The Assessment of Risk and Predictors of Sleep Disorders in Patients with Psoriasis—A Questionnaire-Based Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
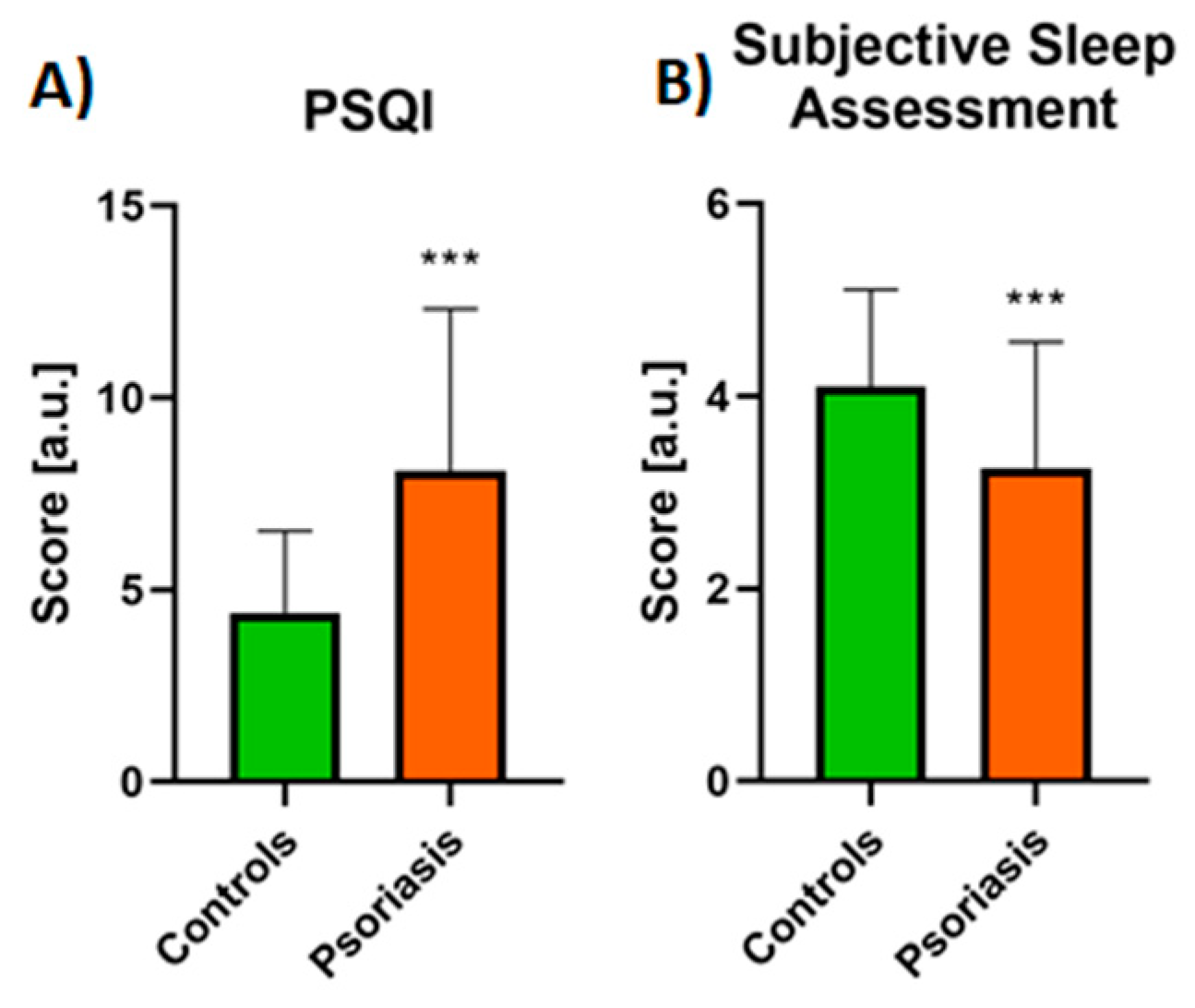
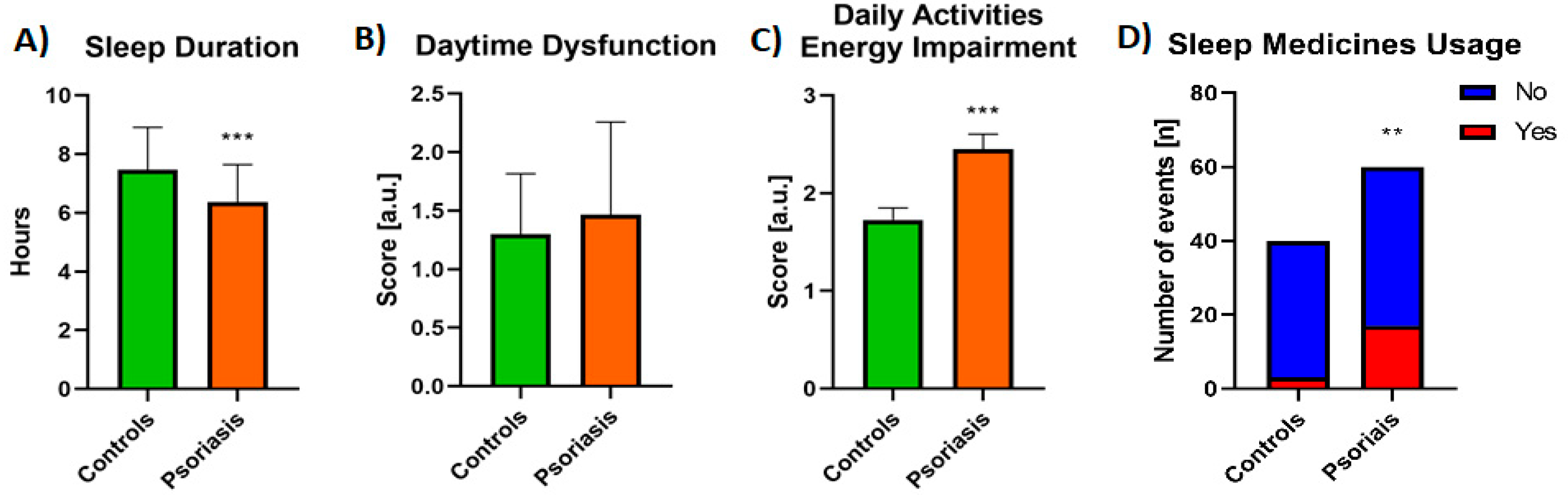
3.1. Sleep Quality Assessment
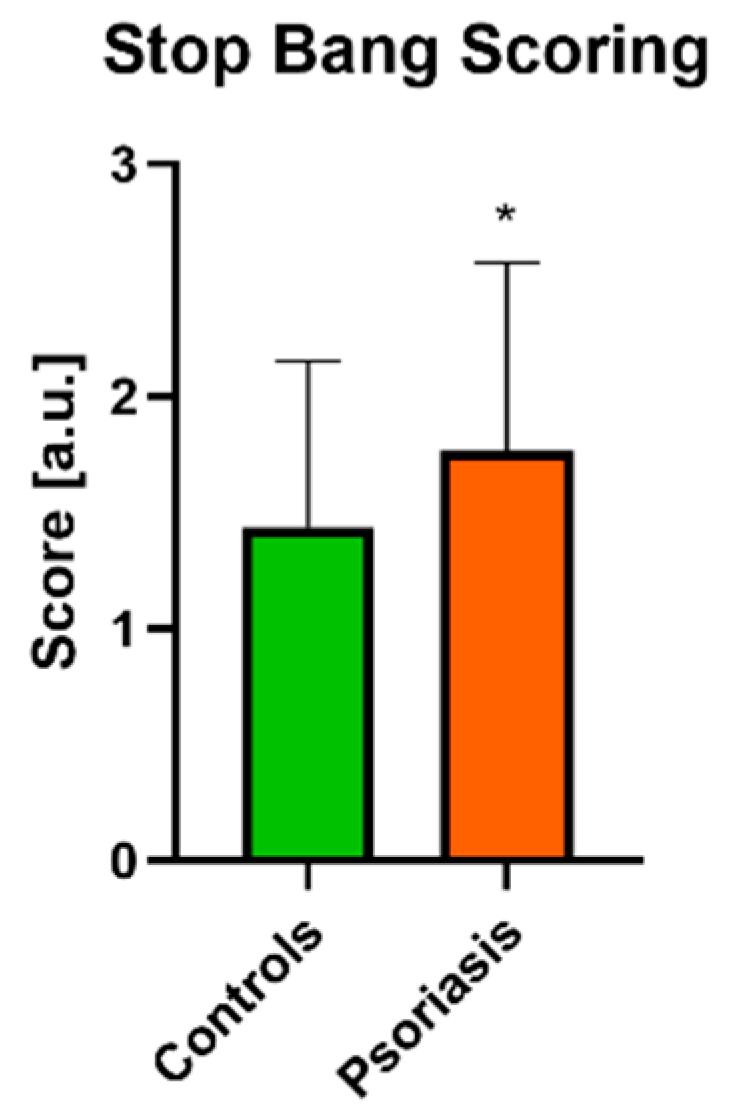
3.2. OSAS Assessment
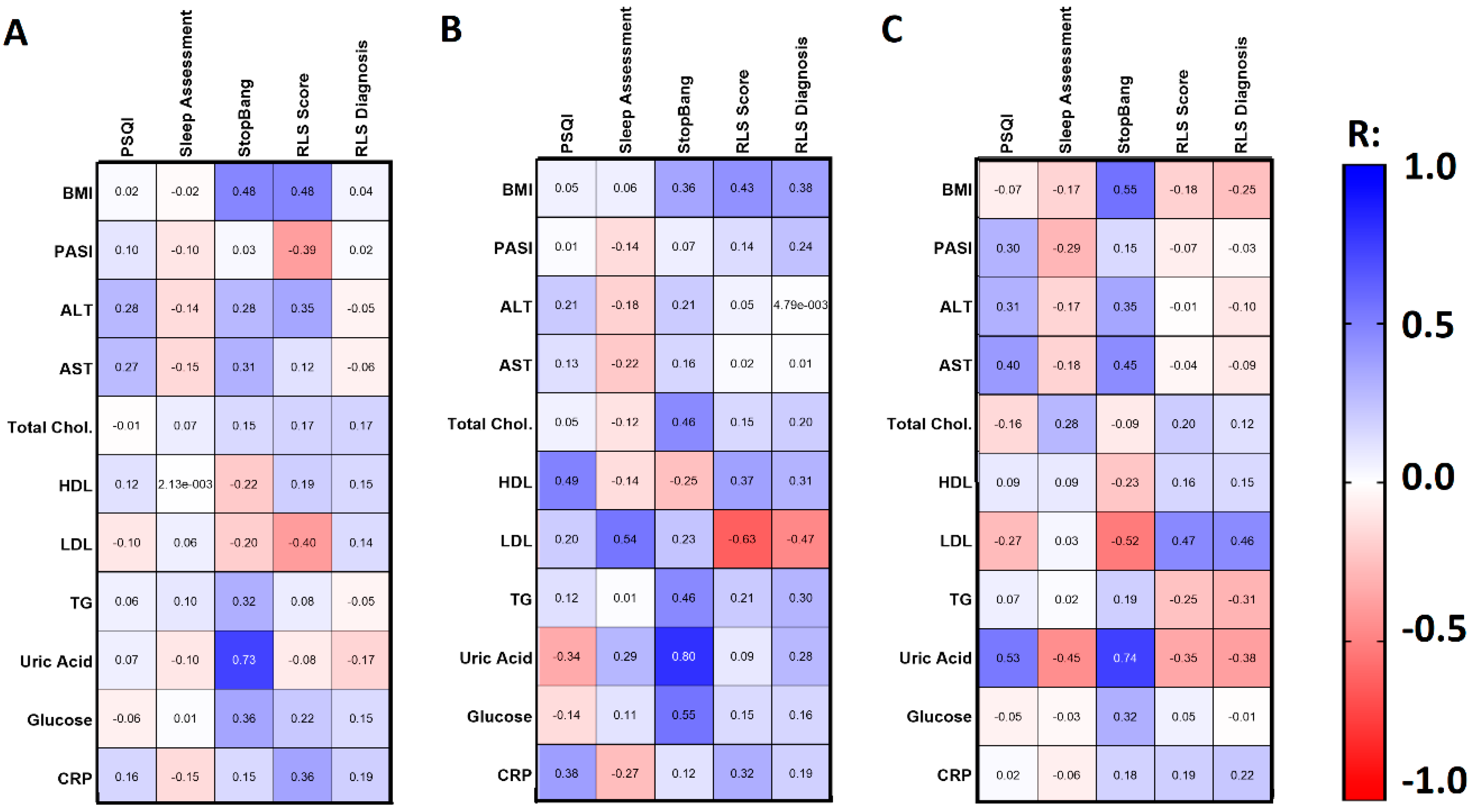
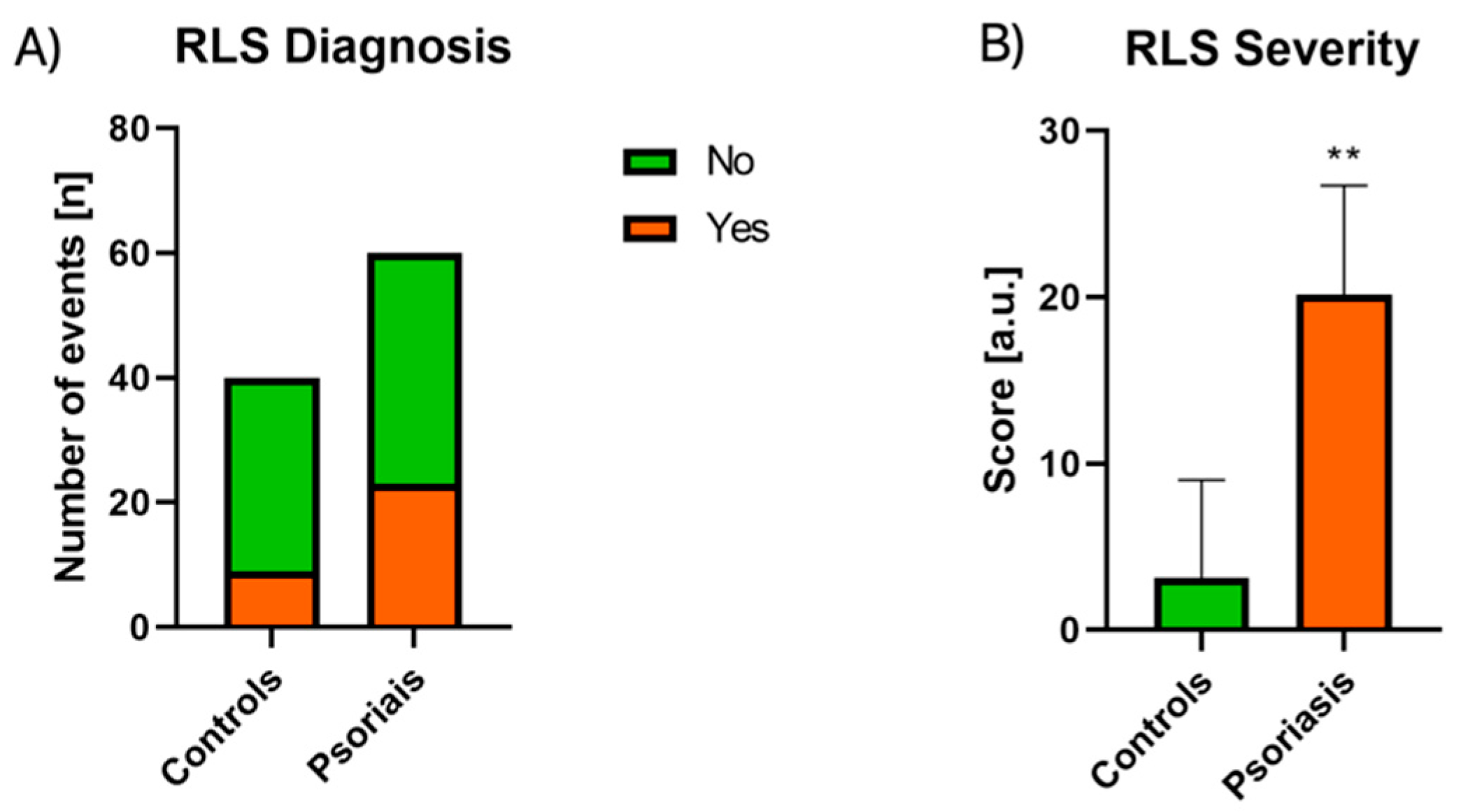
3.3. RLS Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Sleep quality among adult patients with chronic dermatoses. Adv. Dermatol. Allergol. 2019, 36, 659–666. [Google Scholar] [CrossRef]
- Gupta, M.A.; Simpson, F.C.; Gupta, A.K. Psoriasis and sleep disorders: A systematic review. Sleep Med. Rev. 2016, 29, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Kirby, B. Psoriasis Is a Systemic Disease with Multiple Cardiovascular and Metabolic Comorbidities. Dermatol. Clin. 2015, 33, 41–55. [Google Scholar] [CrossRef]
- Li, X.; Kong, L.; Li, F.; Chen, C.; Xu, R.; Wang, H.; Li, B. Association between psoriasis and chronic obstructive pulmonary disease: A system-atic review and meta-analysis. PLoS ONE 2015, 10, e0145221. [Google Scholar] [CrossRef]
- Salman, A.; Yucelten, A.D.; Sarac, E.; Saricam, M.H.; Perdahli-Fis, N. Impact of psoriasis in the quality of life of children, adolescents and their families: A cross-sectional study. An. Bras. Dermatol. 2018, 93, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-N.; Han, K.; Song, S.-W.; Lee, J.H. Hypertension and risk of psoriasis incidence: An 11-year nationwide population-based cohort study. PLoS ONE 2018, 13, e0202854. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Nowowiejska, J.; Krahel, J.A.; Kamiński, T.W.; Maciaszek, M.; Flisiak, I. Higher Serum Selenoprotein P Level as a Novel Inductor of Metabolic Complications in Psoriasis. Int. J. Mol. Sci. 2020, 21, 4594. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Krahel, J.; Kaminski, T.W.; Krawiel, M.; Maciaszek, M.; Flisiak, I. Elevated circulating Krüppel-like fac-tor 4 level as a novel independent marker of the proatherogenic risk in patients with psoriasis: A preliminary study. Adv. Dermatol. Allergol. 2020, 10. in press. [Google Scholar]
