) | 24 (15) | 7 (27) | 31 (17) | 0.134 |
| Salvage pancreatectomy, n (%) | 13 (8) | 4 (15) | 17 (9) | 0.432 |
| Bile leak, n (%) | 4 (3) | 0 (0) | 4 (2) | 0.414 |
| Gastrointestinal leak, n (%) | 2 (1) | 0 (0) | 2 (1) | 0.565 |
| Postoperative hemorrhage, n (%) | 7 (4) | 5 (19) | 12 (7) | 0.004 |
| Wound infection, n (%) | 34 (21) | 7 (27) | 41 (22) | 0.528 |
| Pulmonary complication, n (%) | 20 (13) | 6 (23) | 26 (14) | 0.153 |
| Cardiovascular complication, n (%) | 6 (4) | 6 (23) | 12 (7) | <0.0001 |
| Renal complication, n (%) | 4 (3) | 2 (8) | 6 (3) | 0.167 |
| Variable | <80 Years (n = 125) | ≥80 Years (n = 25) | All Patients (n = 150) | p Value |
|---|---|---|---|---|
| Gender, n (%) | 0.306 | |||
| Female | 56 (45) | 14 (56) | 70 (47) | |
| Male | 69 (55) | 11 (44) | 80 (53) | |
| Cost recovery, %, median (range) | 103 (47–233) | 90 (46–172) | 100 (46–233) | 0.021 |
| Heart disease, n (%) | 72 (58) | 21 (84) | 93 (62) | 0.13 |
| Kidney disease, n (%) | 18 (14) | 9 (36) | 27 (18) | 0.010 |
| Smoking, n (%) | 48 (38) | 3 (12) | 51 (34) | 0.011 |
| Alcohol consumption, n (%) | 42 (34) | 5 (20) | 47 (31) | 0.181 |
| ASA status, n (%) | 0.037 | |||
| 1 | 2 (2) | 0 (0) | 2 (1) | |
| 2 | 33 (26) | 1 (4) | 34 (23) | |
| 3 | 80 (64) | 19 (76) | 99 (66) | |
| 4 | 10 (8) | 5 (20) | 15 (10) | |
| CA 19-9 preoperative, kU/L, median (range) | 360 (2–63690) | 311 (4–11184) | 343 (2–63690) | 0.692 |
| IRE, n (%) | 20 (16) | 2 (8) | 22 (15) | 0.302 |
| Operating time, h, median (range) | 6.0 (2–9) | 6.4 (4–9) | 6.0 (2–9) | 0.484 |
| T stage, n (%) | 0.065 | |||
| T1 | 5 (4) | 0 (0) | 5 (3) | |
| T2 | 45 (36) | 16 (64) | 61 (41) | |
| T3 | 74 (59) | 9 (36) | 83 (55) | |
| T4 | 1 (1) | 0 (0) | 1 (1) | |
| N stage, n (%) | 0.221 | |||
| N0 | 22 (18) | 8 (32) | 30 (20) | |
| N1 | 74 (59) | 11 (44) | 85 (57) | |
| N3 | 29 (23) | 6 (24) | 35 (23) | |
| Lymph node ratio, median (range) | 3 (0–37)/ 28 (6–107) | 2 (0–10)/ 23 (19–62) | 2 (0–37)/ 28 (6–107) | 0.169 |
| Lymphagiosis carcinomatosa, n (%) | 25 (20) | 32 (8) | 33 (22) | 0.186 |
| Venous invasion, n (%) | 16 (13) | 7 (28) | 23 (15) | 0.054 |
| Perineural invasion, n (%) | 6 (5) | 2 (8) | 8 (5) | 0.516 |
| Tumor differentiation, n (%) | 0.484 | |||
| G1 | 18 (14) | 3 (12) | 21 (14) | |
| G2 | 50 (40) | 14 (56) | 64 (43) | |
| G3 | 55 (44) | 8 (32) | 63 (42) | |
| G4 | 2 (2) | 0 (0) | 2 (1) | |
| Tumor margins, n (%) | 1.0 | |||
| R1 | 40 (32) | 8 (32) | 48 (32) | |
| R0 | 85 (68) | 17 (68) | 102 (68) | |
| Length of ICU stay, days, median (range) | 1 (0–37) | 1 (1–19) | 1 (0–37) | 0.269 |
| Length of hospital stay, days, median (range) | 13 (2–70) | 12 (3–66) | 13 (2–70) | 0.667 |