- Peralta, C.; Hamid, P.; Batool, H.; Al Achkar, Z.; Maximus, P. Psoriasis and Metabolic Syndrome: Comorbidities and Environmental and Therapeutic Implications. Cureus 2019, 11, e6369. [Google Scholar] [CrossRef]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Sleep disturbances in psoriasis. Dermatol. Rev. 2020, 107, 273–280. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W. Sleep disorders in patients with psoriatic ar-thritis and psoriasis. Reumatologia 2018, 56, 301–306. [Google Scholar] [CrossRef]
- Khalid, U.; Ahlehoff, O.; Gislason, G.; Kristensen, S.L.; Skov, L.; Torp-Pedersen, C.; Hansen, T.W. Psoriasis and risk of heart failure: A nationwide cohort study. Eur. J. Heart Fail. 2014, 16, 743–748. [Google Scholar] [CrossRef]
- Zhao, Q.; Deng, N.; Chen, S.; Cui, Y.; Du, X.; Gu, Z. Systemic lupus erythematosus is associated with negatively variable impacts on domains of sleep disturbances: A systematic review and meta-analysis. Psychol. Health Med. 2018, 23, 685–697. [Google Scholar] [CrossRef]
- Kabeloglu Ilbay, V.; Tas, B.; Altuntas, M.; Atakli, H.D.; Soysal, A. Risk of Obstructive Sleep Apnea Syndrome in Psoriasis Patients. Arch. Iran. Med. 2019, 22, 137–143. [Google Scholar]
- Gonzaga, C.; Bertolami, A.; Bertolami, M.; Amodeo, C.; Calhoun, D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J. Hum. Hypertens. 2015, 29, 705–712. [Google Scholar] [CrossRef]
- Laratta, C.R.; Ayas, N.T.; Povitz, M.; Pendharkar, S.R. Diagnosis and treatment of obstructive sleep apnea in adults. Can. Med. Assoc. J. 2017, 189, E1481–E1488. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical Guideline for the Evaluation, Management and Long-term Care of Obstructive Sleep Apnea in Adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [PubMed]
- Castello-Branco, R.C.; Cerqueira-Silva, T.; Andrade, A.L.; Gonçalves, B.M.; Pereira, C.B.; Felix, I.F.; Santos, L.S.; Porto, L.M.; Marques, M.E.; Catto, M.B.; et al. Association between risk of obstructive sleep ap-nea and cerebrovascular reactivity in stroke patients. J. Am. Heart Assoc. 2020, 9, e015313. [Google Scholar] [CrossRef]
- Vakil, M.; Park, S.; Broder, A. The complex associations between obstructive sleep apnea and auto-immune dis-orders: A review. Med. Hypotheses 2018, 110, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Schell, C.; Schleich, R.; Walker, F.; Yazdi, A.S.; Lerche, H.; Röcken, M.; Axmann, D.; Ghoreschi, K.; Eberle, F.C. Restless legs syndrome in psoriasis: An unexpected comorbidity. Eur. J. Dermatol. 2015, 25, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Trotti, L.M.; Rye, D.B.; De Staercke, C.; Hooper, W.C.; Quyyumi, A.; Bliwise, D.L. Elevated C-reactive protein is associated with severe periodic leg movements of sleep in patients with restless legs syndrome. Brain Behav. Immun. 2012, 26, 1239–1243. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Allen, R.; Högl, B.; Clemens, S.; Patton, S.; Schormair, B.; Winkelmann, J. Comorbidities, treatment, and pathophysiology in restless legs syn-drome. Lancet Neurol. 2018, 17, 994–1005. [Google Scholar]
- Ponikowska, M.; Tupikowska, M.; Kasztura, M.; Jankowska, E.A.; Szepietowski, J.C. Deranged iron status in pso-riasis: The impact of low body mass. J. Cachexia Sarcopenia Muscle 2015, 6, 358–364. [Google Scholar] [CrossRef]
- Allen, R.P. Restless Leg Syndrome/Willis-Ekbom Disease Pathophysiology. Sleep Med. Clin. 2015, 10, 207–214. [Google Scholar] [CrossRef]
- Güler, S.; Tekatas, A.; Arican, O.; Kaplan, O.S.; Dogru, Y. Restless legs syndrome and insomnia frequency in patients with psoriasis. Ideggyógyászati Szle. 2015, 68, 331–336. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K. Psoriasis Is Associated With a Higher Prevalence of Obstructive Sleep Apnea and Restless Legs Syndrome: A Possible Indication of Autonomic Activation in Psoriasis. J. Clin. Sleep Med. 2018, 14, 1085. [Google Scholar] [CrossRef]
- Cicek, D.; Halisdemir, N.; Dertioglu, S.B.; Berilgen, M.S.; Ozel, S.; Colak, C. Increased frequency of restless legs syndrome in atopic dermatitis. Clin. Exp. Dermatol. 2012, 37, 469–476. [Google Scholar] [CrossRef]
- Martínez-Ortega, J.M.; Nogueras, P.; Muñoz-Negro, J.E.; Gutiérrez-Rojas, L.; González-Domenech, P.; Gurpegui, M. Quality of life, anxiety and depressive symptoms in patients with psoriasis: A case-control study. J. Psychosom. Res. 2019, 124, 109780. [Google Scholar] [CrossRef]