| Readmission within 90 days, n (%) | 22 (18) | 3 (13) | 25 (17) | 0.571 |
| Postoperative morbidity, n (%) | 70 (56) | 15 (60) | 86 (57) | 0.713 |
| Major postoperative morbidity, n (%) | 25 (20) | 8 (32) | 33 (22) | 0.186 |
| Postoperative mortality, n (%) | 3 (2.4) | 5 (20) | 8 (5.3) | <0.0001 |
| POPF, n (%) | 0.077 | |||
| None | 104 (83) | 21 (84) | 125 (83) | |
| Type A | 10 (8) | 1 (4) | 11 (7) | |
| Type B | 8 (7) | 0 (0) | 8 (6) | |
| Type C | 3 (2) | 3 (12) | 6 (4) | |
| Re-operation, n (%) | 17 (13) | 7 (28) | 24 (16) | 0.073 |
| Postoperative hemorrhage, n (%) | 6 (5) | 6 (24) | 12 (8) | 0.001 |
| Wound infection, n (%) | 30 (24) | 4 (16) | 34 (23) | 0.383 |
| Pulmonary complication, n (%) | 16 (13) | 6 (24) | 22 (15) | 0.148 |
| Cardiovascular complication, n (%) | 7 (6) | 5 (20) | 12 (8) | 0.015 |
| Renal complication, n (%) | 9 (7) | 2 (8) | 11 (7) | 0.889 |
| Neoadjuvant chemotherapy, n (%) | 13 (10) | 0 (0) | 13 (9) | 0.092 |
| Adjuvant chemotherapy, n (%) | 99 (79) | 12 (48) | 111 (74) | 0.001 |
| Adjuvant radiotherapy, n (%) | 1 (1) | 0 (0) | 1 (1) | 0.654 |
| Variable | Postoperative Mortality (n = 12) | No Postoperative Mortality (n = 334) | All Patients (n = 346) | p Value | MV p Value, OR (CI) |
|---|---|---|---|---|---|
| Male, n (%) | 7 (58) | 177 (53) | 184 (53) | 0.716 | |
| Age ≥80 years, n (%) | 6 (50) | 33 (10) | 39 (11) | <0.0001 | 0.013, 6.71 (1.5–30.3) |
| Heart disease, n (%) | 8 (67) | 182 (55) | 190 (55) | 0.411 | |
| Kidney disease, n (%) | 2 (17) | 52 (16) | 54 (16) | 0.918 | |
| Pulmonary disease, n (%) | 5 (42) | 74 (22) | 79 (23) | 0.114 | |
| Alcohol consumption, n (%) | 2 (17) | 110 (33) | 112 (32) | 0.237 | |
| ASA status 4, n (%) | 1 (8) | 16 (5) | 7 (6) | 0.577 | |
| Resection type, n (%) | 0.295 | ||||
| Pancreatoduodenectomy | 5 (42) | 180 (54) | 185 (53) | ||
| Distal pancreatectomy | 3 (25) | 66 (20) | 69 (20) | ||
| Total pancreatectomy | 4 (33) | 41 (12) | 45 (13) | ||
| Enucleation | 0 (0) | 3 (1) | 3 (1) | ||
| Duodenal-preserving resection | 0 (0) | 24 (7) | 24 (7) | ||
| Segmental pancreatic resection | 0 (0) | 20 (6) | 20 (6) | ||
| Histologic type, n (%) | 0.097 | ||||
| Adenocarcinoma | 8 (67) | 142 (43) | 150 (43) | ||
| other | 4 (33) | 192 (56) | 196 (57) | ||
| Vascular reconstruction, n (%) | 0.001 | NS | |||
| none | 4 (34) | 264 (79) | 268 (78) | ||
| venous | 6 (50) | 60 (18) | 66 (19) | ||
| arterial | 1 (8) | 7 (2) | 8 (2) | ||
| combined | 1 (8) | 3 (1) | 4 (1) | ||
| POPF, n (%) | 0.036 | NS | |||
| None | 7 (59) | 245 (73) | 252 (73) | ||
| Type A | 1 (8) | 45 (14) | 46 (13) | ||
| Type B | 1 (8) | 27 (8) | 28 (8) | ||
| Type C | 3 (25) | 17 (5) | 20 (6) | ||
| Postoperative hemorrhage, n (%) | 6 (50) | 16 (5) | 22 (6) | <0.0001 | 0.001, 12.21 (2.7–55.9) |