- Hawro, T.; Hawro, M.; Zalewska-Janowska, A.; Weller, K.; Metz, M.; Maurer, M. Pruritus and sleep disturbances in patients with psoriasis. Arch. Dermatol. Res. 2020, 312, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Hsieh, C.F.; Chiang, Y.T.; Tsai, Y.W.; Huang, W.F.; Li, C.Y.; Wang, T.S.; Tsai, T.F. Concomitant sleep disorders significantly increase the risk of cardio-vascular disease in patients with Psoriasis. PLoS ONE. 2016, 11, e0146462. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.J.; Ricotta, D.N.; Freed, J.; Smith, C.C.; Huang, G.C. Adapting Maslow’s Hierarchy of Needs as a frame-work for resident wellness. Teach. Learn Med. 2019, 31, 109–118. [Google Scholar] [CrossRef]
- Sacmaci, H.; Gürel, G. Sleep disorders in patients with psoriasis: A cross-sectional study using non-polysomnographical methods. Sleep Breath. 2019, 23, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Melikoglu, M. Sleep Quality and its Association with Disease Severity in Psoriasis. Eurasian J. Med. 2017, 49, 124–127. [Google Scholar] [CrossRef]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.; Gelfand, J.M.; Lichten, J.; Mehta, N.N.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Elewski, B.E.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef]
- Reich, A.; Adamski, Z.; Chodorowska, G.; Kaszuba, A.; Krasowska, D.; Lesiak, A.; Maj, J.; Narbutt, J.; Osmola-Mańkowska, A.J.; Owczarczyk-Saczonek, A.; et al. Psoriasis. Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Part 1. Dermatol. Rev. 2020, 107, 92–108. [Google Scholar] [CrossRef]
- Guo, S.; Sun, W.; Liu, C.; Wu, S. Structural validity of the Pittsburgh Sleep Quality Index in Chinese undergradu-ate students. Front Psychol. 2016, 7, 1126. [Google Scholar] [CrossRef]
- Buysse, D.J.; Hall, M.L.; Strollo, P.J.; Kamarack, T.W.; Owens, J.; Lee, L.; Reis, S.E.; Matthews, K.A. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Ep-worth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J. Clin. Sleep Med. 2008, 4, 563–571. [Google Scholar] [CrossRef]
- Stinco, G.; Trevisan, G.; Piccirillo, F.; Di Meo, N.; Nan, K.; Deroma, L.; Bergamo, S.; Patrone, P. Psoriasis vulgaris does not adversely influence the quality of sleep. G. Ital. Dermatol. Venereol. 2013, 148, 655–659. [Google Scholar] [PubMed]
- Choi, C.W.; Kim, B.R.; Park, J.S.; Youn, S.W. Both educational lectures and reference photographs are necessary to improve the accuracy and reliability of Psoriasis Area and Severity Index (PASI) assessment: Results from Kore-an nation-wide PASI educational workshop. Ann. Dermatol. 2018, 30, 284–289. [Google Scholar] [CrossRef]
- Bożek, A.; Reich, A. The reliability of three psoriasis assessment tools: Psoriasis area and severity index, body surface area and physician global assessment. Adv. Clin. Exp. Med. 2017, 26, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Tas, B.; Kabeloglu, V.; Soysal, A.; Atakli, D. Sleep quality in psoriasis patients and its relations with possible af-fecting factors. Med Bull. Sisli Etfal Hosp. 2020, 54, 181–187. [Google Scholar]
- Chang, S.P.; Chen, Y.-H. Relationships between sleep quality, physical fitness and body mass index in college freshmen. J. Sports Med. Phys. Fit. 2014, 55, 1234–1241. [Google Scholar]
- Wang, J.; Chen, Y.; Jin, Y.; Zhu, L.; Yao, Y. Sleep quality is inversely related to body mass index among university students. Rev. Assoc. Médica Bras. 2019, 65, 845–850. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Lorenzi-Filho, G.; De Paula, L.K.; Oliveira, C.M.; Alvim, R.D.O.; Beijamini, F.; Negrão, A.B.; Von Schantz, M.; Knutson, K.; Krieger, J.E.; et al. Poor sleep quality and lipid profile in a rural cohort (The Baependi Heart Study). Sleep Med. 2019, 57, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Gjevre, R.M.; Gjevre, J.A.; Nair, B.V.; Skomro, R.P.; Lim, H.J. Improved sleep efficiency after anti-tumor necrosis factor α therapy in rheumatoid arthritis patients. Ther. Adv. Musculoskelet. Dis. 2011, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, C.; Nogueira, H.; Albuquerque, R.G.; Tomimori, J.; Tufik, S.; Andersen, M.L. The bidirectional interactions between psoriasis and obstructive sleep apnea. Int. J. Dermatol. 2015, 54, 1352–1358. [Google Scholar] [CrossRef]
- Papadavid, E.; Dalamaga, M.; Vlami, K.; Koumaki, D.; Gyftopoulos, S.; Christodoulatos, G.S.; Papiris, S.; Rigopoulos, D. Psoriasis is associated with risk of obstructive sleep apnea independently from metabolic parameters and other comorbidities: A large hospital-based case-control study. Sleep Breath. 2017, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.J.; Ren, J. Obstructive sleep apnoea and cardiovascular complications: Perception versus knowledge. Clin. Exp. Pharmacol. Physiol. 2012, 39, 995–1003. [Google Scholar] [CrossRef]
- Goes, A.C.J.; Reis, L.A.B.; Silva, M.B.G.; Kahlow, B.S.; Skare, T. Rheumatoid arthritis and sleep quality. Rev. Bras. Reum. English Ed. 2017, 57, 294–298. [Google Scholar] [CrossRef]