| Wound infection, n (%) | 3 (25) | 65 (20) | 68 (20) | 0.635 | |
| Pulmonary complication, n (%) | 6 (50) | 38 (11) | 44 (13) | <0.0001 | NS |
| Cardiovascular complication, n (%) | 5 (42) | 18 (5) | 23 (7) | <0.0001 | NS |
| Renal complication, n (%) | 6 (50) | 12 (4) | 18 (5) | <0.0001 | 0.001, 15.80 (3.1–81.4) |
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Gender | Male | Male | Female | Male | Female | Female |
| Age | 81 | 85 | 80 | 82 | 81 | 84 |
| Comorbidities | no | cardiac | cardiac | cardiac | renal | cardiac |
| ASA status | 2 | 3 | 3 | 3 | 3 | 3 |
| Type of resection | Distal pancreatectomy | Distal pancreatectomy | Whipple procedure | Whipple procedure | Whipple procedure | total pancreatectomy |
| Pancreatic anastomosis | - | - | pancreato-jejunostomy | pancreato-jejunostomy | pancreato-jejunostomy | - |
| Pancreas texture | soft | soft | hard | soft | hard | soft |
| Pancreatic duct diameter (mm) | - | - | 8 | 3 | 4 | - |
| POPF | type A | type C | no | type C | type C | no |
| Postoperative hemorrhage | no | yes | yes | yes | no | yes |
| Re-operation | no | yes | yes | yes | yes | yes |
| Other postoperative complications | myocardial infarction | pneumonia, acute kidney failure | thrombosis of common hepatic artery | aspiration pneumonia | aspiration pneumonia, acute kidney failure | pneumonia, acute kidney failure |
| ICU stay, days | 8 | 19 | 3 | 4 | 7 | 11 |
| Postoperative day of death | 18 | 67 | 3 | 22 | 11 | 52 |
| Cause of death | cardiac arrest | sepsis | liver failure | respiratory failure | sepsis | sepsis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreou, A.; Aeschbacher, P.; Candinas, D.; Gloor, B. The Impact of Patient Age ≥80 Years on Postoperative Outcomes and Treatment Costs Following Pancreatic Surgery. J. Clin. Med. 2021, 10, 696. https://doi.org/10.3390/jcm10040696
Andreou A, Aeschbacher P, Candinas D, Gloor B. The Impact of Patient Age ≥80 Years on Postoperative Outcomes and Treatment Costs Following Pancreatic Surgery. Journal of Clinical Medicine. 2021; 10(4):696. https://doi.org/10.3390/jcm10040696
Chicago/Turabian StyleAndreou, Andreas, Pauline Aeschbacher, Daniel Candinas, and Beat Gloor. 2021. "The Impact of Patient Age ≥80 Years on Postoperative Outcomes and Treatment Costs Following Pancreatic Surgery" Journal of Clinical Medicine 10, no. 4: 696. https://doi.org/10.3390/jcm10040696
APA StyleAndreou, A., Aeschbacher, P., Candinas, D., & Gloor, B. (2021). The Impact of Patient Age ≥80 Years on Postoperative Outcomes and Treatment Costs Following Pancreatic Surgery. Journal of Clinical Medicine, 10(4), 696. https://doi.org/10.3390/jcm10040696