- Buslau, M.; Benotmane, K. Cardiovascular complications of psoriasis: Does obstructive sleep apnea play a role? Acta Derm Venereol. 1999, 79, 234. [Google Scholar] [PubMed]
- Atan, D.; Köseoğlu, S.; Özcan, K.M.; Ikincioğulları, A.; Topak, A.B.; Özcan, I.; Dere, H. Evaluation of Liver Functions Based on Serum Aminotransferase Enzyme Levels in Patients with Obstructive Sleep Apnea Syndrome. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 1679–1682. [Google Scholar] [CrossRef]
- Hirotsu, C.; Tufik, S.; Guindalini, C.; Mazzotti, D.R.; Bittencourt, L.R.A.; Andersen, M.L. Association Between Uric Acid Levels and Obstructive Sleep Apnea Syndrome in a Large Epidemiological Sample. PLoS ONE 2013, 8, e66891. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Nakatsumi, Y.; Sugiyama, Y.; Hamaoka, T.; Murai, H.; Takamura, M.; Kaneko, S.; Takata, S.; Takamura, T.; Takada, S. Severity Indices for Obstructive Sleep Apnea Syndrome Reflecting Glycemic Control or Insulin Resistance. Intern. Med. 2019, 58, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, M.S.; Berkowitz, R.I.; Tapia, I.E. Effects of obesity therapies on sleep disorders. Metabolism 2018, 84, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Debbaneh, M.; Millsop, J.W.; Bhatia, B.K.; Koo, J.; Liao, W. Diet and psoriasis, part I: Impact of weight loss inter-ventions. J. Am. Acad Dermatol. 2014, 71, 133–140. [Google Scholar] [CrossRef]
- Holzknecht, E.; Hochleitner, M.; Wenning, G.K.; Högl, B.; Stefani, A. Gender differences in clinical, laboratory and polysomnographic features of restless legs syndrome. J. Sleep Res. 2020, 29, e12875. [Google Scholar] [CrossRef] [PubMed]
- Yazar, H.O.; Yazar, T.; Özdemir, S.; Arici, Y.K. Serum C-reactive protein/albumin ratio and restless legs syn-drome. Sleep Med. 2019, 58, 61–65. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Li, L.; Yang, J.; Ma, S. Evaluation of Cardiovascular Risk Factors and Restless Legs Syndrome in Women and Men: A Preliminary Population-Based Study in China. J. Clin. Sleep Med. 2018, 14, 445–450. [Google Scholar] [CrossRef]
- Etindele-Sosso, F. Insomnia, excessive daytime sleepiness, anxiety, depression and socioeconomic status among customer service employees in Canada. Sleep Sci. 2020, 13, 54–64. [Google Scholar] [PubMed]
- Silva-Perez, L.J.; Gonzalez-Cardenas, N.; Surani, S.; Etindelle Sosso, F.A.; Surani, S.R. Socioeconomic Status in Pregnant Women and Sleep Quality During Pregnancy. Cureus 2019, 11, e6183. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Sosso, F.E.; Khoury, T.; Surani, S.R. Sleep Disturbances Are Mediators between Socioeconomic Status and Health: A Scoping Review. Int. J. Ment. Health Addict. 2020, 1–25. [Google Scholar] [CrossRef]
| Parameter | Controls n = 40 | Psoriatic Patients n = 60 |
|---|---|---|
| Sex (M/F) | 21/19 | 31/29 NS |
| Age (years) | 49.78 ± 17.9 | 49.75 ± 17.03 NS |
| BMI | 25.38 (20.01–35.5) | 25.45 (17.01–42.10) NS |
| PASI before treatment | - | 14.23 (2–44.4) |
| PASI after treatment | - | 8.6 (0–25) *** |
| Parameters | Under 45 n = 21 | Over 45 n = 39 |
|---|---|---|
| Sex (M/F) | 10/11 | 21/18 NS |
| BMI | 24.03 (17.3–41.21) | 26.75 (17.01–42.1) NS |
| PASI before | 10.7 (4–27.3) | 12.6 (2–44.4) NS |
| ALT | 17 (6–77) | 19 (6–98) NS |
| AST | 24.38 ± 8.9 | 28.1 ± 13.01 NS |
| Total cholesterol | 154.2 ± 36.76 | 159.2 ± 41.74 NS |
| HDL | 39.71 ± 10.98 | 45.75 ± 15.28 NS |
| LDL | 92 ± 31.96 | 90.55 ± 51.81 NS |
| TG | 119.6 ± 37.24 | 140 ± 70.99 NS |
| Uric acid | 4.41 ± 0.93 | 6.46 ± 1.85 ** |
| Glucose | 84.81 ± 13 | 97.08 ± 32.89 * |
| CRP | 2.44 (0.66–46.2) | 4.395 (1.03–91) NS |
| PSQI | 7.47 ± 4.4 | 8.43 ± 4.15 NS |
| Subjective sleep assessment | 3.38 ± 1.28 | 3.18 ± 1.33 NS |
| STOP BANG | 1.28 ± 0.71 | 2.04 ± 0.71 * |
| RLS diagnosis | 6/14 | 17/22 NS |
| RLS severity | 19.33 ± 8.52 | 20.41 ± 6.05 NS |
| Parameter | PASI I n = 23 | PASI II n = 37 |
|---|---|---|
| Sex (M/F) | 8/15 | 23/14 * |
| BMI | 28.63 (18.11–41.21) | 25.16 (17.01–42.1) NS |
| Psoriatic arthritis | 1/22 | 9/28 * |
| ALT | 17 (10–77) | 20 (6–98) NS |
| AST | 21 (10–100) | 24 (12–81) NS |
| Total cholesterol | 160.3 ± 38.41 | 155.7 ± 41.1 NS |
| HDL | 43.11 ± 11.04 | 44.72 ± 16.01 NS |
| LDL | 81 (41–197) | 74 (24–154) NS |
| TGs | 124.4 ± 53.12 | 138.1 ± 66.7 NS |
| Uric acid | 4.6 (3.3–11.17) | 6.1 (3.2–8.9) NS |
| Glucose | 94.91 ± 28.81 | 91.46 ± 27.94 NS |
| CRP | 4 (1.03–46.2) | 3.66 (0.66–91) NS |
| PSQI | 7.91 ± 4.06 | 8.22 ± 4.31 NS |
| Subjective sleep assessment | 3.22 ± 1.13 | 3.27 ± 1.43 NS |
| STOP BANG | 1.74 ± 0.81 | 1.78 ± 0.82 NS |
| RLS diagnosis | 8/15 | 15/22 NS |
| Parameter | Topical Treatment n = 25 | Systemic Treatment n = 35 |
|---|---|---|
| BMI | 25.27 (17.01–42.1) | 28.81 (17.56–41.21) NS |
| PASI Before | 12.14 ± 6.41 | 15.63 ± 7.2 NS |
| ALT | 19.5 (7–92) | 16.5 (6–98) NS |
| AST | 24.5 (10–41) | 22 (10–100) NS |
| Cholesterol | 163.7 ± 40.51 | 152.7 ± 39.21 NS |
| HDL | 46.75 ± 17.58 | 42.13 ± 11.36 NS |
| LDL | 43 (24–88) | 39 (25–197) NS |
| TG | 116.5 ± 46.72 | 145.4 ± 69.2 * |
| Uric acid | 5.3 (3.2–11.17) | 6.2 (3.3–8.4) NS |
| Glucose | 90.73 ± 19.96 | 94.35 ± 33.2 NS |
| CRP | 3.31 (1.03–46.2) | 4.44 (0.66–91) NS |
| PSQI | 7 (1–17) | 9 (3–18) ** |
| Subjective sleep assessment | 3.615 ± 1.17 | 2.971 ± 1.36 * |
| STOP BANG | 1.654 ± 0.85 | 1.85 ± 0.78 NS |
| RLS diagnosis | 8/17 | 15/16 NS |
| RLS Scoring | 16.88 ± 6.71 | 21.87 ± 6.03 * |
| Parameters | |t| | p Value | p Value Summary |
|---|---|---|---|
| Total cholesterol | 2.899 | 0.0338 | * |
| Glucose | 3.227 | 0.0233 | * |
| CRP | 3.481 | 0.0176 | * |
| PSQI | 2.666 | 0.0446 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowowiejska, J.; Baran, A.; Lewoc, M.; Grabowska, P.; Kaminski, T.W.; Flisiak, I. The Assessment of Risk and Predictors of Sleep Disorders in Patients with Psoriasis—A Questionnaire-Based Cross-Sectional Analysis. J. Clin. Med. 2021, 10, 664. https://doi.org/10.3390/jcm10040664
Nowowiejska J, Baran A, Lewoc M, Grabowska P, Kaminski TW, Flisiak I. The Assessment of Risk and Predictors of Sleep Disorders in Patients with Psoriasis—A Questionnaire-Based Cross-Sectional Analysis. Journal of Clinical Medicine. 2021; 10(4):664. https://doi.org/10.3390/jcm10040664
Chicago/Turabian StyleNowowiejska, Julia, Anna Baran, Marta Lewoc, Paulina Grabowska, Tomasz W. Kaminski, and Iwona Flisiak. 2021. "The Assessment of Risk and Predictors of Sleep Disorders in Patients with Psoriasis—A Questionnaire-Based Cross-Sectional Analysis" Journal of Clinical Medicine 10, no. 4: 664. https://doi.org/10.3390/jcm10040664
APA StyleNowowiejska, J., Baran, A., Lewoc, M., Grabowska, P., Kaminski, T. W., & Flisiak, I. (2021). The Assessment of Risk and Predictors of Sleep Disorders in Patients with Psoriasis—A Questionnaire-Based Cross-Sectional Analysis. Journal of Clinical Medicine, 10(4), 664. https://doi.org/10.3390/jcm10040664









